Abstract
The neurobiology of methamphetamine (MA) remains largely unknown despite its high abuse liability. The present series of studies explored the role of adenosine receptors on MA reward and reinforcement and identified alterations in the expression of adenosine receptors in dopamine terminal areas following MA administration in rats. We tested whether stimulating A1 or A2A adenosine receptor subtypes would influence MA-induced place preference or MA self-administration on fixed and progressive ratio schedules in male Sprague-Dawley rats. Stimulation of either adenosine A1 or A2A receptors significantly reduced the development of MA-induced place preference. Stimulating A1, but not A2A, adenosine receptors reduced MA self-administration responding. We next tested whether repeated experimenter-delivered MA administration would alter the expression of adenosine receptors in the striatal areas using immunoblotting. We observed no change in the expression of adenosine receptors. Lastly, rats were trained to self-administer MA or saline for 14 days and we detected changes in adenosine A1 and A2A receptor expression using immunoblotting. MA self-administration significantly increased adenosine A1 in the nucleus accumbens shell, caudate-putamen and prefrontal cortex. MA self-administration significantly decreased adenosine A2A receptor expression in the nucleus accumbens shell, but increased A2A receptor expression in the amygdala. These findings demonstrate that MA self-administration produces selective alterations in adenosine receptor expression in the nucleus accumbens shell and that stimulation of adenosine receptors reduces several behavioral indices of MA addiction. Together, these studies shed light onto the neurobiological alterations incurred through chronic MA use that may aid in the development of treatments for MA addiction.
1. INTRODUCTION
The acute rewarding and reinforcing effects of many drugs of abuse, including methamphetamine (MA) result from elevations in dopamine (DA) in the mesocorticolimbic system, which is comprised of DA cells in the ventral tegmental area (VTA) that terminate in forebrain regions such as the nucleus accumbens (NAc). MA has multiple, well-defined actions that amplify synaptic activity of DA in the mesocorticolimbic system (McCann et al., 2008, Pereira et al., 2002, Pereira et al., 2006). Importantly, MA potently reverses the activity of both the DA transporter and the intracellular vesicular monoamine transporter 2. This results in high intracellular concentration of DA that is transported through non-vesicular transport from the cytoplasm into the synaptic cleft via reverse action of the DA transporter (Vergo et al., 2007, Volz et al., 2007).
MA induces robust neurobiological changes in the mesolimbic system. For example, MA abuse in humans is associated with striatal DA D1 receptors upregulation and striatal DA D2 receptor downregulation (Volkow et al., 2001a, Volkow et al., 2001b, Volkow et al., 2001c, Worsley et al., 2000). Animal studies show somewhat different effects following MA administration in that MA produces reductions in both DA D1 and D2 receptor expression with seemingly greater reductions in DA D1 receptors following repeated experimenter-delivered MA (McCabe et al., 1987, Segal et al., 2005, Stefanski et al., 1999). More recent data suggests that DA receptor downregulation is offset by increases in high affinity DA D1 and D2 receptors following chronic MA treatment (Shuto et al., 2008). Together, these findings suggest that both acute and chronic actions of MA alter the mesocorticolimbic system to produce the behavioral effects of MA.
There has been recent interest in pursuing adenosine as a negative modulator of DA receptor signaling. Adenosine is a nucleoside neurotransmitter found ubiquitously in the brain. Under basal conditions, adenosine levels are quite low (nM range), but sufficient for tonic receptor binding and observable physiological effects (Ballarin et al., 1991, Dunwiddie and Masino, 2001, Snyder et al., 1981). Phasic increases in adenosine levels can arise from increased neuronal metabolic activity and co-release of adenosine triphosphate (ATP) with vesicular neurotransmitter release (Cass et al., 1987, Fredholm et al., 1982, Thorn and Jarvis, 1996, White, 1977). Vesicular release of DA, for example, is accompanied by the release of ATP that can be metabolized to adenosine and act on postsynaptic adenosine receptors (Cass et al., 1987, Fredholm et al., 1982, Thorn and Jarvis, 1996, White, 1977). Under physiological conditions, DA and adenosine receptor subtypes have antagonistic interactions through the formation of receptor-receptor complexes (i.e. heteromeric receptors) and/or opposing G protein mediated signaling cascades. However, these antagonistic receptor interactions may not be fully appreciated in the presence of MA. Thus, non-vesicular release of DA by MA can potently and aberrantly stimulate postsynaptic DA receptors in the absence of important regulators such as adenosine. This lack of complementary regulation by adenosine may promote the development and persistence of MA-induced neurobiological changes and subsequent abuse.
Here, we explore how the stimulation of the two primary neuronal adenosine receptor subtypes (A1 and A2A) affects the development of a conditioned place preference for MA and affects MA intake using self-administration procedures. Additionally, we identify how experimenter-delivered MA or MA self-administration alters the expression of these adenosine receptor subtypes that are robustly expressed in DA terminal areas such as the NAc, prefrontal cortex and amygdala (Dixon et al., 1996).
2. MATERIALS AND METHODS
2.1 Animals
Male Sprague–Dawley rats (Charles River, Wilmington, MA) weighing 275–325g were individually housed with ad libitum food and water upon arrival. All experiments were conducted during the light period of a (12:12) light/dark cycle. All procedures were completed in accordance with the Guide for the Care and Use of Animals and approved the Institutional Animal Care and Use Committee at the University of Colorado Boulder. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize available alternatives to in vivo techniques.
2.2 Place Conditioning
Place conditioning was conducted according to previously published procedures (Merritt and Bachtell, 2013). A custom-built, unbiased three chambered apparatus was used for place conditioning procedures. The two conditioning chambers (15 cm X 25 cm X 35 cm) were distinct in wall patterns (gray vs. vertical white and black stripes) and floor textures (grid vs. hole). The center compartment (15 cm X 10 cm) had white walls and a plexiglass floor. Chambers were equipped with infrared photocells to detect animal position and movement in the apparatus. A three-phase procedure was conducted as follows: Day 1–20 min preconditioning session, Days 2–4- six 30 min conditioning sessions (3 AM saline; 3 PM MA) and Day 5–20 min post-conditioning session. Locomotor activity during the conditioning sessions was measured by photocell beam breaks. During the pre- and post-conditioning session, time spent in each compartment was also measured by calculating the duration of photocell beam breaks within each chamber. The animal’s preference was determined using a conditioned place preference (CPP) score that was calculated by subtracting the time in the saline-paired compartment from the time in drug-paired compartment. We tested the effects of adenosine A1 agonist (CPA: 0.03 and 0.1 mg/kg, i.p., n=9/dose) and A2A receptor agonist (CGS 21680: 0.01 and 0.03 mg/kg, i.p., n=9–10/dose) on the development of a place preference induced by MA (1.0 mg/kg, i.p.) by administering the agonist 5 min prior to MA during conditioning. Vehicle pretreatment (n=16) served as a control. Dosing and timing of injections was determined by previous studies examining the behavioral effects of adenosine agonists on psychostimulant-induced behaviors (Bachtell and Self, 2009, Golembiowska and Zylewska, 1998, 2000, Hobson et al., 2013, O’Neill et al., 2012, Shimazoe et al., 2000, Yoshimatsu et al., 2001).
2.3 Methamphetamine Treatments for Tissue Collection
We tested the effects of both experimenter-delivered MA and self-administered MA on the expression of adenosine receptors in dopamine terminal areas. For the experimenter-delivered experiment, rats randomly divided into two treatment groups (saline or 1.5 mg/kg MA, ip). Rats were treated for 7 consecutive days in the home cage. Twenty-four hours after the last injection, animals were sacrificed by rapid decapitation and tissue was processed and analyzed as described below. For the self-administration experiment, animals were trained to lever-press for sucrose pellets in standard operant test chambers (Med Associates Inc, St. Albans, VT) under food-restricted conditions. Rats were then fed ad libitum and surgically implanted with chronic indwelling intra-jugular catheters (O’Neill et al., 2012). Rats were randomly assigned to either a saline (n=6) or MA (n=10) self-administration group following recovery from surgery. MA self-administering animals were able to lever press for MA (0.05 mg/kg/injection) on an fixed ratio 1:time-out 15 sec (FR1:TO15) schedule in daily 2 hr sessions over 14 days. Saline self-administering animals were treated identically, however, saline was substituted for MA. Twenty-four hours after the last self-administration session, animals were sacrificed by rapid decapitation and tissue was processed and analyzed as described below.
2.4 Methamphetamine Self-administration Behavioral Procedures
Separate groups of animals were run through the self-administration procedure to test the effects of adenosine receptor stimulation on FR and progressive ratio (PR) responding. To facilitate acquisition of MA self-administration, rats were trained to lever-press for sucrose pellets in standard operant test chambers under food-restricted conditions. Rats were then fed ad libitum and surgically implanted with chronic indwelling intra-jugular catheters. Following recovery, rats self-administered MA (FR1:TO20 sec) in daily 2 hr sessions. Separate groups of animals were trained with either 0.05 mg/kg/infusion MA or 0.1 mg/kg/infusion MA. After 1 week of FR1:TO20 sec responding, rats were advanced to an FR5:TO20 sec schedule until stable (MA intake varies <10% over 3 consecutive days). On the test day, animals received an adenosine agonist (CPA: 0.03 & 0.1 mg/kg, ip; CGS 21680: 0.01 & 0.03 mg/kg, ip) pretreatment 5 minutes prior to the start of the session. The breakdown of the groups administering 0.05 MA mg/kg/infusion on an FR5 schedule was as follows: Veh (n=12), 0.01 CGS (n=8), 0.03 CGS (n=6), 0.03 CPA (n=9), 0.1 CPA (n=10). The breakdown of the groups administering 0.1 MA mg/kg/infusion on an FR5 schedule was as follows: Veh (n=7), 0.01 CGS (n=9), 0.03 CGS (n=6), 0.03 CPA (n=9), 0.1 CPA (n=7). Dosing and timing of injections was determined by previous studies examining the behavioral effects of adenosine agonists on psychostimulant-induced behaviors (Bachtell and Self, 2009, Golembiowska and Zylewska, 1998, 2000, Hobson et al., 2013, O'Neill et al., 2012, Shimazoe et al., 2000, Yoshimatsu et al., 2001). Rats were tested in counterbalanced fashion across all doses and baseline performance in the absence of a pretreatment served as a control for repeated testing. Thus, between each treatment animals were re-stabilized on the FR5:TO20 schedule prior to receiving the next treatment. All animals received at least 1 treatment and up to 4 treatments total, but no animals were successfully administered all test doses.
Testing on the PR schedule was conducted identically to the FR5 testing except that after being advanced to an FR5:TO20 sec schedule and achieving stability (MA intake varies <10% over 3 consecutive days) rats were advanced to the PR schedule. The progression for response/injection ratios was determined according to [5e(injection number x 0.2)]-5 (e.g. 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50 etc.). Separate groups of animals were trained with either 0.05 mg/kg/infusion MA or 0.1 mg/kg/infusion MA. Stable baseline performance on the PR schedule was evaluated over several days prior to administration of CPA (0, 0.03, 0.1 mg/kg, ip) or CGS 21680 (0, 0.01, 0.03 mg/kg, ip). On the test day, animals received a pretreatment 5 minutes prior to the start of the session. The breakdown of the groups administering 0.05 MA mg/kg/infusion on an PR schedule was as follows: Veh (n=11), 0.01 CGS (n=7), 0.03 CGS (n=6), 0.03 CPA (n=6), 0.1 CPA (n=16). The breakdown of the groups administering 0.1 MA mg/kg/infusion on an PR schedule was as follows: Veh (n=10), 0.01 CGS (n=10), 0.03 CGS (n=16), 0.03 CPA (n=10), 0.1 CPA (n=16). Rats were tested in counterbalanced fashion across all doses and baseline performance in the absence of a pretreatment served as a control for repeated testing. Thus, between each treatment animals were re-stabilized on the PR schedule prior to receiving the next treatment. All animals received at least 1 treatment and up to 4 treatments total, but no animals were successfully administered all test doses.
2.5 Sucrose Self-administration Procedures
The effects of adenosine receptor agonists were also tested on fixed ratio in separate groups of rats. Rats were initially trained to lever-press for 45 mg sucrose pellets (Bio-Serv, Flemington, NJ) in standard operant test chambers on an FR1:TO20 sec schedule of reinforcement under food-restricted conditions. Self-administration sessions were terminated after the acquisition of 100 sucrose pellets or at 1 hr. Rats were advanced to an FR5:TO20 sec schedule when they consistently reached the criterion of >75 sucrose pellets earned during the 1 hr session. Rats were tested when they established stable responding for sucrose on the FR5 schedule (sucrose pellets earned or latency to acquire 100 pellets varied <10% over 3 consecutive days). On the test day, animals received an adenosine agonist (vehicle, 0.1 mg/kg CPA, or 0.03 mg/kg CGS 21680, ip) pretreatment 5 minutes prior to the start of the session. Each rat was repeatedly tested across vehicle and either CPA or CGS 21680 and the ordering was counterbalanced. Between each treatment animals were re-stabilized on the PR schedule prior to receiving the next treatment. The breakdown of the groups tested on the sucrose FR5 schedule was as follows: Veh (n=22), 0.1 CPA (n=11), 0.03 CGS (n=11).
2.6 Immunoblotting
Bilateral 1 mm3 tissue punches of the caudate-putamen nucleus accumbens core, nucleus accumbens shell, prefrontal cortex and amygdala were taken from chilled tissue slices and were homogenized immediately in lysis buffer. Samples were stored at −80°C until protein levels were quantified by a Lowry protein assay (BioRad, Hercules, CA). Samples (15 µg/well) from each animal were separated by SDS-PAGE and electrophoretically transferred to PVDF membranes. Blots were run with equal numbers of control and MA-treated samples per gel and loaded in an alternating fashion. The membranes were blocked overnight in 5% bovine serum albumin at 4°C and incubated in primary antibody for adenosine A1 (1:1000 Calbiochem/EMD Millipore, Billerica, MA) and adenosine A2A (1:2000 Millipore, Billerica, MA) receptors for 24 hours at 4°C. The membranes were washed and labeled with species-specific peroxidase-conjugated secondary (1:10–20 K; Bio-Rad) for one hour at 25°C. Following chemiluminescence detection (SuperSignal Dura West; Thermoscientific), blots were stripped for 20 minutes at room temperature (Restore, Thermo Scientific, Rockford, IL, USA) and reprobed. All blots were stripped and re-probed for the loading control protein, β-Tubulin (1:1000 Millipore, Billerica, MA). Immunoreactivity was quantified by densitometry (ImageJ) under conditions linear over at least a threefold concentration range. The optical density for the proteins was normalized to β Tubulin and a percentage change from control was derived.
2.7 Drugs
The selective adenosine A1 receptor agonist, CPA (N6-Cyclopentyladenosine), and selective adenosine A2A receptor agonist CGS 21680 hydrochloride, (4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride), were purchased from Tocris Bioscience (Bristol, UK). Methamphetamine hydrochloride was obtained from Sigma-Aldrich (St Louis, MO). All drugs were dissolved in sterile-filtered physiological saline.
2.8 Data Analysis
The effects of MA administration on the optical density of each protein of interest from immunoblotting was normalized to β Tubulin and then analyzed separately using a t-test. Conditioned place preference (CPP) was evaluated using a CPP score (time in drug-paired minus time in saline-paired) and analyzed using a 2-way between subjects ANOVA with adenosine agonist dose and conditioning (pre-conditioning vs. post-conditioning) as factors. The effect of adenosine receptor stimulation during self-administration test sessions was analyzed separately for each MA dose using a one-way ANOVA across adenosine agonist doses. A percentage score was calculated by dividing the number of infusions (or pellets/latency) during the test session by the number of infusions (or pellets/latency) during baseline, multiplying by 100 and subtracted 100. This score was used as the dependent variable in these tests since there was a high degree of variability between animals that obscured the effects of the treatments. Similarly, a percentage score was used to evaluate the effect of adenosine receptor stimulation on sucrose self-administration, although separate paired t-tests for each adenosine agonist were used for analysis. In all cases, significant interactions and main effects were followed by appropriate post hoc tests.
3. RESULTS
3.1 Stimulation of adenosine receptors impairs the development of methamphetamine-induced conditioned place preference
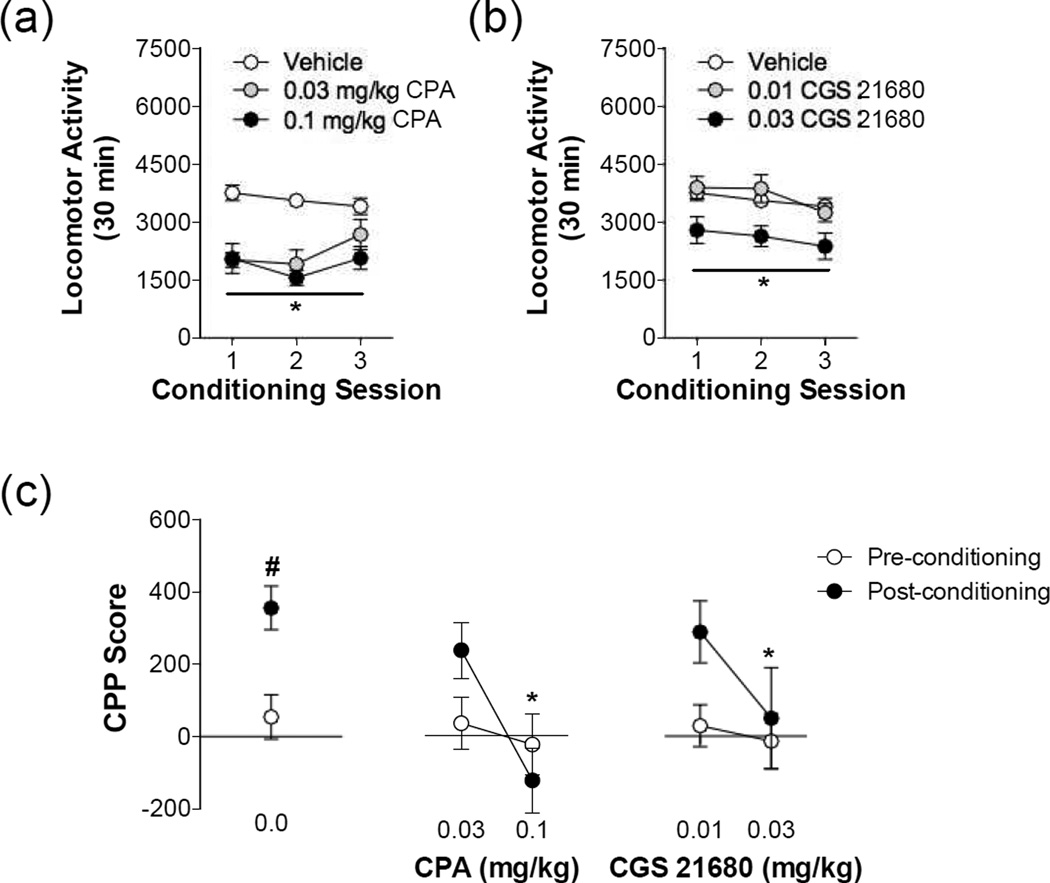
We first determined whether adenosine receptor stimulation would impair the acute rewarding effects of MA using a place conditioning procedure. Stimulation of both A1 and A2A receptors prior to MA administration blunted MA-induced locomotion during the conditioning sessions (Figure 1). Adenosine A1 receptor stimulation significantly reduced MA-induced locomotion during the conditioning trials as revealed by a significant main effect of CPA dose (F(2,86) = 21.85, P < 0.0001). Likewise, a main effect of CGS 21680 dose reveals that A2A stimulation dose-dependently reduced MA-induced locomotion, where only 0.03 mg/kg CGS 21680 significantly reduced MA-induced locomotion (F(2,88) = 5.660, P = 0.0065). As shown in Figure 1, stimulation of either adenosine A1 or A2A receptors also dose-dependently inhibited the development of MA place preference (CPA: F(2,31) = 6.444, P = 0.0284; CGS 21680: F(2,32) = 3.333, P = 0.0484). Thus, a pretreatment of the 0.1 mg/kg CPA and 0.03 mg/kg CGS 21680 impaired the development of MA place preference. These findings suggest that stimulation of either adenosine A1 or A2A receptors is sufficient to reduce MA reward conditioning in drug naïve animals.
Figure 1. Adenosine receptor stimulation impairs methamphetamine-induced locomotion and reward.
Methamphetamine (1.0 mg/kg, ip) produced locomotor stimulation that was impaired by (a) the adenosine A1 selective agonist, CPA, and (b) the adenosine A2A selective agonist, CGS 21680 (* significant from vehicle, P < 0.05). (c) A significant place preference was observed in rats pretreated with vehicle on conditioning days (# significant from preconditioning, P < 0.05). Both adenosine A1 receptor and adenosine A2A receptor stimulation during conditioning dose-dependently impaired the development of a methamphetamine place preference (* significant from vehicle post-conditioning score, P < 0.05) N = 9–17/group
3.2 Experimenter-delivered methamphetamine does not affect the expression of adenosine receptors in the mesocorticolimbic dopamine system
Given the ability of adenosine receptor stimulation to impair the development of MA-induced CPP, we next investigated the effects of experimenter-delivered MA on the expression of adenosine receptors in several DA terminal areas, including the NAc core and shell, and the caudate putamen. Protein expression was measured in bilateral punches taken from tissue slices collected 24 hours after the last MA administration. Experimenter-delivered MA produced no changes in adenosine A1 or adenosine A2A receptor expression in the NAc shell, NAc core or the caudate-putamen (Table 1).
Table 1.
Effects of experimenter-delivered methamphetamine on adenosine receptor expression in the striatum
| Control | Methamphetamine | |
|---|---|---|
| Nucleus Accumbens Shell | ||
| Adenosine A1 | 100.0 ± 8.9 | 114.9 ± 11.4 |
| Adenosine A2A | 100.0 ± 3.7 | 94.9 ± 5.8 |
| Nucleus Accumbens Core | ||
| Adenosine A1 | 100.0 ± 5.9 | 115.5 ± 13.4 |
| Adenosine A2A | 100.0 ± 5.2 | 101.6 ± 4.5 |
| Caudate-Putamen | ||
| Adenosine A1 | 100.0 ± 4.6 | 96.5 ± 8.4 |
| Adenosine A2A | 100.0 ± 8.7 | 96.3 ± 6.3 |
Values are reported were normalized to β-tubulin and are expressed as % of saline control.
3.3 Stimulation of adenosine A1, but not A2A, receptors impairs methamphetamine self-administration
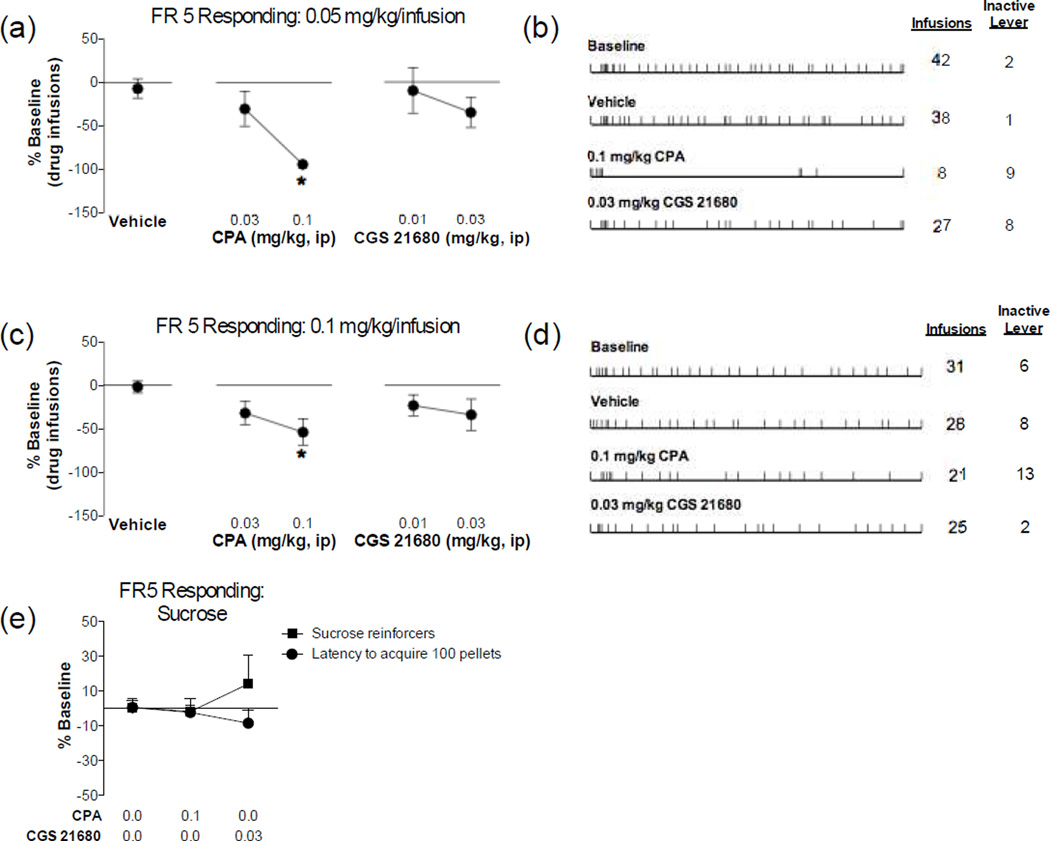
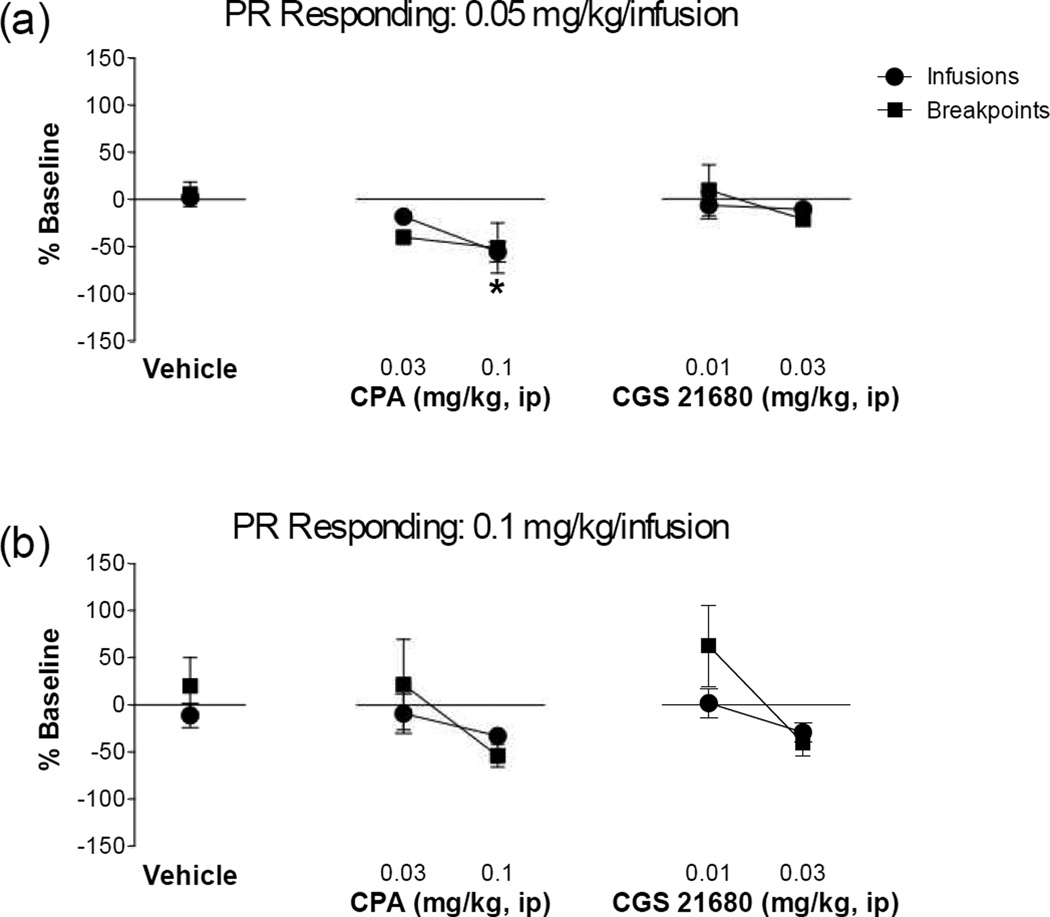
The next series of experiments tested whether stimulation of adenosine receptors diminished the reinforcing effects of MA using a self-administration model. As shown in Figure 2, adenosine A1 receptor stimulation dose-dependently blunted responding for both 0.05 mg/kg/infusion MA on the FR5 schedule (F(2,28) = 13.00, P < 0.0001) and for 0.1 mg/kg/infusion MA on the FR5 schedule (F(2,20) = 3.861, P = 0.0382). Despite showing a slight reduction in FR5 responding, the adenosine A2A receptor stimulation did not significantly affect FR5 responding for either 0.05 mg/kg/infusion MA (F(2,23) < 1 , NS) or 0.1 mg/kg/infusion MA (F(2,19) < 1.471, P = 0.2548). A similar adenosine receptor selectivity was observed on PR responding (Figure 3). Thus, adenosine A1 receptor stimulation dose-dependently blunted responding for 0.05 mg/kg/infusion MA on the PR schedule (Infusions: F(2,30) = 11.170, P = 0.0002; Breakpoint: F(2,30) = 1.622, P = 0.2137) and had no effect on responding for 0.1 mg/kg/infusion MA (Infusions: F(2,34) = 1.027, p = 0.3690; Breakpoint: F(2,32) = 2.582, P = 0.0913). Adenosine A2A receptor stimulation did not have any effect on PR responding for either 0.05 mg/kg/infusion MA (Infusions: F(2,23) = 1.705 , P = 0.2039; Breakpoint: F(2,23) = 1.343, P = 0.2808) or 0.1 mg/kg/infusion MA (Infusions: F(2,33) = 1.599, p = 0.2174; Breakpoint: F(2,33) = 2.928, P < 0.0675). Stimulation of neither adenosine A1 nor adenosine A2A receptors significantly altered responding for sucrose as measured by the number of sucrose pellets earned (CPA: t10 = 0.853, p = 0.4135, CGS 21680: t10 = 0.5630, p = 0.5858) or the latency to acquire 100 pellets (CPA: t10 = 0.6068, p = 0.5575, CGS 21680: t10 = 1.580, p = 0.1453). These findings suggest that stimulation of adenosine A1 receptors specifically reduces MA reinforcement, while stimulation of adenosine A2A receptors is without effect.
Figure 2. Adenosine A1 receptor stimulation impairs fixed ratio responding for methamphetamine.
Rats were trained to self-administer (a, b) 0.05 mg/kg/infusion or (c, d) 0.1 mg/kg/infusion methamphetamine on a fixed ratio 5 schedule of reinforcement. After establishing stable responding rats were administered a pretreatment of the adenosine A1 receptor agonist, CPA, or the adenosine A2A receptor agonist, CGS21680. Adenosine A1 receptor, but not A2A, stimulation dose-dependently impaired methamphetamine responding at both the (a) 0.05 mg/kg/infusion and (c) 0.1 mg/kg/infusion doses (* Significant from vehicle controls, P < 0.05). Representative response patterns under baseline and pretreatment conditions (vehicle, 0.1 mg/kg CPA and 0.03 mg/kg CGS 231680) are displayed for (b) 0.05 mg/kg/infusion and (d) 0.1 mg/kg/infusion methamphetamine. (e) Rats trained to self-administer sucrose pellets on a fixed ratio 5 schedule of reinforcement did not show impairments in the number of sucrose reinforcers earned or the latency to acquire 100 sucrose pellets during the 60 min session. N=6–12/group
Figure 3. Adenosine A1 receptor stimulation impairs progressive ratio responding for methamphetamine.
Rats were trained to self-administer (a) 0.05 mg/kg/infusion or (b) 0.1 mg/kg/infusion methamphetamine on a progressive ratio schedule of reinforcement. After establishing stable responding, rats were administered a pretreatment of the adenosine A1 receptor agonist, CPA, or the adenosine A2A receptor agonist, CGS21680. Adenosine A1 receptor, but not A2A, stimulation dose-dependently impaired the number of methamphetamine infusions earned at the (a) 0.05 mg/kg/infusion (* Infusions significant from vehicle controls, P < 0.05). No effects on breakpoints were observed. (b) Neither adenosine A1 receptor nor A2A, stimulation altered responding or infusions delivered when responding for the 0.1 mg/kg/infusion dose. N=6–16/group
3.4 Methamphetamine self-administration alters the expression of adenosine receptors in the mesocorticolimbic dopamine system
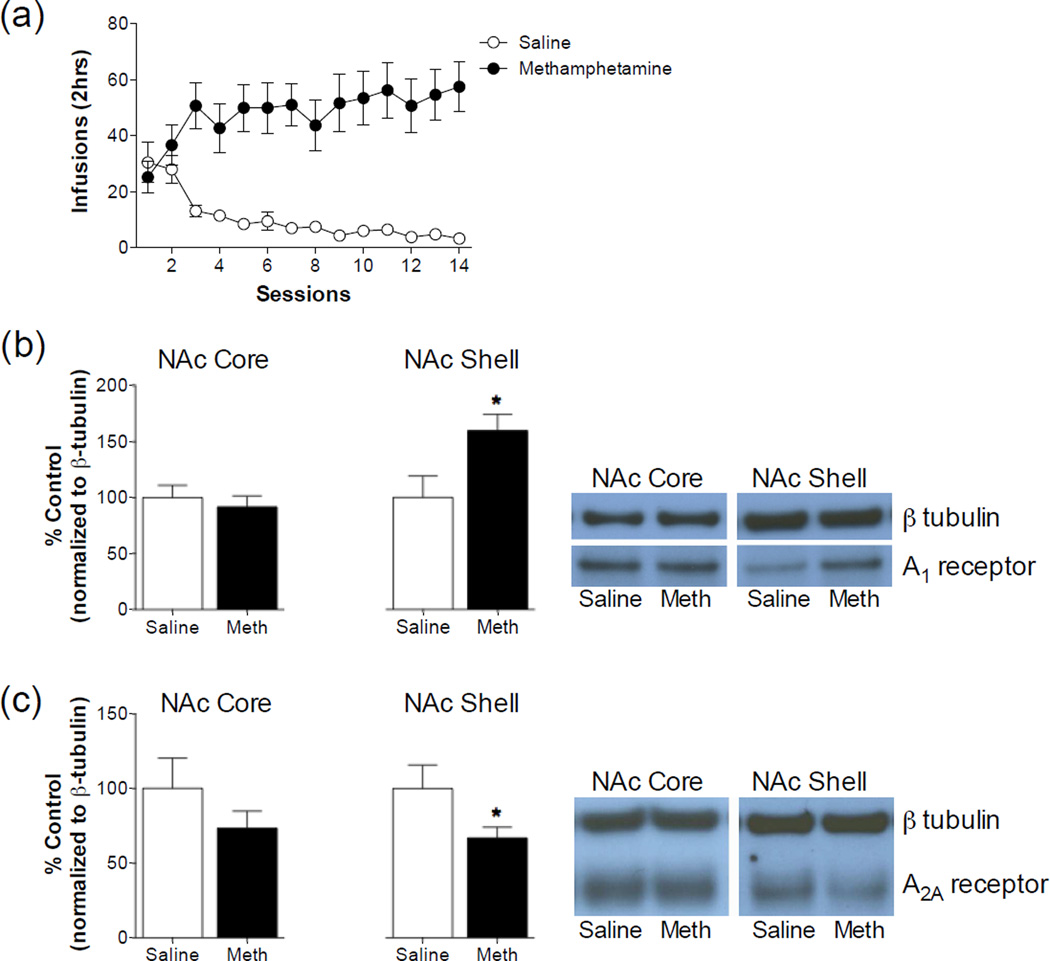
Given the subtype selective effects of adenosine receptor stimulation on methamphetamine reinforcement using an operant self-administration paradigm, we next assessed whether MA self-administration would produce a differential effect on the expression of adenosine receptors. Over the course of the self-administration procedure, rats averaged 48.21 ± 2.31 MA infusions compared with 10.32 ± 2.28 saline infusions in the control animals (Figure 4). This resulted in an average MA consumption of 2.41 ± 2.28 mg/kg/session. Protein expression was measured in bilateral punches taken from tissue slices collected 24 hours after the last self-administration session. MA self-administration produced an approximate 60% increase in adenosine A1 receptor expression in the NAc shell (t12 = 2.448, P = 0.0307) and no change in the NAc core (t12 = 0.5289, NS). Interestingly, we observed a 35% reduction in adenosine A2A receptor expression in the NAc shell (t12 = 2.206, P = 0.0476) and no change in the NAc core (t12 = 1.250, P = 0.2351). We also analyzed additional dopamine terminal areas including the caudate putamen, amygdala and prefrontal cortex (Table 2). MA self-administration produced a robust increase in adenosine A1 receptor expression (t12 = 4.317, P = 0.001) and no change in adenosine A2A receptor expression (t12 = 1.508, P = 0.1575) in the caudate-putamen. MA self-administration had no effect on adenosine A1 receptor expression (t13 = 1.260, P = 0.2299), but produced a robust increase in adenosine A2A receptor expression (t12 = 2.560, P = 0.0205) in the amygdala. A small, but significant increase in the expression of adenosine A1 receptors (t13 = 2.663, P = 0.0195) was observed in the prefrontal cortex of animals that had self-administered MA. Adenosine A2A receptor expression was undetectable in prefrontal cortex samples and was therefore not analyzed. These findings suggest that 24 hours after MA self-administration adenosine receptors are altered throughout dopamine terminal areas.
Figure 4. Methamphetamine self-administration produces bi-directional alterations in adenosine receptor subtypes in the nucleus accumbens shell.
(a) Rats were trained to self-administer either saline or 0.05 mg/kg/infusion on a fixed ratio schedule of reinforcement. Twenty-four hours after the last self-administration session, tissue was collected from the nucleus accumbens core and shell and adenosine receptor expression was determined using immunoblotting. (b) Methamphetamine self-administration produced an increase in adenosine A1 receptor expression in the nucleus accumbens shell, but not the nucleus accumbens core (* Significant from saline self-administration group, P <0.05). Representative blots depicting detections of adenosine A1 receptor and β tubulin are shown to the right of the figure. (c) Methamphetamine self-administration produced a decrease in adenosine A2A receptor expression in the nucleus accumbens shell, but not the nucleus accumbens core (* Significant from Saline self-administration group, P <0.05). Representative blots depicting detections of adenosine A2A receptor and β tubulin are shown to the right of the figure. N = 5–9
Table 2.
Effects of methamphetamine self-administration on adenosine receptor expression in caudate-putamen, prefrontal cortex and amygdale
| Control | Methamphetamine | |
|---|---|---|
| Caudate-Putamen | ||
| Adenosine A1 | 100.0 ± 3.9 | 152.7 ± 10.0* |
| Adenosine A2A | 100.0 ± 6.6 | 114.5 ± 6.7 |
| Prefrontal cortex | ||
| Adenosine A1 | 100.0 ± 5.8 | 115.5 ± 2.8* |
| Adenosine A2A | ND | ND |
| Amygdala | ||
| Adenosine A1 | 100.0 ± 10.3 | 83.8 ± 5.5 |
| Adenosine A2A | 100.0 ± 10.2 | 160.7 ± 18.9* |
Values are reported were normalized to β-tubulin and are expressed as % of saline self-administering control.
Significant from control
4. DISCUSSION
These findings suggest that the adenosine system plays an integral role in the development of MA reward and reinforcement. The development of MA CPP was uniformly decreased by stimulation of both adenosine A1 and A2A receptors suggesting that both receptor subtypes are likely involved in the acute rewarding properties of MA. Interestingly, we observed a reduction in MA self-administration with adenosine A1 receptor stimulation, but not with the stimulation of adenosine A2A receptors. We hypothesize that this adenosine receptor subtype selectivity results from alterations occurring after MA self-administration, but not experimenter-delivered MA. Thus, consistent increases in adenosine A1 receptor expression were observed in the NAc shell, caudate-putamen and prefrontal cortex following MA self-administration, but not experimenter-administered MA. MA self-administration decreased adenosine A2A receptor expression in the NAc shell and increased expression occurring in the amygdala. Together, these findings suggest that the initial rewarding effects of MA may be tempered with adenosine A1 or A2A receptor stimulation; however, prolonged volitional MA administration produces selective adenosine receptor changes that render adenosine A1 receptor stimulation more effective at reducing the reinforcing effects of MA in the self-administration paradigm.
Our behavioral findings are largely congruent with previous work, although the specific effects of adenosine receptor stimulation on MA CPP and self-administration have not been explicitly explored. Utilizing other paradigms, it was observed that stimulation of both adenosine A1 or A2A receptors reduces MA-induced DA release in the striatum of MA-sensitized animals (Golembiowska and Zylewska, 1998, 2000). Adenosine A1 and A2A receptor stimulation also reduces the behavioral expression of MA sensitization (Shimazoe et al., 2000, Yoshimatsu et al., 2001). Our findings that adenosine A1 and A2A receptor stimulation reduces the development of MA CPP suggest that activation of adenosine receptors may offset excessive, unregulated, DA release and subsequent DA receptor stimulation that contributes to MA reward.
Given the ability of adenosine receptor stimulation to reduce MA CPP, and potentially interfere with DA receptor signaling, it is important to consider potential adverse consequences of adenosine receptor stimulation. Previous studies demonstrate that adenosine receptor stimulation alone either does not produce any appetitive or aversive place conditioning (Brockwell and Beninger, 1996), or produces a place preference when administered at higher doses than those used in our studies (Poleszak and Malec, 2002). These findings suggest that the inhibition of MA CPP produced by adenosine A1 or A2A receptor stimulation is not attributable to inherent aversive effects of the agonists. Special consideration should also be made to the locomotor inhibitory effects of adenosine receptor stimulation. We observed that stimulation of either adenosine A1 and A2A receptors inhibited MA-induced locomotion during the CPP conditioning sessions. It is unclear how locomotor responses relate to the development of CPP (Bardo et al., 1999, Swerdlow and Koob, 1984), although it seems plausible that suppression of MA-induced locomotion may indicate impaired subjective effects of MA prohibiting the development of CPP. This concern is minimized with regard to the effects of adenosine receptor stimulation on MA self-administration since adenosine receptor stimulation impaired lever responding for 0.1 mg/kg/infusion MA, but did not abolish it entirely. In fact, responding was distributed throughout the self-administration session suggesting that animals were not sedate. Finally, we demonstrate that adenosine receptor stimulation does not impair the number of sucrose pellets earned or the latency to acquire the maximum sucrose pellets in self-administration sessions corroborating previous work using food self-administration and sucrose seeking in extinguished animals (Bachtell and Self, 2009, Hobson et al., 2013, O'Neill et al., 2012, Wydra et al., 2014). While the locomotor suppressive effects of adenosine receptor agonists may factor into the observed behavioral outcomes, they do not appear to be the sole factor in the production of these effects.
We observed adenosine receptor changes in a portion of the dopamine terminal areas examined including the caudate-putamen, NAc shell, prefrontal cortex and amygdala 24 hours following the last MA self-administration. Interestingly, no changes in adenosine receptor expression were observed in these same areas 24 hours after experimenter-administered MA. It is well documented that experiential factors, such as whether the drug is voluntarily or forcibly administered, can play a major role in determining both qualitative and quantitative neurobiological responses to the drug (Dworkin et al., 1995, Graziella De Montis et al., 1998, Hemby et al., 1997, Mark et al., 1999, Porrino et al., 2002, Wilson et al., 1994). It is unclear whether the differential effects that we observed between the two procedures was due to the experiential nature of MA administration or several other procedural variables including the route of administration (intraperitoneal vs. intravenous), dosing (1.5 mg/kg vs. approx. 2.5 mg/kg), and duration of MA treatments (7 vs. 14). It was previously reported that subchronic administration (11 days) of a relatively high MA dose (4.6 mg/kg, ip) produced no changes in adenosine A1 or adenosine A2A receptor binding or densities in the striatum (Shirayama et al., 2001). Together these findings suggest that the volitional intake of MA may, in part, play a role in the adenosine receptor subtype changes observed following MA self-administration.
Previous work suggests that impairments in adenosine signaling may underlie individuals’ susceptibility to the effects of MA. Recent studies have shown that polymorphisms in the A2A, but not A1, receptor are associated with MA abuse suggesting that impairments in functional A2A receptors may predispose individuals to MA abuse (Kobayashi et al., 2010, 2011). We observed a significant decrease in adenosine A2A receptor expression the NAc shell that may mimic functional impairments in the adenosine A2A receptor gene. We also observed a significant increase in adenosine A2A receptors in the amygdala and it is unclear how this may be interpreted behaviorally. In addition, repeated MA administration decreases the expression of adenosine transporters, consequently impairing extracellular adenosine transport and perhaps leave DA receptors further unregulated (Escubedo et al., 1998). These findings suggest that impairments in adenosine signaling may render individuals more vulnerable to MA’s potentiation of DA signaling contributing to initial drug taking and/or the development of compulsive drug use and possible relapse.
Adenosine interacts with the A1 and A2A subtypes that are highly expressed in striatal regions such as the NAc (Dixon et al., 1996). Adenosine receptor subtypes are differentiated by their pharmacology, signal transduction and cellular localization. Thus, adenosine A1 receptors are coupled to inhibitory G proteins that inhibit adenylyl cyclase and exert depressant effects on cellular functions (Dunwiddie and Fredholm, 1989, Fredholm and Dunwiddie, 1988). Adenosine A2A receptors, on the other hand, are coupled to stimulatory G proteins that activate adenylyl cyclase and possess excitatory actions on neuronal activity (Cunha et al., 1995, Kirkpatrick and Richardson, 1993, Popoli et al., 1995, Sebastiao and Ribeiro, 1996). The cellular distribution of postsynaptic adenosine receptors in the NAc has an expression profile where A1 receptors exist primarily on dopamine D1-containing substance P/dynorphin expressing neurons, whereas A2A receptors exist primarily on dopamine D2-containing enkephalin expressing neurons (Schiffmann et al., 1991, Svenningsson et al., 1999). This expression profile enables adenosine receptors to provide antagonist influences on postsynaptic DA neurotransmission through either intracellular signaling cascades or the formation of receptor protein complexes (e.g. heteromeric receptors). For example, the stimulatory effects of dopamine D1 receptor agonism are offset by simultaneous activation of adenosine A1 receptor agonism (Ferre et al., 1996a, Ferre et al., 1994, Ferre et al., 1996b, Ferre et al., 1998, Hobson et al., 2013) while adenosine A1 receptor stimulation in an A1-D1 heteromeric complex induces conformational changes in the D1 receptor that alters the ligand recognition site, binding characteristics, and activity of intracellular signaling cascades (Ferre et al., 1996a, Ferre et al., 1994, Ferre et al., 1996b, Ferre et al., 1999, Ferre et al., 1998).
MA potently reverses the activity of both the DA transporter and the intracellular vesicular monoamine transporter 2 resulting in non-vesicular DA release into the synaptic cleft (Vergo et al., 2007, Volz et al., 2007). We hypothesize that the antagonistic receptor interactions between DA and adenosine receptors may therefore not be fully appreciated in the presence of MA. This may be especially true after prolonged MA self-administration when adenosine receptor expression changes are most evident. The initial rewarding effects of MA are tempered through the stimulation of both adenosine A1 and A2A receptors, when adenosine receptor populations in the striatal areas remain intact and functional. However, upon chronic MA self-administration, the effect of non-vesicular release of DA by MA in the absence of adenosine regulation begins to alter the expression of adenosine receptor systems and produce dysfunctional reciprocal antagonism between the adenosine and DA receptor systems. Our findings suggest that MA self-administration produces robust increases in adenosine A1 receptor expression in the NAc shell that may explain the ability of adenosine A1 receptor stimulation to inhibit MA reinforcement. On the other hand, MA self-administration decreases the expression of adenosine A2A receptors in the NAc shell and stimulation of this receptor subtype is therefore without effect. Importantly, we do not see these same receptor alterations following experimenter-delivered MA and we also do not see the adenosine receptor subtype selectivity in the absence of these receptor changes.
Interestingly, the effects of adenosine receptor agonists on MA behaviors are quite similar to the effects observed with the administration of DA receptor antagonists. Thus, both DA D1 and D2 receptor antagonists inhibit MA locomotor sensitization and CPP (Kuribara and Uchihashi, 1993, Kurokawa et al., 2010a, Kurokawa et al., 2010b, Mizoguchi et al., 2004). Similar to our results, there is DA receptor subtype selectivity when assessing the reinforcement properties of MA using a self-administration paradigm. Thus, DA D1 receptor antagonism disrupts FR MA self-administration, whereas DA D2 receptor antagonism had little effect (Brennan et al., 2009). Likewise, DA D1 receptors are also necessary for the reinstatement of MA seeking in extinguished rats, while DA D2 receptors are not (Carati and Schenk, 2011). The preferential effects of DA D1, but not DA D2, receptors in these MA-mediated behaviors associated with chronic volitional intake is congruent with our observations that adenosine A1 receptor stimulation impairs operant responding for MA. This DA and adenosine receptor subtype selectivity is important since postsynaptic A1 and D1 receptors are co-localized in striatal neurons where the alterations in receptor interactions may be an important contributor to the development of MA abuse.
5. CONCLUSIONS
These findings illuminate the importance of the adenosine system in the development of MA reward and reinforcement. It is thought that the adenosine receptor changes that are observed following MA self-administration, but not experimenter-delivered MA, are a critical element in the transition from acute MA reward to later stages of MA use that may involve additional reinforcement mechanisms. Based on these findings we hypothesize that the initial rewarding effects of MA are reduced by stimulation of either adenosine A1 or A2A receptors, when adenosine receptor signaling is still capable of tempering MA-induced excessive DA neurotransmission. However, more prolonged volitional MA administration produces selective adenosine receptor changes that renders the system more sensitive to MA reinforcement through DA D1 receptor activity, and more sensitive to the inhibitory effects of adenosine A1 receptor stimulation.
Table 3.
Raw methamphetamine infusions during baseline and pharmacological testing
| Methamphetamine | ||
|---|---|---|
| 0.05 mg/kg/infusion | 0.1 mg/kg/infusion | |
| FR 5 Schedule | ||
| Baseline | 28.95 ± 2.03 (n=25) | 22.09 ± 2.60 (n=15) |
| Vehicle | 24.86 ± 4.84 (n=8) | 19.11 ± 4.15 (n=7) |
| 0.03 mg/kg CPA | 23.44 ± 8.36 (n=9) | 16.56 ± 4.30 (n=9) |
| 0.1 mg/kg CPA | 1.90 ± 0.55 (n=10)*# | 11.17 ± 4.59 (n=7) |
| 0.01 mg/kg CGS 21680 | 31.50 ± 10.58 (n=8) | 19.00 ± 3.62 (n=9) |
| 0.03 mg/kg CGS 21680 | 22.50 ± 7.36 (n=6) | 14.00 ± 5.55 (n=6) |
| PR Schedule | ||
| Baseline | 10.80 ± 0.67 (n=28) | 13.66 ± 0.80 (n=27) |
| Vehicle | 13.15 ± 0.94 (n=11) | 11.18 ± 2.12 (n=10) |
| 0.03 mg/kg CPA | 11.83 ± 1.83 (n=6) | 10.30 ± 2.29 (n=10) |
| 0.1 mg/kg CPA | 4.25 ± 1.21 (n=11)*# | 11.00 ± 1.83 (n=16) |
| 0.01 mg/kg CGS 21680 | 13.14 ± 2.54 (n=7) | 11.90 ± 1.96 (n=10) |
| 0.03 mg/kg CGS 21680 | 10.33 ± 1.12 (n=6) | 10.56 ± 1.43 (n=16) |
Values are reported as the average number of infusions (± SEM) during the self-administration session.
FR: Fixed Ratio; PR: Progressive ratio
Significant from baseline (p < 0.05),
Significant from vehicle (p < 0.05)
Highlights.
Adenosine A1 and A2A receptor stimulation inhibits methamphetamine place preference
Adenosine A1 receptor stimulation reduces methamphetamine self-administration
Methamphetamine self-administration increases adenosine A1 receptors in accumbens shell
Methamphetamine self-administration decreases adenosine A2A receptors in accumbens shell
Acknowledgements
This work was supported by United States Public Health Service grants DA029240 and DA 033358.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bachtell RK, Self DW. Effects of adenosine A(2A) receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2009;206:469–478. doi: 10.1007/s00213-009-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administration in rats. Behavioural pharmacology. 2009;20:688–694. doi: 10.1097/FBP.0b013e328333a28d. [DOI] [PubMed] [Google Scholar]
- Brockwell NT, Beninger RJ. The differential role of A1 and A2 adenosine receptor subtypes in locomotor activity and place conditioning in rats. Behav Pharmacol. 1996;7:373–383. doi: 10.1097/00008877-199608000-00009. [DOI] [PubMed] [Google Scholar]
- Carati C, Schenk S. Role of dopamine D1- and D2-like receptor mechanisms in drug-seeking following methamphetamine self-administration in rats. Pharmacology, biochemistry, and behavior. 2011;98:449–454. doi: 10.1016/j.pbb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Cass CE, Belt JA, Paterson AR. Adenosine transport in cultured cells and erythrocytes. Prog Clin Biol Res. 1987;230:13–40. [PubMed] [Google Scholar]
- Cunha RA, Johansson B, Fredholm BB, Ribeiro JA, Sebastiao AM. Adenosine A2A receptors stimulate acetylcholine release from nerve terminals of the rat hippocampus. Neurosci Lett. 1995;196:41–44. doi: 10.1016/0304-3940(95)11833-i. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989;249:31–37. [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Co C, Smith JE. Rat brain neurotransmitter turnover rates altered during withdrawal from chronic cocaine administration. Brain Res. 1995;682:116–126. doi: 10.1016/0006-8993(95)00327-m. [DOI] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jimenez A, Pubill D, Pallas M, et al. Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain research. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Gimenez-Llort L, Finnman UB, Martinez E, Scotti de Carolis A, et al. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994;6:73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- Ferre S, O'Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, et al. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996a;8:1545–1553. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Tinner-Staines B, Fuxe K. Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neuroscience letters. 1996b;208:109–112. doi: 10.1016/0304-3940(96)12577-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, et al. Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. The Journal of biological chemistry. 1998;273:4718–4724. doi: 10.1074/jbc.273.8.4718. [DOI] [PubMed] [Google Scholar]
- Ferre S, Rimondini R, Popoli P, Reggio R, Pezzola A, Hansson AC, et al. Stimulation of adenosine A1 receptors attenuates dopamine D1 receptor-mediated increase of NGFI-A, c-fos and jun-B mRNA levels in the dopamine-denervated striatum and dopamine D1 receptor-mediated turning behaviour. Eur J Neurosci. 1999;11:3884–3892. doi: 10.1046/j.1460-9568.1999.00810.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Fried G, Hedqvist P. Origin of adenosine released from rat vas deferens by nerve stimulation. Eur J Pharmacol. 1982;79:233–243. doi: 10.1016/0014-2999(82)90629-x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Zylewska A. Agonists of A1 and A2A adenosine receptors attenuate methamphetamine-induced overflow of dopamine in rat striatum. Brain Res. 1998;806:202–209. doi: 10.1016/s0006-8993(98)00743-4. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Zylewska A. Effect of adenosine kinase, adenosine deaminase and transport inhibitors on striatal dopamine and stereotypy after methamphetamine administration. Neuropharmacology. 2000;39:2124–2132. doi: 10.1016/s0028-3908(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Graziella De Montis M, Co C, Dworkin SI, Smith JE. Modifications of dopamine D1 receptor complex in rats self-administering cocaine. Eur J Pharmacol. 1998;362:9–15. doi: 10.1016/s0014-2999(98)00731-6. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hobson BD, O'Neill CE, Levis SC, Monteggia LM, Neve RL, Self DW, et al. Adenosine A and Dopamine D Receptor Regulation of AMPA Receptor Phosphorylation and Cocaine-Seeking Behavior. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick KA, Richardson PJ. Adenosine receptor-mediated modulation of acetylcholine release from rat striatal synaptosomes. Br J Pharmacol. 1993;110:949–954. doi: 10.1111/j.1476-5381.1993.tb13905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Ujike H, Iwata N, Inada T, Yamada M, Sekine Y, et al. The adenosine A2A receptor is associated with methamphetamine dependence/psychosis in the Japanese population. Behavioral and brain functions : BBF. 2010;6:50. doi: 10.1186/1744-9081-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Ujike H, Iwata N, Inada T, Yamada M, Sekine Y, et al. Association analysis of the adenosine A1 receptor gene polymorphisms in patients with methamphetamine dependence/psychosis. Current neuropharmacology. 2011;9:137–142. doi: 10.2174/157015911795016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y. SCH 23390 equivalently, but YM-09151-2 differentially reduces the stimulant effects of methamphetamine, MK-801 and ketamine: assessment by discrete shuttle avoidance in mice. Japanese journal of pharmacology. 1993;62:111–114. doi: 10.1254/jjp.62.111. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Mizuno K, Shibasaki M, Ohkuma S. Regulation of ryanodine receptors by dopamine D1 receptors during methamphetamine-induced place conditioning. Journal of neurochemistry. 2010a;115:1206–1214. doi: 10.1111/j.1471-4159.2010.07010.x. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Shibasaki M, Ohkuma S. Methamphetamine-induced up-regulation of alpha2/delta subunit of voltage-gated calcium channels is regulated by DA receptors. Synapse. 2010b;64:822–828. doi: 10.1002/syn.20797. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- McCabe RT, Hanson GR, Dawson TM, Wamsley JK, Gibb JW. Methamphetamine-induced reduction in D1 and D2 dopamine receptors as evidenced by autoradiography: comparison with tyrosine hydroxylase activity. Neuroscience. 1987;23:253–261. doi: 10.1016/0306-4522(87)90287-9. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Merritt KE, Bachtell RK. Initial d2 dopamine receptor sensitivity predicts cocaine sensitivity and reward in rats. PLoS ONE. 2013;8:e78258. doi: 10.1371/journal.pone.0078258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y, et al. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Molecular pharmacology. 2004;65:1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- O'Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology. 2012;37:1245–1256. doi: 10.1038/npp.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FC, Imam SZ, Gough B, Newport GD, Ribeiro CF, Slikker W, Jr, et al. Acute changes in dopamine release and turnover in rat caudate nucleus following a single dose of methamphetamine. Journal of neural transmission. 2002;109:1151–1158. doi: 10.1007/s00702-002-0754-z. [DOI] [PubMed] [Google Scholar]
- Pereira FC, Lourenco ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR, et al. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60:185–193. doi: 10.1002/syn.20285. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Malec D. Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol J Pharmacol. 2002;54:119–126. [PubMed] [Google Scholar]
- Popoli P, Betto P, Reggio R, Ricciarello G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol. 1995;287:215–217. doi: 10.1016/0014-2999(95)00679-6. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol. 1996;48:167–189. doi: 10.1016/0301-0082(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O'Neil ML, Melega WP, Cho AK. Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterization. Psychopharmacology. 2005;180:501–512. doi: 10.1007/s00213-005-2188-4. [DOI] [PubMed] [Google Scholar]
- Shimazoe T, Yoshimatsu A, Kawashimo A, Watanabe S. Roles of adenosine A(1) and A(2A) receptors in the expression and development of methamphetamine-induced sensitization. Eur J Pharmacol. 2000;388:249–254. doi: 10.1016/s0014-2999(99)00899-7. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Higuchi T, Minabe Y. Subchronic treatment with methamphetamine and phencyclidine differentially alters the adenosine A1 and A2A receptors in the prefrontal cortex, hippocampus, and striatum of the rat. Neurochemical research. 2001;26:363–368. doi: 10.1023/a:1010994913749. [DOI] [PubMed] [Google Scholar]
- Shuto T, Seeman P, Kuroiwa M, Nishi A. Repeated administration of a dopamine D1 receptor agonist reverses the increased proportions of striatal dopamine D1High and D2High receptors in methamphetamine-sensitized rats. Eur J Neurosci. 2008;27:2551–2557. doi: 10.1111/j.1460-9568.2008.06221.x. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. European journal of pharmacology. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Restrained rats learn amphetamine-conditioned locomotion, but not place preference. Psychopharmacology (Berl) 1984;84:163–166. doi: 10.1007/BF00427440. [DOI] [PubMed] [Google Scholar]
- Thorn JA, Jarvis SM. Adenosine transporters. Gen Pharmacol. 1996;27:613–620. doi: 10.1016/0306-3623(95)02053-5. [DOI] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain research. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. The American journal of psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE. The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. Journal of neurochemistry. 2007;101:883–888. doi: 10.1111/j.1471-4159.2006.04419.x. [DOI] [PubMed] [Google Scholar]
- White TD. Direct detection of depolarisation-induced release of ATP from a synaptosomal preparation. Nature. 1977;267:67–68. doi: 10.1038/267067a0. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res. 1994;668:39–45. doi: 10.1016/0006-8993(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Molecular psychiatry. 2000;5:664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Suder A, Kaminska K, Fuxe K, Filip M. On the role of adenosine (A) receptors in cocaine-induced reward: a pharmacological and neurochemical analysis in rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3675-2. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu A, Shimazoe T, Kawashimo A, Shuto T, Doi Y, Fukumoto T, et al. Effects of adenosine A1- and A2A-receptor agonists on enhancement of dopamine release from the striatum in methamphetamine-sensitized rats. Jpn J Pharmacol. 2001;86:254–257. doi: 10.1254/jjp.86.254. [DOI] [PubMed] [Google Scholar]