Abstract
Aim
An increase of late sodium Na+ current (INaL) in cardiac myocytes can raise the cytosolic Na+ concentration and is associated with activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and alterations of mitochondrial metabolism and Ca2+ handling by sarcoplasmic reticulum (SR). We tested the hypothesis that augmentation of INaL can increase mitochondrial reactive oxygen species (ROS) production and oxidation of CaMKII, resulting in spontaneous SR Ca2+ release and increased diastolic Ca2+ in myocytes.
Methods and results
Confocal fluorescent imaging was used to study effects of INaL and intracellular Na+ load on Ca2+ transients, mitochondrial ROS production, and SR-associated Ca2+ releases in intact and membrane-permeabilized rabbit ventricular myocytes. Anemone toxin-II (ATX-II) and ranolazine were used to enhance and inhibit INaL, respectively. ATX-II increased cytosolic Na+, diastolic Ca2+, ROS formation, oxidation of CaMKII, and frequency of SR Ca2+ release events. Effects of ATX-II were inhibited by ranolazine, antioxidants, and CaMKII inhibitors. Elevation of cytosolic Na+ in membrane-permeabilized myocytes increased ROS production in mitochondria and caused spontaneous Ca2+ releases from the SR. Inhibitions of CaMKII and/or ROS production with KN93 and coenzyme Q10 (CoQ10), respectively, eliminated the effects of ATX-II and Na+ overload to cause increases of ROS formation, diastolic Ca2+, and spontaneous SR Ca2+ release events. In myocytes isolated from failing mouse hearts, ranolazine and CoQ10 reduced levels of cytosolic Na+ and intracellular ROS.
Conclusion
Increases of INaL and/or of the cytosolic Na+ concentration led to mitochondrial ROS production and oxidation of CaMKII to cause dysregulation of Ca2+ handling in rabbit cardiac myocytes.
Keywords: Late sodium current, ATX-II, RyRs, CaMKII, ROS, mitochondria
1. INTRODUCTION
Regulation of cellular sodium (Na+) and calcium (Ca2+) handling is essential for the maintenance of normal excitation-contraction coupling [1-3]. Cardiac Na+ channels open transiently and inactivate rapidly, causing a large, brief peak of Na+ current that initiates the cardiac action potential [4, 5]. Under pathological conditions, including heart failure and myocardial ischemia, Na+ channel inactivation is slowed and/or incomplete, resulting in a sustained Na+ current, referred to as persistent or late Na+ current (INaL) [3-5]. Increased INaL promotes intracellular Na+ overload, thereby reducing the electrochemical gradient for Ca2+ extrusion by the Na+-Ca2+ exchanger (NCX), causing cellular Ca2+ overload, contractile dysfunction, and arrhythmias [3-5].
In addition to elevating cytosolic Ca2+, intracellular Na+ overload also perturbs mitochondrial Ca2+ homeostasis, resulting in mitochondrial reactive oxygen species (ROS) generation [6-8]. Ca2+ is an important regulator of the mitochondrial redox state [6-10]. Ca2+ activates key rate-limiting enzymes of the Krebs cycle (pyruvate, isocitrate, and malate dehydrogenases) required for producing NADH (the main electron donor for ATP in the respiratory chain) and for regenerating NADPH (to maintain the antioxidative capacity of mitochondrial matrix enzymes) [9]. When cardiac work increases, mitochondrial Ca2+ uptake is elevated to increase Krebs cycle activity and ATP production to meet an increased metabolic demand [9]. An elevation of the cytosolic [Na+], on the other hand, decreases mitochondrial Ca2+ uptake and NADH levels [6]. In myocytes isolated from failing hearts, cytosolic Na+ elevation promotes mitochondrial Ca2+ efflux via the mitochondrial Na+- Ca2+ exchanger, and thereby reduces Krebs cycle activity and levels of NADH and NADPH [6,7]. A decrease in the level of NADPH impairs the capacity of antioxidant enzymes such as thioredoxin and glutathione to reverse protein oxidation, thus increasing ROS-induced cellular dysfunction. Indeed, the elevated ROS in cardiomyocytes isolated from the failing heart can be normalized either by lowering cytosolic [Na+] or by inhibiting mitochondrial Na+- Ca2+ exchange [7,8,10]. Conversely, elevating cytosolic Na+ increases ROS generation in normal cardiomyocytes [7]. These studies suggest that mitochondrial ROS generation may play a critical contributory role in mediating the pathological effects of cytosolic Na+ overload [2,7,8,10].
Elevated ROS production promotes adverse cardiac remodeling by activating intracellular signaling pathways that induce cardiomyocyte cell death, hypertrophy, and arrhythmia [11]. We previously demonstrated that the multi-functional Ca2+/calmodulin-dependent protein kinase II delta (CaMKIIδ) is activated in response to enhanced INaL and mediates downstream pathological processes that include necrotic and apoptotic cardiac cell death and ventricular arrhythmia [12]. CaMKIIδ phosphorylates a number of substrates involved in cellular Ca2+ homeostasis, including phospholamban (PLN), the sarcoplasmic reticulum (SR) Ca2+ATPase, ryanodine receptor 2 (RyR2), and the cardiac Na+ channel, Nav1.5. Phosphorylation of these proteins disrupts normal SR Ca2+ cycling, promotes cellular Ca2+ overload, induces spontaneous Ca2+ release, and alters excitation-contraction coupling and propagation of electrical impulses [13-18]. CaMKIIδ activity is regulated by Ca2+/calmodulin and depends on the amplitude and frequency of cytosolic Ca2+ waves. CaMKIIδ activity is also regulated by direct oxidation of methionine 281/282 at its regulatory domain [11,15]. Based on its dual modes of activation, CaMKIIδ is centrally poised to sense elevations in either Ca2+ or ROS, and to initiate downstream molecular events that promote Ca2+ mishandling. CaMKIIδ may therefore serve as a nodal point of convergence for pathological signaling events induced by an increased INaL [12,18].
Despite the well-established link between cytosolic Na+ overload and altered intracellular Ca2+ handling [3-8,12,19-21], the mechanism of Na+-dependent modulation of Ca2+ dynamics is not fully understood [2]. In this study we tested the hypothesis that intracellular Na+ overload caused by an enhanced INaL increases mitochondrial ROS production, resulting in oxidation-induced activation of CaMKIIδ, that in turn promotes Ca2+ mishandling. Our experimental approaches to study the pathway between intracellular Na+ overload and Ca2+ mishandling included intact and permeabilized adult rabbit cardiomyocytes exposed to an enhancer of INaL and an increased superfusate Na+ concentration, respectively, and a mouse model of pressure overload-induced heart failure. Fluorescent dyes sensitive to Na+, Ca2+, and ROS were used to measure the effects of increased INaL and intracellular Na+ to alter ROS formation and cellular Ca2+ handling. Anemonia sulcata toxin II (ATX-II) and ranolazine were used to increase and inhibit INaL, respectively [22,4].
2. MATERIALS AND METHODS
2.1 Chemicals
ATX-II and tetrodotoxin were purchased from Alomone Labs and Tocris Bioscience, respectively. Dithiothreitol (DTT) and coenzyme Q10 (CoQ10) were purchased from Sigma Chemical. The CaMKII inhibitor KN-93 and its inactive analog KN-92 were purchased from EMD Millipore. Ranolazine and the selective INaL blocker GS-967 [23] were provided by Gilead Sciences. The sources of fluorescent dyes and antibodies are indicated in the relevant sections below.
2.2 Cell Isolation
Experimental protocols using animals were approved by the Gilead Institutional Animal Care and Use Committee following criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Adult female 2-3 kg New Zealand White rabbits (Western Oregon Rabbit Company) were sedated with intramuscular injections of 35 mg/kg ketamine and 5 mg/kg xylazine. The respiratory rate, muscle tone, and righting reflex were monitored to ensure sedation. Once sedated, rabbits were given 1 mL of heparin (1000 USP units/mL) into the ear vein and then deeply anesthetized by intravenous injection of 15 mg/mL ketamine and 3 mg/mL xylazine. Hearts were removed and mounted on a Langendorff apparatus for retrograde perfusion (17-20 mL/min) of the aorta with a perfusion solution containing (in mmol/L): 140 NaCl, 4.4 KCl, 1.5 MgCl2, 1 NaH2PO4, 5 HEPES, 7.5 glucose, 16 taurine, 5 Na pyruvate, and NaOH to adjust the pH to 7.3. Cardiac myocytes were enzymatically isolated from left ventricles using as previously described by Brunner et al. [24]. Perfusion solution was supplemented with collagenase type II enzyme (0.8 mg/mL, Worthington), 0.2% 2,3-butanedione monoxime, and 0.2% bovine serum albumin. Left ventricles were dissected, chopped, and subjected to postdigestion in a shaker with periodical changes of the isolation solution. The isolation solution contained (in mmol/L): 140 NaCl, 4.6 KCl, 1.1 MgCl2, 1 NaH2PO4, 10 HEPES, 10 glucose, and NaOH to adjust the pH to 7.4. Isolation solution was supplemented with a low concentration (0.344 mg/mL) of collagenase and 0.975 % albumin. All procedures were performed at 37°C. Isolated cardiomyocytes were used within 6 h after isolation.
Adult male mice were heparinized and sacrificed by cervical dislocation. The hearts were perfused on a Langendorff apparatus with the following solution (in mmol/L): 135 NaCl, 4.6 KCl, 1.1 MgCl2, 1 NaH2PO4, 10 HEPES, 10 glucose, and NaOH to adjust the pH to 7.4. Then cardiomyocytes were perfused with the solution supplemented with 0.896 mg/mL collagenase, 0.08 mg/mL protease type XIV, and 0.68 mg/mL bovine serum albumin for 15-20 min at 29°C. The left ventricles were dissected and gently triturated. Isolated cardiomyocytes were used within 4 h after isolation.
2.3. Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record INa in the voltage-clamp mode using a Multiclamp 700B amplifier (Molecular Devices) and pClamp 10.2 data acquisition software (Molecular Devices). The digital data were analyzed using pClampfit 10, Microcal Origin (OriginLab), and GraphPad Prism (Graph Pad Software) programs. Patch pipettes were pulled from borosilicate glass (World Precision Instruments) using a DMZ Universal puller (Dagan). For recording of INa, cardiomyocytes were superfused with bath solution containing (in mM): 135 NaCl, 4.6 CsCl, 1.8 CaCl2, 1.1 MgSO4, 10 HEPES, 10 glucose, and 0.01 nitrendipine (pH 7.4 adjusted with NaOH). Pipette resistance was 1.5–2 MΩ when filled with a pipette (internal) solution containing (in mM): 120 aspartic acid, 20 CsCl, 1 MgSO4, 4 ATPNa2, 0.1 GTPNa3, and 10 HEPES (pH 7.3 adjusted with CsOH). The recordings of INa were performed at 22-24°C following a stabilization period (5-10 min) after establishing a patch with whole-cell configuration. The myocytes were held at −120 mV and depolarizing pulses (−20 mV, 220 ms) were applied at a rate of 1 Hz. The rate of decay (inactivation) of INa was fitted using two exponential terms, and the slower component was used to describe INaL.
2.4 Measurement of Intracellular Dye Fluorescence
Experiments were performed using a LSM 5 PASCAL (Carl Zeiss) laser scanning confocal system equipped with a Zeiss Plan-Apochromat 40× 1.4 numerical aperture oil immersion objective. Myocytes were maintained in bath solution containing (in mmol/L): 140 NaCl, 5.4 KCl, 2.0 CaCl2, 2.5 MgCl2, 10 HEPES and 5.6 glucose, pH 7.3. Cells were incubated for 25 min at 23 °C in the bath solution supplemented with 0.25 mmol/L CaCl2, 2.5 μmol/L pluronic acid (Life Technologies) and the appropriate fluorescent dye, either 5 μmol/L Fluo-4 AM (Life Technologies), 5 μmol/L Asante NaTRIUM Green AM, Asante NaTRIUM Green-2 AM (Teflabs), 10 μmol/L 2′,7′-dichlorofluorescein diacetate (DCFDA; Life Technologies), or 2 μmol/L MitoSOX Red (Life Technologies). Myocytes were permeabilized as previously described [25], using saponin (0.01%) added to a solution containing (in mmol/L): 120 K+ L-aspartate, 20 KCl, 3 MgATP, 10 phosphocreatine, 1 KH2PO4, 5 NaCl, 0.1 EGTA, 0.7 MgCl2, 0.019 CaCl2, 20 HEPES (pH 7.2), 5 units/mL creatine phosphokinase, and 5 μmol/L Fluo-3 K+ salt (Life Technologies). The free Ca2+ concentration in a solution was calculated using MAXChelator software (Stanford University).
Fluo-3, Fluo-4, DCFDA, and MitoSOX Red were excited at 488 nm, and fluorescence signals were acquired at wavelengths >505 nm in the line scan mode or XY mode of the confocal system. Asante NaTRIUM Green and Asante NaTRIUM Green 2 were excited at 543 nm, and fluorescence was acquired at wavelengths >560 nm in the XY scan mode. Fluorescent images were analyzed using ImageJ software (NIH) and Origin 8.1 (OriginLab).
We used exogenous CaMKII (New England Biolabs) to potentiate Ca2+ signals in membrane-permeabilized cardiomyocytes [26]. CaMKII was pre-activated by incubation at 30°C in CaMKII reaction buffer supplemented with 100 μmol/L ATP, 1.2 μmol/L calmodulin, and 2 mmol/L CaCl2 for 10 min.
In the experiments on intact cells, myocytes were paced at 1 Hz. Fluorescent signals were recorded after 2 min of pacing before or after drug application. For experiments using membrane-permeabilized cells, fluorescence images were recorded within 5 min after membrane permeabilization. All measurements were done at a temperature of 22-24° C.
2.5 Western Blot Analysis
Western blot analyses were performed by a standard method. Antibodies for phospho-CaMKIIδ at threonine 286 and for GAPDH were purchased from Santa Cruz Biotechnology. Antibodies for PLN phosphorylated at threonine 17 and RyR2 phosphorylated at serine 2814 were purchased from Badrilla. To detect oxidized CaMKIIδ, an immune serum with antibodies directed against oxidized M281/M282 (ox-CaMKII) was used (polyclonal rabbit, 1:2500 dilution). Secondary antibody was horseradish peroxidase-linked goat anti-rabbit (1:1000) (PerkinElmer Life and Analytical Sciences). Relative intensity of individual bands from Western blots was quantitated using ImageJ software and normalized to GAPDH. The ratio for control was assigned a value of 1.
2.6 TAC Mouse Model of HF
Transverse aortic constriction (TAC) was performed on 3-month-old male CD-1 mice as previously described [27]. The presence of a TAC-induced pressure gradient across the aortic constriction was verified using echocardiography. Serial echocardiography was done for data acquisition. Two-dimensional guided m-mode and 2D images were used to quantify and estimate LV luminal dimensions, systolic and diastolic function, and fractional shortening. Only mice with a pressure gradient >30 mmHg were used for experiments. Sham-operated animals were used as controls.
2.7 Statistics
All values were expressed as mean ± SEM. Statistical comparisons were made using unpaired Student’s t-test or 2-way ANOVA for repeated measurements (p<0.05).
3. RESULTS
3.1 ATX-II increases INaL in adult rabbit ventricular myocytes
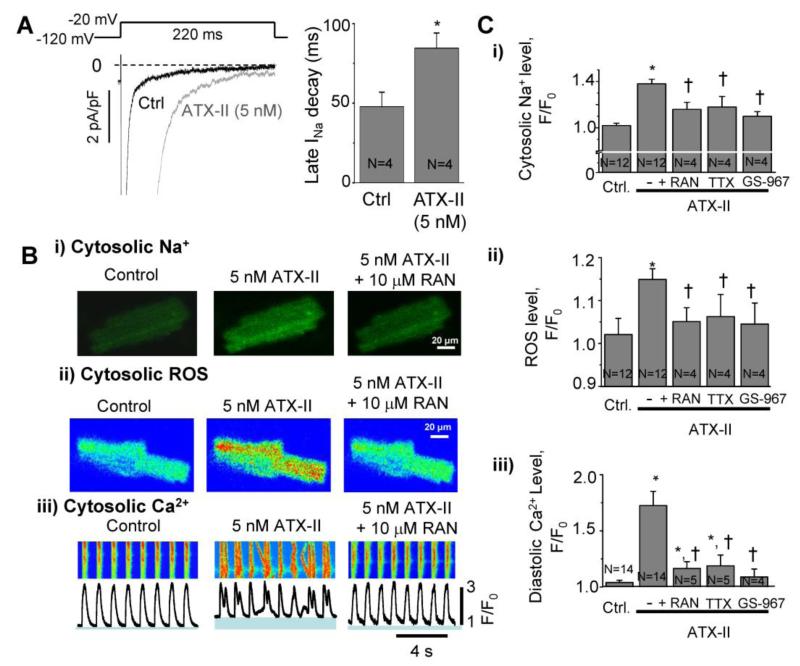
To increase INaL in rabbit ventricular myocytes, we incubated cells with 5 nM ATX-II for 30 minutes. Treatment with ATX-II caused a slowing of the rate of decay of Na+ current induced by a depolarizing voltage-clamp pulse, thereby increasing INaL (Figure 1A). The time constants of INa decay were 84.6 ± 9.5 ms in ATX-II treated cells and 47.8 ± 9.1 ms in control (vehicle-treated) cells. These results are consistent with previous findings that ATX-II causes slowing and incomplete inactivation of Na+ channels, and increased INaL [12,19,28].
Figure 1. Na+i overload induces Ca2+i mishandling and ROS generation in adult rabbit cardiomyocytes.
A. Activation of INaL by Anemone toxin (ATX-II) in rabbit ventricular myocytes, i) representative recordings of INaL in the absance of drug (control) and after superfusion with 5 nM ATX-II. ii) Mean values of INaL decay time in control (n=4) and after superfusion with 5 nM ATX-II (n=4). B.) Confocal images of a rabbit ventricular myocyte paced at 1 Hz and loaded with Na+ dye Asante NaTRIUM Green AM. Application of ATX-II for 2 min increased cytosolic Na+ compared to control (Ctrl), which was reversed by ranolazine (RAN, 10 μM). ii) Cytosolic ROS, measured using DCFDA, was increased in response to ATX-II, and was also reduced by treatment with RAN. iii) Line-scan recordings of Ca2+ transients using the dye Fluo-4 AM. ATX-II increased spontaneous Ca2+ release and increased diastolic Ca2+ level (shown in blue under each Ca2+ transient profile). ATX-II effects on Ca2+ transients were reversed by RAN. C. Quantification of results for ATX-ll-induced changes in i) cytosolic Na+, ii) cytosolic ROS level, and iii) diastolic Ca2+ levels in the absence and presence of RAN (10 μM), TTX (1 μM) and GS-967 (1 μM). Data are normalized to F0 - the baseline fluorescence signal at the beginning of an experiment. N=3-14, * p<0.05 vs control; † P<0.05 vs ATX-II.
3.2 ATX-II increases Na+ and ROS production, and causes Ca2+ mishandling in rabbit ventricular myocytes
The INaL enhancer ATX-II markedly increased the intracellular Na+ concentration measured with the fluorescent dye Asante NaTRIUM Green in cardiomyocytes paced at a rate of 1 Hz. The value of F/F0, the intensity of dye fluorescence in the presence of ATX-II normalized to that before pacing and drug application, was 1.38±0.04 (Figure 1Bi and 1Ci). Treatment with the late Na+ current blockers ranolazine (10 μmol/L), tetrodotoxin (1 μmol/L), and GS-967 (1 μmol/L) in the presence of ATX-II reduced the values of F/F0 to 1.16±0.06, 1.18±0.09, and 1.1±0.04, respectively (Figure 1Ci).
ATX-II also significantly elevated the cytosolic ROS level (Figure 1Bii and 1Cii). The F/F0 value of DCFDA fluorescence was significantly increased from 1.02±0.04 in control to 1.15±0.02 in the presence of ATX-II (Figure 1Cii). Application of ranolazine, TTX or GS-967 significantly reduced cellular ROS production in the presence of ATX-II (F/F0 values were 1.05±0.03, 1.06±0.06 and 1.05±0.5, respectively).
Line-scan confocal images of cardiomyocytes loaded with Fluo-4 AM and paced at 1 Hz revealed an increase in the diastolic Ca2+ level in the presence of ATX-II. The value of F/F0 was increased from a control of 1.0±0.01 in control to 1.78±0.09 in the presence of ATX-II (Figure 1Biii and 1Ciii). Inhibition of Na+ influx with ranolazine, TTX or GS-967 significantly decreased the diastolic Ca2+ level in the presence of ATX-II; values of F/F0 were 1.16±0.06 (RAN), 1.19±0.09 (TTX) and 1.09±0.07 (GS-967), respectively (Figure 1Ciii). The effect of ATX-II to cause spontaneous arrhythmic Ca2+ releases was also blocked by ranolazine (see Ca2+ transients in Figure 1Biii).
3.3 Both ROS production and CaMKIIδ activation are essential for ATX-II-induced abnormal intracellular Ca2+ handling
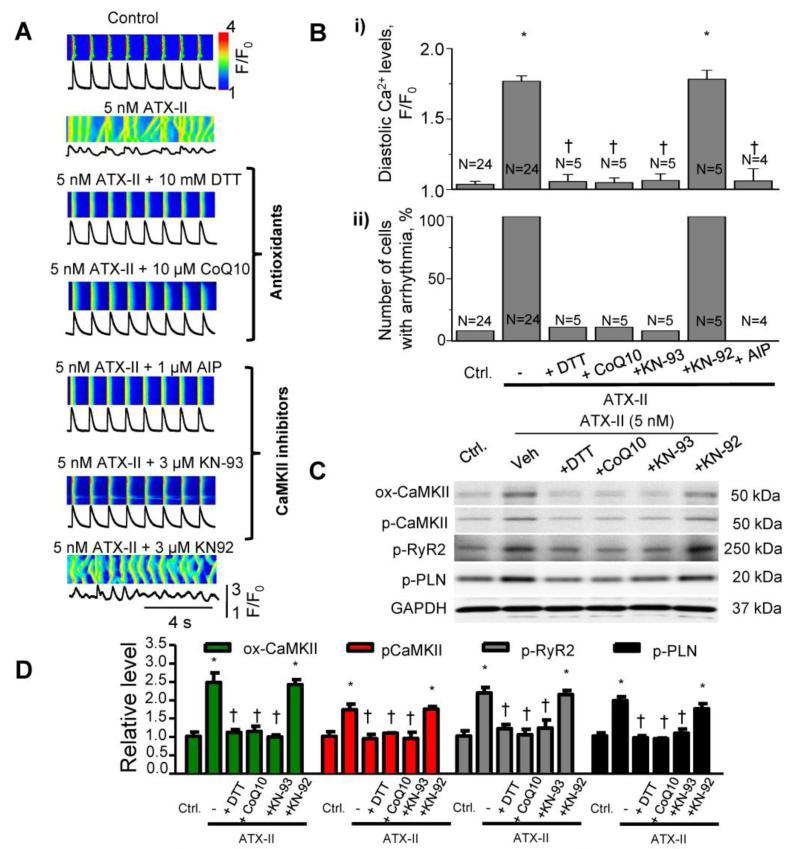
To determine whether intracellular Ca2+ mishandling depends on Na+-induced oxidative stress, we applied antioxidants to ATX-II-treated rabbit cardiomyocytes (Figure 2A,B). ATX-II alone increased the number and amplitude of Ca2+ transients and the diastolic Ca2+ level (Figure 2A,B). ATX-II also increased the rate of leak of Ca2+ from the SR (Supplemental Figure 1). Either dithiothreitol (DTT, 5 mmol/L) or coenzyme Q10 (CoQ10, 10 μmol/L) significantly attenuated an ATX-II-induced increase in diastolic Ca2+ concentration, restored Ca2+ transient amplitude, and eliminated cellular arrhythmias (Figure 2A,Bi,Bii). CoQ10 also reduced cytosolic Na+ levels in ATX-II-treated cardiomyocytes (Supplemental Figure 2).
Figure 2. ATX-II induced Ca2+ mishandling is reversed by antioxidants or CaMKII inhibition.
A. Line-scan confocal images of Ca2+transients recorded from paced adult rabbit cardiomyocytes. The antioxidants CoQ10 (10μM) and DTT (10mM) or the CaMKII inhibitors, AIP (1 μM) or KN-93 (3) μM), were applied after induction of arrhythmic Ca2+ transients with 5 nM ATX-II. Treatment with antioxidants or CaMKII inhibitors restored normal Ca2+ dynamics and attenuated arrhythmic Ca2+ transients. KN-92 (3 μM) was used as a negative control for KN-93. B. Quantification of i) diastolic Ca2+, and ii) number of cells with arrhythmic Ca2+ transients (%). N=4-24; * p<0.05 vs control; †P<0.05 vs ATX-II alone. C. Western blots for oxidation of CaMKII at Met281/282 (ox-CaMKII), phosphorylation of CaMKII at Tyr287 (p-CaMKII), phosphorylation of RyR2s at Ser2814 (p-RyR2) and phosphorylation of PLN at Thr17 (p-PLN). Cardiomyocytes were treated with 5 nM ATX-II, in the absence and presence of antioxidants CoQ10 (10 (μM), DTT (10 (μM), the CaMKII inhibitor KN-93 (3 μM) and the inactive analog, KN92 (3 μM). D. Western Blot quantification, normalized to GAPDH (N=3, * p<0.05 vs control and † p<0.05 vs ATX-II alone).
Next we determined whether CaMKIIδ is involved in ATX-II-induced Ca2+ mishandling. Application of a CaMKII inhibitor, either autocamtide inhibitory peptide (AIP, 1 μmol/L) or KN-93 (3 μmol/L), improved intracellular Ca2+ handling in ATX-II-treated cells (Figure 2A,B). By contrast, KN-92, a non-active analog of KN-93, had no effect. Western Blot analysis revealed an increase in oxidation of CaMKIIδ at methionines 281/282 and autophosphorylation of CaMKIIδ at serine 287 (Figure 2C, D) in the presence of 5 nmol/L ATX-II (Figure 2C). Antioxidants DTT and CoQ10 prevented the oxidation and phosphorylation of CaMKII in ATX-II-treated cells (Figure 2C,D). The effect of KN-93 (but not KN-92) was similar to that of the antioxidants. Furthermore, DTT, CoQ10 and KN-93 reduced phosphorylations of CaMKIIδ targets, RyR2 at serine 2814 and PLN at serine 17 (Figure 2C, 2D), in the presence of ATX-II. These results suggest that ATX-II-induced ROS production may activate CaMKII.
3.4 Elevated Na+ modulates Ca2+ handling in membrane-permeabilized cardiomyocytes
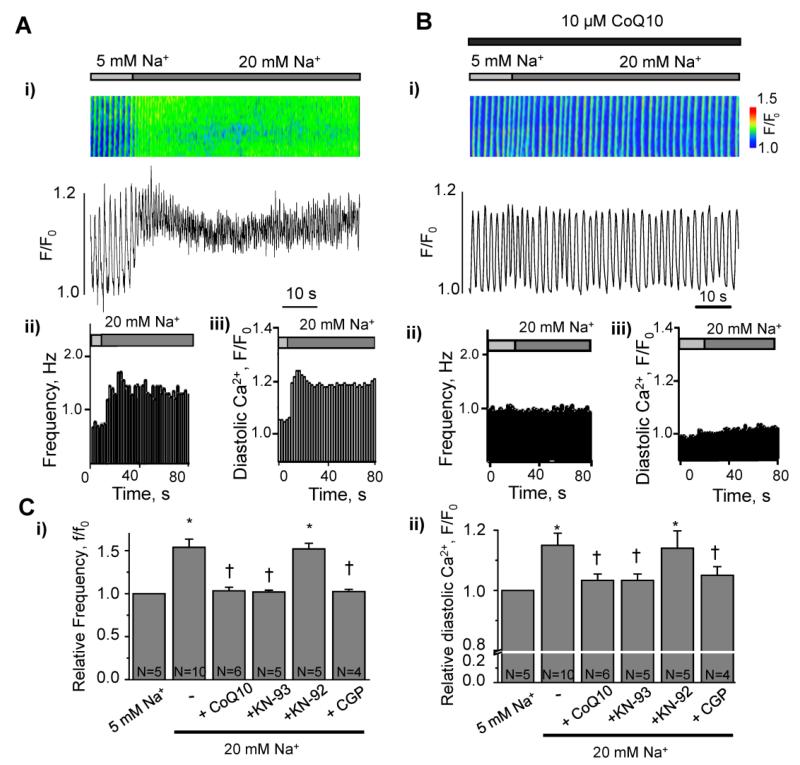
To rule out the possibility that ion channels, Ca2+ pumps and NCX in the sarcolemmal membrane may explain the effects of ATX-II and increased cytosolic [Na+]i on intracellular Ca2+ handling in this study, we used membrane-permeabilized cardiomyocytes that allow us to control cytosolic Na+ and Ca2+ concentrations. We clamped the cytosolic free Ca2+ concentration at 100 nmol/L with 50 μmol/L EGTA. In the presence of 5 mmol/L Na+, a rhythmic pattern of spontaneous Ca2+ transients is observed in membrane-permeabilized myocytes (Figure 3A and reference 25). Elevation of the intracellular Na+ concentration from a physiological (5 mmol/L) to a pathological (20 mmol/L) level resulted in an accelerated rate of spontaneous Ca2+ waves (Figure 3A). The presence of CoQ10 prevented such an acceleration (Figure 3B). The finding suggests that ROS play a role in activation of Ca2+ release from intracellular stores (e.g., sarcoplasmic reticulum) when the intracellular Na+ concentration is raised. Activation of CaMKII by ROS is a potential mechanism of the effect. To verify the involvement of CaMKII in the response to 20 mmol/L Na+, we pre-treated cells with the CaMKII inhibitor KN-93 (3 μmol/L). KN-93 (but not KN-92) abolished acceleration of spontaneous Ca2+ waves induced by 20 mmol/L Na+ (Figure 3C). As positive controls, permeabilized myocytes were incubated with exogenous pre-activated CaMKII (2 units activity/L) or H2O2 (100 μmol/L); these agents also induced an acceleration of the frequency of spontaneous Ca2+ releases (Supplemental Figures 3 and 4).
Figure 3. Role of ROS, mitochondrial Na+/Ca2+ exchange, and CaMKII in maladaptive Ca2+ handling induced by elevation of [Na+]i in membrane-permeabilized cardiomyocytes.
A. i) Confocal line-scan image and profile of Ca2+ transients in a membrane-permeabilized rabbit ventricular myocyte, recorded during elevation of cytosolic Na+ from 5 to 20 mM; ii) spontaneous Ca2+ wave frequency and iii) diastolic Ca2+ level for the experiment shown in panel (i). B. i, Confocal line-scan image and profile of Ca2+ transients in a membrane-permeabilized rabbit ventricular myocyte during elevation of cytosolic Na+ from 5 to 20 mM in the presence of the antioxidant CoQ10 (10 μM); ii) spontaneous Ca2+ wave frequency and iii) diastolic Ca2+ level for the experiment shown in (i). C. Quantification of spontaneous Ca2+ wave frequency f/f0(i) and the diastolic Ca2+ level (ii), induced by elevation of [Na+]i in the absence and presence of the antioxidat CoQ10 (10 μM), the CaMKII inhibitor KN-93 (3 μM), the inactive analog KN-92 (3 μM) or mitochondrial Na+/Ca2+ exchange inhibitor CGP-37157 (CGP, 10 (μM) N=4-10 * p<0.05 vs control; †p<0.05 vs 20 mM Na+. f0, the frequency of spontaneous Ca2+ waves at the beginning of experiment.
An increase in intracellular Na+ can cause Ca2+ efflux from mitochondria, and has the potential to increase mitochondrial ROS production. Therefore, we used the mitochondria-specific ROS indicator MitoSOX Red to assess ROS production in mitochondria in membrane-permeabilized cardiomyocytes exposed to 5 and 20 mmol/L Na+. The fluorescence of MitoSOX Red was increased to 173 ± 15% of initial fluorescence intensity in response to elevation of Na+ from 5 to 20 mmol/L (Supplemental Figure 5). Inhibition of mitochondrial Na+- Ca2+ exchange with 10 μmol/L CGP-37157 abolished the effect of 20 mmol/L Na+ on the frequency of intracellular Ca2+ transients (Figure 3C). These data are in accordance with previous observations [6,10].
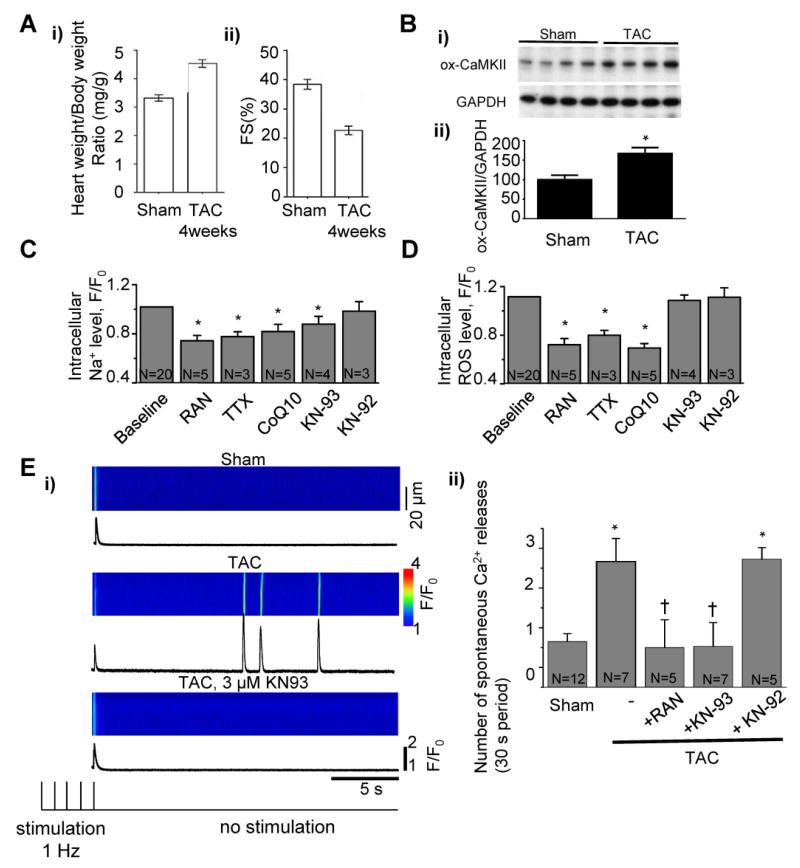
3.5 Na+ overload and oxidative stress in mouse TAC model of HF
To confirm whether Na+ overload can cause ROS production and increase in diastolic Ca2+ in the failing heart, we isolated cardiomyocytes from TAC mice with failing hearts. At 4 weeks after TAC, the mice developed cardiac hypertrophy (a 38% increase in the ratio of heart/body weight) and fractional shortening of LV myocardium was reduced (Figure 4A). It has been shown previously that the level of phosphorylation of CaMKII is increased in TAC hearts (29). To determine whether TAC intervention changed oxidation of CaMKII we carried out Western Blot analysis, which showed a significant increase in ox-CaMKII in TAC cardiac tissue when compared to sham tissue (Figure 4B). The level of intracellular Na+ in paced myocytes isolated from TAC animals was significantly reduced by ranolazine (10 μmol/L) or TTX (1 μmol/L) (Figure 4C) as indicated by decreases in fluorescence of the Na+-sensitive dye Asante NaTRIUM Green-2 to 74% and 78% of baseline, respectively. Neither ranolazine nor TTX reduced dye fluorescence in myocytes isolated from sham-operated animals (Supplemental Figure 6). The antioxidant CoQ10 (10 μmol/L) and the CaMKII inhibitor KN-93 (3 μmol/L), but not KN-92, also significantly decreased Asante NaTRIUM Green-2 fluorescence relative to baseline in paced myocytes isolated from TAC mice (Figure 4C). Treatment of TAC myocytes with ranolazine (10 μmol/L) or TTX (1 μmol/L) also significantly decreased the fluorescence of DCFDA (a ROS-sensitive fluorescent indicator) by 72% and 80% relative to baseline, respectively (Figure 4D). The fluorescence of DCFDA was also decreased to 69% of baseline by CoQ10 (10 μmol/L) (Figure 4D). However, inhibition of CaMKII with KN93 (3 μmol/L) did not significantly change the ROS level (Figure 4D). None of the studied drugs had an effect on basal Na+ and ROS levels in myocytes isolated from sham-operated animals (Supplemental Figure 6).
Figure 4. Pathological contributions of INaL, CaMKII and ROS to maladaptive Ca2+ handling in cardiomyocytes isolated from mouse failing hearts.
A. i) Heart weight/body weight ratio and ii) fractional shortening (FS, %) of the left ventricular wall in male CD-1 mice subjected to transverse aortic constriction (TAC) for 4 weeks, compared to sham-operated, age- matched controls (sham). B. i) Western blots for oxidation of CaMKII at Met281/282 (ox-CaMKII) and Western Blot quantification, normalized to GAPDH (ii). Effects of ranolazine (10 μM), TTX (1 μM), CoQ10 (10 μM), KN-93 (3 μM), and KN-92 (3 μM) on levels of intracellular Na+ (C) and ROS (D) in myocytes isolated from TAC mice and paced at a rate of 1 Hz (N = 3-20, p<0.05), compared to baseline. E. Spontaneous Ca2+ release events following termination of 1 Hz stimulation of myocytes isolated from Sham-operated and TAC mice in the absence or presence of RAN (10μM), KN-93 (3 μM) or its inactive analog KN-92 (3 μM). *p<0.05 vs sham and †p<0.05 vs TAC without drugs.
Analysis of Ca2+ transients in myocytes isolated from sham-operated and TAC animals indicated that the rise time of the transient was significantly longer in the TAC group (Supplemental Table 1). Spontaneous Ca2+ releases following the termination of 1-Hz stimulation occurred more often in myocytes from TAC than in those isolated from sham animals (Figure 4E). Inhibition of INaL by ranolazine (1 μmol/l) or CaMKII with KN-93 (3 μmol/L) normalized Ca2+ transients and diminished spontaneous Ca2+ waves in TAC myocytes (Figure 4E, Supplemental Table I) whereas KN-92 (3 μmol/L) had no effect.
4. DISCUSSION
The goal of the present study was to elucidate potential pathways that connect increases of INaL and of the cytosolic Na+ concentration (i.e., Na+ overload) to disruption of intracellular Ca2+ storage and release (dysfunctional Ca2+ handling). The major findings were: 1) increases of INaL and [Na+]i promoted ROS generation, CaMKII oxidation and phosphorylation, and phosphorylation of CaMKII substrates, including RyR2; 2) an increase of [Na+]i caused increases of diastolic Ca2+ and the frequency of spontaneous Ca2+ waves in membrane-permeabilized myocytes, i.e., in the absence of sarcolemmal membrane regulation of the intracellular Ca2+ concentration; 3) Ca2+ mishandling induced by Na+ overload was reversed by ranolazine and tetrodotoxin, CaMKII inhibitors, antioxidants, and inhibition of the mitochondrial Na+- Ca2+ exchanger; 4) in cardiomyocytes isolated from failing hearts of TAC mice, intracellular levels of Na+ and ROS were reduced by inhibitors of INaL and by CoQ10, and inhibition of CaMKII reduced the frequency of spontaneous Ca2+ release events. Taken together, the results suggest a pathological pathway (Figure 5) whereby cardiomyocyte Na+ overload promotes mitochondrial ROS generation, resulting in CaMKII oxidation and phosphorylation, and CaMKII-mediated phosphorylation of substrates including RyR2, which increases Ca2+ leak from SR (Supplemental Figure 1) and the frequency of SR Ca2+ release events (Figures 1,2), resulting in an increase of the cytosolic Ca2+ concentration during diastole (Figures 1,2,5). This pathway appears to be operative in myocytes of failing hearts. Our findings are consistent with and expand on the previously reported links between cytosolic Na+ overload and mitochondrial ROS generation [6, 7] and between oxidized CaMKII and Ca2+ overload [2,11,12,18,30,31].
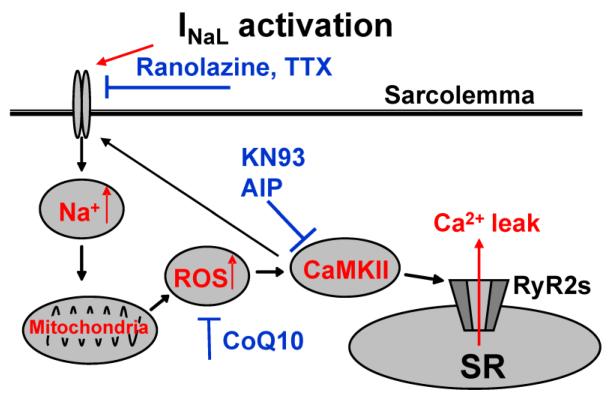
Figure 5. Proposed mechanism for INaL-mediated Na+ overload-induced disruption of myocyte Ca2+ handling.
An increase of INaL leads to an increase of [Na+]i Cytosolic Na+ overload attenuates mitochondrial Ca2+ uptake via the mitochondrial Na+/Ca2+ exchanger, thereby reducing NADPH regeneration and increasing mitochondrial ROS, ROS oxidize and activate CaMKII, resulting in phosphorylation of the CaMKII substrate RyR2 and increasing Ca2+ leak from sarcoplasmic reticulum (SR).
4.1 Contribution of INaL to Na+ influx
In the present research we used INaL enhancement (Figure 1) as a tool to increase cytosolic Na+ load. INaL that occurs during the plateau of the action potential has been shown by many investigators to cause AP prolongation. INaL can account for 30% or more of Na+ influx through myocyte Na+ channels [32]. Other potential sources of Na+ entry via Na+ channels are “window current” [33], and “background current” near the threshold of Na+ channel activation [34]. They are not usually called INaL, which typically refers to current at plateau potentials due to failure of open state inactivation of Na+ channels, but they can be blocked by ranolazine and TTX and may have contributed to Na+ entry in this study. The effects of CaMKII, oxidation, and diseases on window and background Na+ currents are poorly understood.
Our results indicate that the decay time constant of INaL in ATX-II (5 nM)-treated rabbit cardiomyocytes was prolonged 1.5-2-fold. This prolongation is similar in magnitude to that found in myocytes from failing hearts in TAC mice (29). This suggests that the increase of INaL is comparable in both models. Therefore, we believe that the effect of ATX-II on rabbit myocytes in this study is not so large as to be irrelevant to an understanding of its potential pathological effects in failing hearts, and to the mechanisms by which the effect of Na+ overload (regardless of its cause) is mediated.
4.1 Na+ overload induced mitochondrial ROS generation
The detrimental influence of intracellular Na+ overload on cardiac cell function is well recognized [3-5,8,12,19-21]. The prevailing view of the pathway that connects cytosolic Na+ accumulation to altered Ca2+ homeostasis has focused on the role of the sarcolemmal Na+- Ca2+ exchanger in regulation of the cytosolic Ca2+ concentration. In this pathway, Na+ overload decreases Ca2+ extrusion and increases Ca2+ entry via the exchanger, causing Ca2+ accumulation that results in contractile dysfunction and arrhythmias. The results of the present study do not dispute the validity of this mechanism. However, our results suggest that mechanisms independent of membrane Na+- Ca2+ exchange also appear to take part in Na+ overload-induced dysregulation of Ca2+ homeostasis. The finding that ATX-II, an enhancer of INaL, increased cytosolic Na+, diastolic Ca2+, ROS, the frequency of spontaneous Ca2+ release events, and oxidation and phosphorylation of CaMKII strongly suggest that INaL can raise the level of cytosolic Na+ sufficiently to disrupt Ca2+ homeostasis. Importantly, we demonstrate that in the absence of a functional plasma membrane (i.e., in membrane-permeabilized adult rabbit cardiomyocytes) elevation of cytosolic Na+ alone is sufficient to provoke increases in ROS generation, the frequency of Ca2+ waves, and the diastolic Ca2+ concentration (Figure 3).
Our observations that ATX-II promotes elevated ROS production (Figure 1) and that deleterious consequences of Na+ overload are attenuated by antioxidants such as CoQ10 and DTT (Figures 2,3) are in agreement with a growing body of evidence demonstrating that Na+ overload promotes cellular oxidative stress. Kohlhaas et al. [7] recently described a series of experiments demonstrating that elevation of cytosolic Na+ is sufficient to induce mitochondrial ROS generation. In patch clamped guinea-pig cardiomyocytes, elevation of cytosolic Na+ from 5 to 15 mmol/L resulted in mitochondrial Ca2+ extrusion with a concurrent increase in H2O2 generation, effects which were blocked by the mitochondrial Na+- Ca2+ exchange inhibitor CGP-37157 [7]. These authors [7] suggest that Na+ overload promotes mitochondrial Ca2+ efflux, resulting in H2O2 accumulation as a consequence of impaired NADPH-dependent mitochondrial antioxidant capacity. In our study, using permeabilized myocytes, we confirmed that Na+ overload can increase mitochondrial ROS production (Supplemental Figure 5) and that Na+-induced Ca2+ mishandling is attenuated by CGP-37157 (Figure 3C), in agreement with Kohlhaas et al [7]. Involvement of the mitochondrial Na/Ca exchanger in this mechanism assumes that mitochondrial Na+ and Ca2+ concentrations should be altered by cytosolic Na+ overload. Changes in either Na+ or Ca2+ concentration can induce mitochondrial dysfunction. Reduction of mitochondrial Ca2+ efflux in Na+-overloaded myocytes may explain data whereby ranolazine (which can reduce INaL and the cytosolic Na+ concentration [19,20] and can indirectly increase the activity of mitochondrial dehydrogenases such as pyruvate dehydrogenase that rely on mitochondrial Ca2+ for their activity [35,36]). However, it should be noted that the mechanisms of increased mitochondrial ROS generation are only partially understood.
4.2 Elevation of [Na+]i, CaMKII oxidation and phosphorylation, and their relationship to disruption of Ca2+ handling
A novel observation in this study was that CaMKII oxidation was increased subsequent to an elevation by ATX-II of the intracellular Na+ concentration in intact cardiomyocytes (Figure 2C,D) and TAC cardiac tissue (Figure 4B). ATX-II-induced CaMKII oxidation was detected using an antibody that specifically recognizes the oxidized methionine 281/282 site on CaMKII [30]. In parallel to the increase in oxidized-CaMKII, we observed an ATX-II-induced increase in CaMKII phosphorylation at Thr-286, demonstrating increased autophosphorylation (Figure 2C,D). These results are in agreement with previous studies demonstrating that CaMKII oxidation is accompanied by an increase in CaMKII autophosphorylation [11,30,31]. Importantly, the increases in CaMKII oxidation and phosphorylation were accompanied by an elevation in the phosphorylation of CaMKII substrates involved in Ca2+ handling, including PLN and RyR2 (Figure 2C,D), demonstrating functional activation of CaMKII. The ATX-II-induced oxidation/phosphorylation of CaMKII and its substrates was attenuated by inhibitors of INaL, antioxidants (DTT or CoQ10), or the CaMKII inhibitor KN-93. Treatment of myocytes with KN93 or CoQ10 restored normal Ca2+ dynamics and attenuated arrhythmic activity in the presence of ATX-II (Figure 2B). Similarly, KN-93 and CoQ10 also prevented aberrant Ca2+ handling induced by elevated cytosolic Na+ in permeabilized cardiomyocytes (Figure 3). Furthermore, treatment of permeabilized cells with exogenous, pre-activated phosphorylated CaMKII accelerated the frequency of spontaneous Ca2+ waves and increased diastolic Ca2+ (Supplemental Figure 3). The effects of pre-activated CaMKII were strikingly similar to those observed after elevation of the intracellular Na+ concentration in permeabilized myocytes (Figure 3). Taken together, our data provide evidence that CaMKII activity is both necessary and sufficient to induce maladaptive Ca2+ handling in cardiomyocytes, and it acts as both a sensor for, and an effector of responses to, an increase in ROS production [11,30,31].
A high level of oxidized CaMKIIδ has been associated with disruption of intracellular Ca2+ handling, arrhythmias, and contractile dysfunction [18,30,31,37-39]. Recently, Wagner et al [18] demonstrated that ROS-activated CaMKII is required for INaL augmentation leading to cellular Na+ and Ca2+ overload. CaMKII can phosphorylate the cardiac Na+ channel pore-forming alpha subunit and enhance INaL [13,18,40]. Thus, intracellular Na+ -dependent activation of CaMKII could induce further activation of INaL by a positive feedback mechanism. Moreover, CaMKII alters Ca2+ handling in cardiac myocytes via phosphorylation of RyR2 and phospholamban to increase Ca2+ release from, and Ca2+ loading of, the sarcoplasmic reticulum, [41]. The finding that oxidation and phosphorylation of CaMKII were increased in myocytes exposed to the INaL enhancer ATX-II is in accord with previous reports [12]. Thus, CaMKII activation, including activation through an oxidation-dependent pathway, is a key element in a complex interplay between Na+ load and Ca2+ handling under pathological conditions [12,17,18,31,38,39].
4.3 TAC data and relevance to heart failure
Increases of INaL and CaMKII activity are well documented in cardiomyocytes isolated from failing hearts of experimental animals [5,17,20,42] and human patients [20,42]. Toischer et al recently reported roles for INaL and CaMKII in the progression of pressure-overload induced heart disease after TAC in mice [29]. In their study, INaL was not enhanced during the compensatory hypertrophy stage (1 week post-TAC) but was significantly enhanced in hearts that had developed heart failure (5 weeks post-TAC) characterized by a reduced ejection fraction. In parallel to the increase in INaL observed in failing myocytes, there was a significant increase in both CaMKII autophosphorylation and phosphorylation of NaV1.5 at the CaMKII site ser-571, when compared to myocytes isolated from non-failing hearts or from hearts in the compensatory hypertrophy stage [29]. In the present study, we utilized a similar model of pressure overload-induced heart failure in mice to examine the contributions of INaL, CaMKII and ROS to maladaptive Ca2+ handling in the failing heart. Heart failure was induced by 4 weeks TAC, as evidenced by a significant reduction in fractional shortening of left ventricular myocardium, relative to sham-operated mice (Figure 4A). Importantly, the level of ox-CaMKII was increased in cardiac tissue of TAC animals relative to sham animals (Figure 4B). Cardiomyocytes isolated from failing hearts had evidence of maladaptive Ca2+ handling, manifested as an increase in spontaneous Ca2+ release events (Figure 4D). The frequency of Ca2+ release events was normalized after treatment of myocytes with INaL inhibitor ranolazine or CaMKII inhibitor KN93. Treatment with ranolazine, KN-93 or CoQ10 all reduced cellular Na+, whereas ROS was reduced by either CoQ10 or ranolazine, but not by KN-93, because elevation of ROS production can occur in the absence of CAMKII activation (Figure 5). None of these agents had an effect on the cytosolic Na+ concentration or on ROS production in myocytes isolated from non-failing hearts (Supplemental Figure 6). Taken together with the work of Toischer et al. [29] our findings suggest that INaL-induced Na+ overload, oxidative stress, and CaMKII activity likely contribute to pro-arrhythmic Ca2+ mishandling in the failing heart.
Conclusions
Our results provide experimental evidence that CaMKIIδ oxidation subsequent to mitochondrial ROS generations induced by cytosolic Na+ overload is an important component of an intracellular signaling pathway (Figure 5) leading to aberrant Ca2+ handling under pathologic conditions such as heart failure. This pathway is not dependent on, but may operate in parallel with, the “classical” mechanism of Na+-induced Ca2+ overload mediated by the sarcolemmal Na+ - Ca 2+ exchanger. The evidence that Na+ overload-induced mitochondrial ROS formation and CaMKII oxidation are mechanisms of potential importance in the etiology of cardiac disease may provide a conceptual framework for the development of new drug therapies.
Supplementary Material
Highlights.
An increase of INaL increased [Na+]i, mitochondrial ROS, CaMKII activity, and spontaneous SR Ca2+ release in cardiomyocytes
Elevation of [Na+]i in permeabilized myocytes reproduced the effects of increasing INaL
Inhibition of INaL reduced [Na+]i and ROS formation in myocytes from failing hearts
Cytosolic Na+ overload can induce ROS formation and Ca2+ mishandling independently of cell membrane NCX
Acknowledgements
The authors especially thank Sridharan Rajamani (Gilead Sciences Inc.) for experimental assistance.
This project was funded by a grants from National Institutes of Health (NIH) Grants R01-HL 079031, R01-HL096652, and R01-HL070250, R01-HL071140 (all – Marc E. Anderson) and by grant from Gilead Sciences Inc. (C.P. Chang).
Abbreviations
- AIP
autocamtide inhibitory peptide
- ATX-II
Anemonia sulcata toxin-II
- Ca2+
calcium
- CaMKIIδ
calcium/calmodulin dependent protein kinase II delta
- CGP
CGP-37157
- CoQ10
coenzyme Q10
- DTT
dithiothreitol
- INa
sodium channel current
- INaL
sodium channel late current
- Na+
sodium
- NCX
sarcolemmal sodium-calcium exchanger
- PLN
phospholamban
- RAN
ranolazine
- ROS
reactive oxygen species
- RyR2
ryanodine receptor 2
- SR
sarcoplasmic reticulum
- TAC
transverse aortic constriction
- TTX
tetrodotoxin
Glossary
- F/F0
the intensity of dye fluorescence in the presence of an experimental intervention (e.g., drug application) normalized to the intensity of dye fluorescence in the same preparation before the intervention (i.e., control)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Serge Viatchenko-Karpinski, Dmytro Kornyeyev, Nesrine El-Bizri, Grant Budas, Peidong Fan, Zhan Jiang, Luiz Belardinelli and Lina Yao are full time employees of Gilead Sciences Inc. John C. Shryock is a former employee of Gilead Sciences Inc.
REFERENCES
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res. 2009;104:292–303. doi: 10.1161/CIRCRESAHA.108.189050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006;92(Suppl 4):iv1–iv5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Rourke B, Maack C. The role of Na dysregulation in cardiac disease and how it impacts electrophysiology. Drug Discov Today Dis Models. 2007;4:207–217. doi: 10.1016/j.ddmod.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997;80:82–87. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, et al. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chelu MG, Sarma S, Sood S, Wang S, Van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 16.Currie S. Cardiac ryanodine receptor phosphorylation by CaM Kinase II: keeping the balance right. Front Biosci. 2009;14:5134–5156. doi: 10.2741/3591. [DOI] [PubMed] [Google Scholar]
- 17.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, et al. Reactive oxygen species-activated Ca/calmodulin kinase II{delta} is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 20.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schöndube FA, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Shryock JC, Song Y, Rajamani S, Antzelevitch C, Belardinelli L. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovasc Res. 2013;99:600–611. doi: 10.1093/cvr/cvt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chahine M, Plante E, Kallen RG. Sea anemone toxin (ATX II) modulation of heart and skeletal muscle sodium channel alpha-subunits expressed in tsA201 cells. J Membr Biol. 1996;152:39–48. doi: 10.1007/s002329900083. [DOI] [PubMed] [Google Scholar]
- 23.Belardinelli L, Liu G, Smith-Maxwell C, Wang WQ, El-Bizri N, Hirakawa R, Karpinski S, et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. doi: 10.1124/jpet.112.198887. [DOI] [PubMed] [Google Scholar]
- 24.Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Györke S. Dynamic regulation of sarcoplasmic reticulum Ca(2+) content and release by luminal Ca(2+)-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 27.Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulbricht W. Sodium channel inactivation: molecular determinants and modulation. Physiol Rev. 2005;85:1271–1301. doi: 10.1152/physrev.00024.2004. [DOI] [PubMed] [Google Scholar]
- 29.Toischer K, Hartmann N, Wagner S, Fischer TH, Herting J, Danner BC, et al. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J Mol Cell Cardiol. 2013;61:111–122. doi: 10.1016/j.yjmcc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson JR, Joiner MA, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner S, Rokita AG, Anderson ME, Maier LS. Redox regulation of sodium and calcium handling. Antioxid Redox Signal. 2013;18:1063–1077. doi: 10.1089/ars.2012.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makielski J, Farley AL. Na+ current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17:S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 33.Colatsky TJ. Mechanisms of action of lidocaine and quinidine on action potential duration in rabbit cardiac Purkinje fibers. Circ Res. 1982;50:17–27. doi: 10.1161/01.res.50.1.17. [DOI] [PubMed] [Google Scholar]
- 34.Zilberter YI, Starmer F, Starobin J, Grant AO. Late Na channels in cardiac cells: the physiological role of background Na channels. Biophys J. 1994;87:153–160. doi: 10.1016/S0006-3495(94)80464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke B, Spedding M, Patmore L, McCormack JG. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol. 1993;109:748–750. doi: 10.1111/j.1476-5381.1993.tb13637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol. 1996;28:341–350. doi: 10.1006/jmcc.1996.0032. [DOI] [PubMed] [Google Scholar]
- 37.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, et al. Oxidized CaMKII triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rokita AG, Anderson ME. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII) Circulation. 2012;126:2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 42.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.