Abstract
Purpose
To evaluate the feasibility, safety, and utility of intraoperative optical coherence tomography (OCT) for use during ophthalmic surgery.
Design
Prospective, consecutive, case series
Methods
A prospective, single-center, consecutive, case series was initiated to assess intraoperative OCT in ophthalmic surgery. Intraoperative scanning was performed with a microscope mounted spectral domain OCT system. Disease specific or procedure-specific imaging protocols (e.g., scan type, pattern, size, orientation, density) were utilized for anterior and posterior segment applications. A surgeon feedback form was recorded as part of the study protocol to answer specific questions regarding intraoperative OCT utility immediately after the surgical procedure was completed.
Results
During the first 24 months of the PIONEER study, 531 eyes were enrolled (275 anterior segment cases and 256 posterior segment surgical cases). Intraoperative OCT imaging was obtained in 518 of 531 eyes (98%). Surgeon feedback indicated that intraoperative OCT informed surgical decision-making and altered surgeon understanding of underlying tissue configurations in 69/144 (48%) lamellar keratoplasty cases and 63/146 (43%) membrane peeling procedures. The most common anterior segment surgical procedure was descemet stripping automated endothelial keratoplasty (DSAEK, n = 135). Vitrectomy with membrane peeling was the most common procedure for posterior segment surgery (n = 154). The median time that surgery was paused to perform intraoperative OCT was 4.9 minutes per scan session. No adverse events were specifically attributed to intraoperative OCT scanning during the procedure.
Conclusions
Intraoperative OCT is feasible for numerous anterior and posterior segment ophthalmic surgical procedures. A microscope mounted intraoperative OCT system provided efficient imaging during operative procedures. The information gained from intraoperative OCT may impact surgical decision-making in a high frequency of both anterior and posterior segment cases.
Introduction
Optical coherence tomography (OCT) has revolutionized the clinical management of ophthalmic diseases.1 The outstanding cross-sectional anatomic information afforded physicians with OCT imaging has transformed the diagnosis, surveillance, and treatment paradigms for both anterior and posterior segment diseases. The translation of this technology to the operating room theater may have profound implications for the optimal surgical management of ophthalmic diseases.
Intraoperative OCT remains a young and emerging field. The description of intraoperative OCT has been limited to case reports and retrospective case series. These reports have highlighted the potential for intraoperative OCT to impact our understanding of the pathophysiology of surgical diseases, to facilitate optimal decision-making regarding achievement of surgical objectives, and to improve our understanding of the tissue alterations that occur during surgical manipulations. The use of intraoperative OCT has been described for a wide variety of conditions and procedures, including optic-pit related maculopathy, epiretinal membranes, vitreomacular traction syndrome, macular hole, retinal detachments, lamellar keratoplasty, retinopathy of prematurity, and cataract surgery.2–9 These studies were in general small (< 30 patients) and retrospective but offered early insight into intraoperative OCT feasibility and utility, while providing an important foundation for future research.
In order to better understand the feasibility and potential utility of intraoperative OCT, the Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy: PIONEER Study was initiated. The focus of this prospective study is examining the feasibility, utility, and safety of performing intraoperative OCT and perioperative OCT for a wide variety of surgeries to better delineate the role for intraoperative OCT in a large number ophthalmic conditions. This report highlights the two-year results of the feasibility, utility, and safety of the intraoperative OCT portion of this large prospective study.
Methods
PIONEER is a single-site, multi-surgeon, prospective clinical study examining the feasibility, safety, and utility of intraoperative OCT in the surgical management of ophthalmic disease. The study was approved by the Cleveland Clinic IRB and adhered to all the tenets of the Declaration of Helsinki and HIPAA policies. All patients gave written informed consent before enrollment in the study. The primary inclusion criterion was any patient undergoing an ophthalmic incisional surgical procedure willing to undergo intraoperative OCT imaging. Exclusion criteria included any media opacity that precluded OCT scanning of the area of interest and inability to provide written informed consent. Patients were allowed to re-enroll in PIONEER (e.g. fellow eye surgery, same eye reoperation). Additionally, bilateral OCT imaging was allowed for bilateral surgical procedures (e.g., refractive surgery).
Clinical and surgical variables were collected including past ocular history, procedure type, preoperative diagnosis, pertinent surgical maneuvers/techniques (e.g., instrumentation, unique manipulations), number of imaging sessions during surgery, duration of imaging sessions, interruption of surgical procedure time for imaging, and adverse events. Patients were also evaluated on the first postoperative day to assess for clinical variables, perioperative images in select cases, and adverse events. The structured study follow-up ended on postoperative day 1; however, patients were consented for up to two years of surveillance of medical records (e.g., best corrected Snellen visual acuity, OCT outcomes). Any visits beyond the first postoperative visit were based on standard-of-care visits per the individual surgeon.
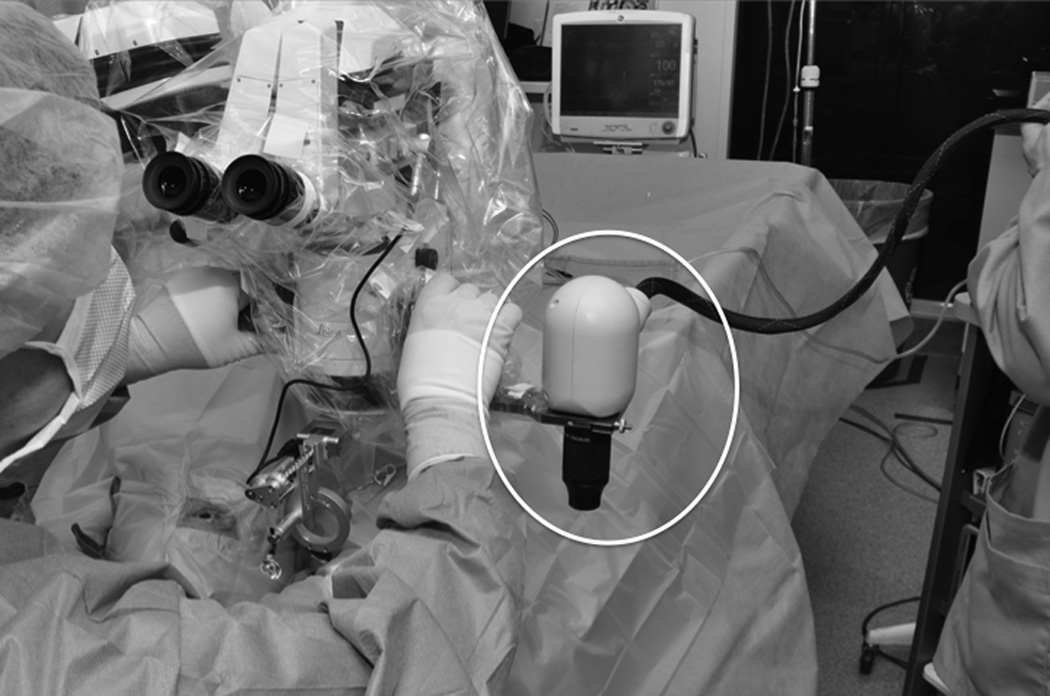
A dedicated intraoperative OCT research coordinator assisted with data acquisition in the operating room. Intraoperative scanning was performed with the Bioptigen SDOIS portable spectral domain OCT (SD-OCT) system (Bioptigen, Research Triangle Park, NC). The portable SD-OCT probe was stabilized with a custom-designed, microscope-mount system (Figure 1) or with handheld scanning. The microscope mount system was developed for use with both Leica and Zeiss microscopes. It also was compatible with the BIOM indirect viewing system as well as contact lens wide-angle viewing systems. When using the microscope mount, the surgeon used the microscope pedal to control the X-Y-Z translation of the probe to image the area of interest.
Figure 1.
Microscope-mounted portable spectral domain optical coherence tomography system (circle).
Disease specific or procedure-specific imaging protocols (e.g., scan type, pattern, size, orientation, density) were outlined for anterior and posterior segment applications. Anterior segment imaging included a 12 mm × 12 mm volumetric cube scan at 0 degree orientation. Posterior segment imaging included a 10 mm × 10 mm volumetric cube with 100 B-scans per volume at both 0 and 90 degrees, a 5 × 10 mm volumetric cube with 7 B-scans per frame for averaging and a total of 175 B-scans per volume at both 0 and 90 degrees, and a 10 mm radial scan with 100 B-scans per volume. Surgeons were provided with guidance for surgical milestones to consider for imaging (e.g., preincision, following membrane peeling, following graft placement), but the specific imaging sessions were at surgeon’s discretion. When possible in vitreoretinal cases, scan sequences were obtained in the operating room prior to any surgical manipulation of the area of interest. Following various surgical milestones, scan sequences were performed to allow for comparative data and change analysis. A “scan” was defined as a single set of images (e.g., volume scan). A “scan session” was defined as a series of scans obtained during a single pause during surgery. Typically, multiple scan sessions were often performed during the surgical procedures.
A specific surgical procedure (i.e., membrane peeling, lamellar keratoplasty) intraoperative OCT feedback form was included as part of the study protocol. This form included questions regarding the impact of the intraoperative OCT on surgical decision-making and on how intraoperative OCT may have impacted the understanding of the surgical disease of interest.
Results
Clinical Demographics
During the first 24 months, 531 eyes were enrolled in the PIONEER study. This included 275 eyes in the anterior segment arm and 256 eyes in the posterior segment arm (Table 1). Overall, 285 right eyes and 246 left eyes were included in the analysis. Successful imaging was obtained in 518 of 531 eyes (98%) at some point during the surgical procedure with variable image quality. Reasons for lack of imaging included poor media clarity and surgeon choice based on logistics (e.g., delay to surgery, timing).
Table 1.
Clinical Characteristics of Patients Participating in the PIONEER Study on Intraoperative Optical Coherence Tomography in Ophthalmic Surgery
| Anterior | Posterior | ||||
|---|---|---|---|---|---|
| Enrollment | 275 | 256 | |||
| Eye | Right | 150 | 135 | ||
| Left | 125 | 121 | |||
| Lens Status | Pseudophakic | 124 | 87 | ||
| Phakic | 147 | 47 | |||
| Aphakic | 4 | 13 | |||
| Concurrent CE/IOL with PPV | 109 | ||||
| Preoperative Diagnosis | Fuchs Dystrophy Cataract Bullous Keratopathy Failed DSAEK Keratoconus Refractive Error Failed PK Other |
82 (30%) 66 (24%) 32 (12%) 23 (8%) 16 (6%) 12 (4%) 11 (4%) 33 (12%) |
Epiretinal membrane Full-thickness macular hole Retinal detachment Proliferative diabetic retinopathy Vitreous hemorrhage Vitreomacular traction Other |
90 (35%) 58 (23%) 44 (17%) 32 (13%) 8 (3%) 6 (2%) 18 (7%) |
|
| Procedures | DSAEK CE/IOL Femto CE/IOL DALK LASIK DSAEK Re-bubble |
135 (49%) 29 (11%) 37 (13%) 19 (7%) 11 (4%) 8 (3%) |
Vitrectomy Vitrectomy and Scleral Buckle Other |
253 15 3 |
|
CE: cataract extraction; IOL: intraocular lens; PPV: pars plana vitrectomy; DSAEK: Descemet stripping automated endothelial keratoplasty; PK: penetrating keratoplasty; DALK: deep anterior lamellar keratoplasty; LASIK: laser assisted insitu keratamileusis
Anterior Segment Intraoperative OCT Summary
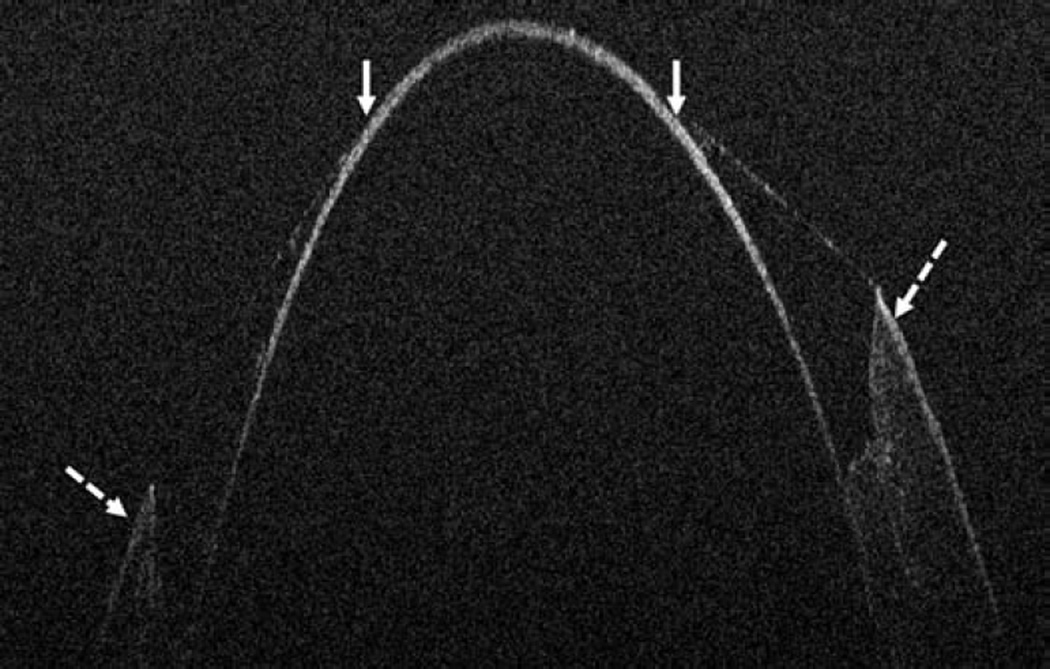
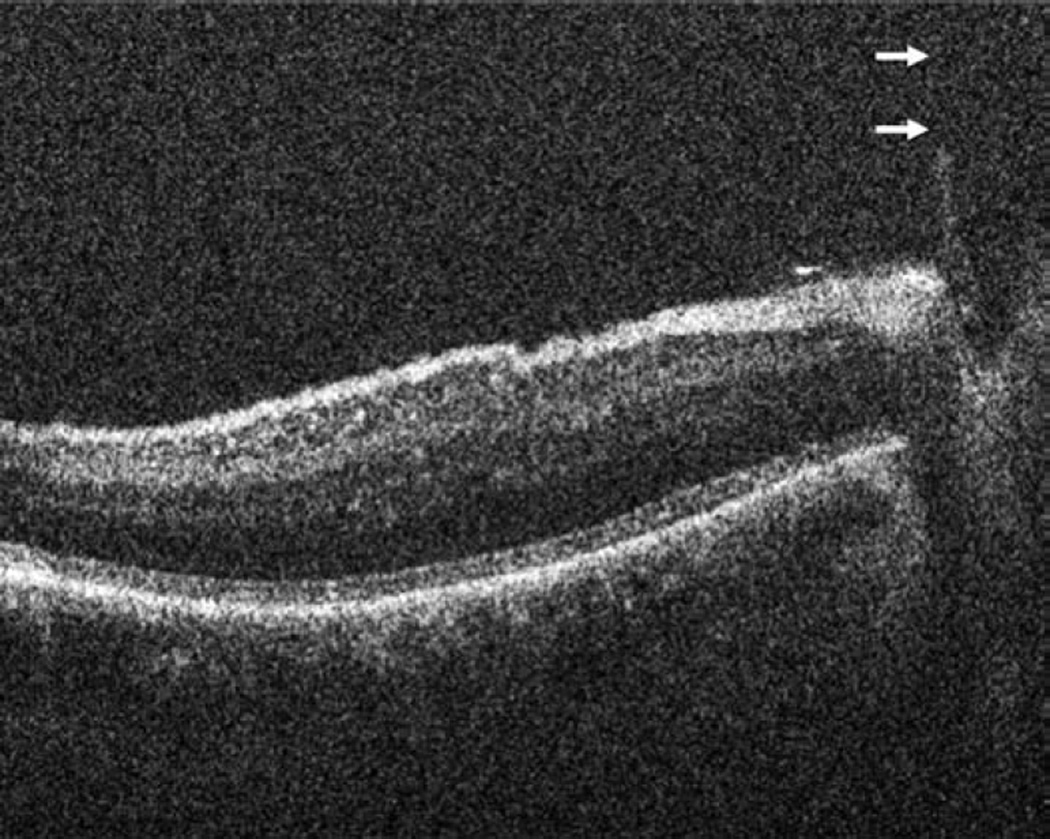
In the anterior segment group, 275 eyes were enrolled. Of these 275 eyes, 124 were pseudophakic, 147 eyes were phakic, and 4 eyes were aphakic. The most common indications for anterior segment surgery were Fuchs endothelial cell dystrophy (30%), cataract (24%), bullous keratopathy (12%), previous failed corneal graft (12%), and keratoconus (6%) (Table 1). The most common surgical procedures performed Descemet stripping automated endothelial keratoplasty (DSAEK), standard phacoemulsification cataract surgery, femtosecond-assisted phacoemulsification cataract surgery, and deep anterior lamellar keratoplasty (DALK)(Table 1). For DSAEK, intraoperative OCT provided excellent visualization of fluid in the graft/host interface (Figure 2). Graft apposition was monitored intraoperatively with intraoperative OCT and the procedure was continued until optimal fluid removal was achieved. The graft dislocation rate on postoperative day 1 was 3% (5/143). In DALK, intraoperative OCT images provided depth information regarding extent of trephination and residual bed information (Figure 2). intraoperative OCT was also utilized for INTACS placement and cataract surgery. Implant location was easily identified with intraoperative OCT was achieved for both INTACS and for intraocular lens placement (Figure 3). Wound architecture was visualized with intraoperative OCT during cataract surgery (Figure 3).
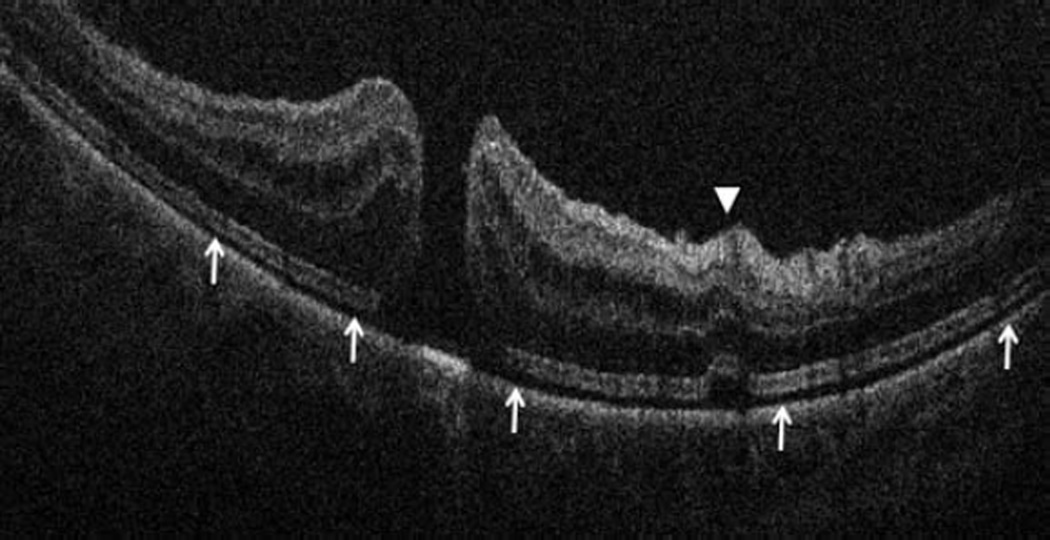
Figure 2.
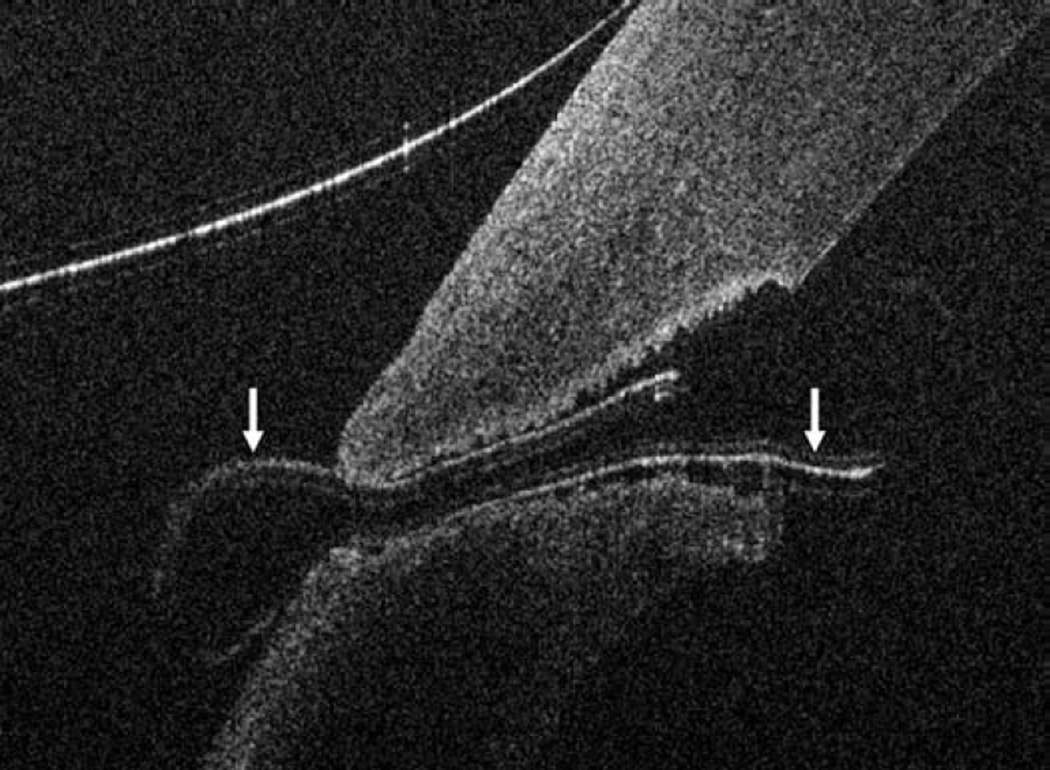
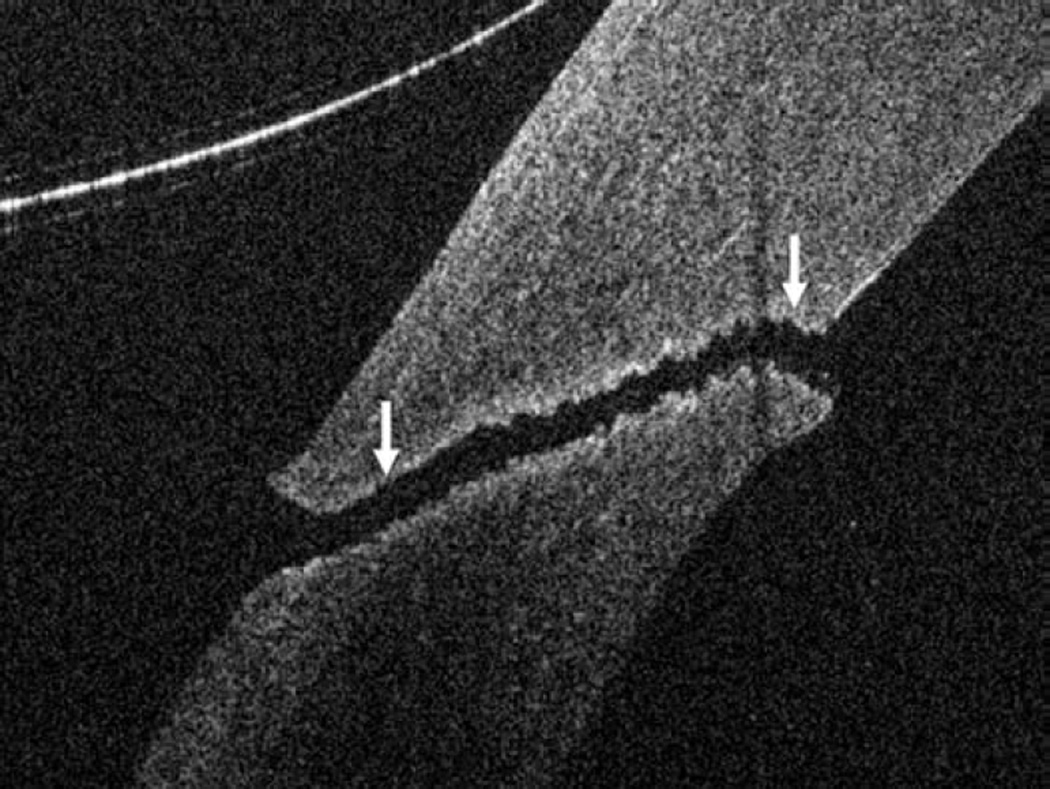
Anterior segment intraoperative optical coherence tomography in lamellar keratoplasty. Intraoperative optical coherence tomography (OCT) B-scan following insertion of graft (dashed arrow) reveals partial apposition with persistent interface fluid (solid arrows) between the graft and the host cornea (arrowhead) during Descemet stripping automated endothelial keratoplasty surgery (Top). Intraoperative OCT B-scan during deep anterior lamellar keratoplasty surgery allows for visualization of edge of full-thickness cornea (dashed arrows) and bare Descemet membrane (solid arrows) following stromal dissection (Bottom).
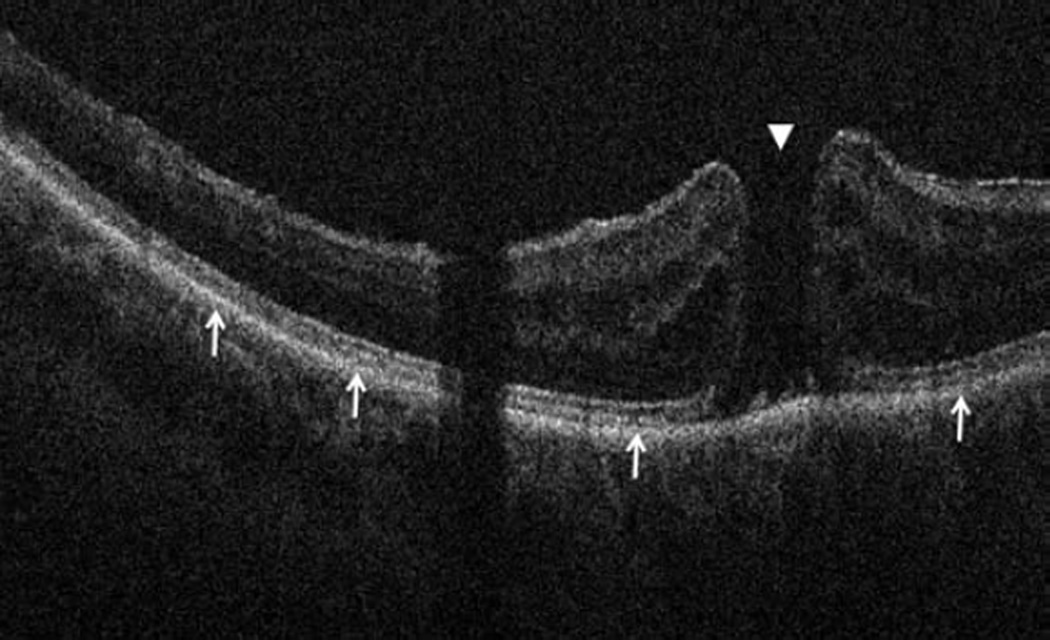
Figure 3.
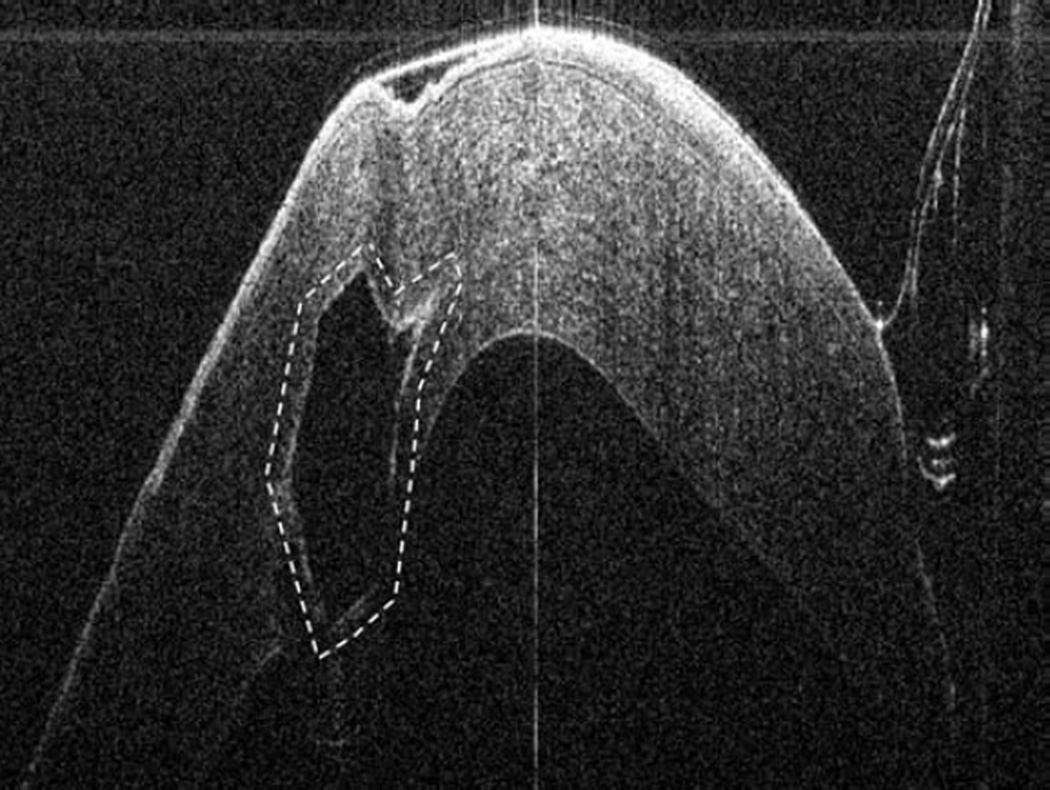
Anterior segment intraoperative optical coherence tomography during corneal and cataract surgery. Intraoperative optical coherence tomography (OCT) B-scan following insertion of INTACS implant (dashed outline) confirms location (Top left). Intraoperative OCT B-scan during cataract extraction and intraocular lens placement verifies optimal location of IOL (solid arrow) behind the anterior capsule (dashed arrow). Irregularity and undulations in the posterior capsule (arrowhead) are visualized (Bottom left). Intraoperative OCT B-scan of clear corneal wound (solid arrows) with associated wound gape is visualized (Top right). Intraoperative OCT B-scan at different location of same corneal incision reveals a capsular remnant (solid arrows) within the wound resulting in wound gape (Bottom right).
Impact of Intraoperative OCT on Surgical Decision-making: Lamellar Keratoplasty
In lamellar keratoplasty procedures, 144 cases included completed surgeon feedback forms. For DSAEK procedures, following graft placement and prior to intraoperative OCT imaging, the surgeon believed the graft was completely apposed in 31/135 (23%) cases, was not apposed in 17/135 (13%), and was unsure of apposition in 87/135 (64%) cases. Additional manipulations were performed (e.g., corneal sweep, varied infusion pressure) based on intraoperative OCT imaging findings of incomplete apposition and persistent interface fluid. In 9/31 (29%) cases that the surgeon believed the graft was entirely apposed, intraoperative OCT revealed persistent fluid that resulted in additional manipulations. In 3/17 (18%) cases that the surgeon thought that the graft was not apposed, the intraoperative OCT revealed complete apposition confirming completion of the surgical objectives. Finally, in cases where the surgeon was unsure of apposition, 42/87 (48%) eyes revealed persistent fluid requiring additional manipulations. Overall following intraoperative OCT, 65/135 (48%) eyes revealed persistent interface fluid requiring additional manipulations. In DALK procedures, the initial trephination was not believed to be deep enough in 3/9 (33%) cases and the surgeon was unsure of depth in 6/9 (67%) cases prior to intraoperative OCT imaging. In 1 of 3 (33%) cases where the surgeon did not believe the trephination was deep enough, intraoperative OCT revealed the depth was optimal and did not require further deepening. In 5/9 (56%) cases intraoperative OCT prompted further manual dissection to deepen the initial trephination.
Posterior Segment Intraoperative OCT Summary
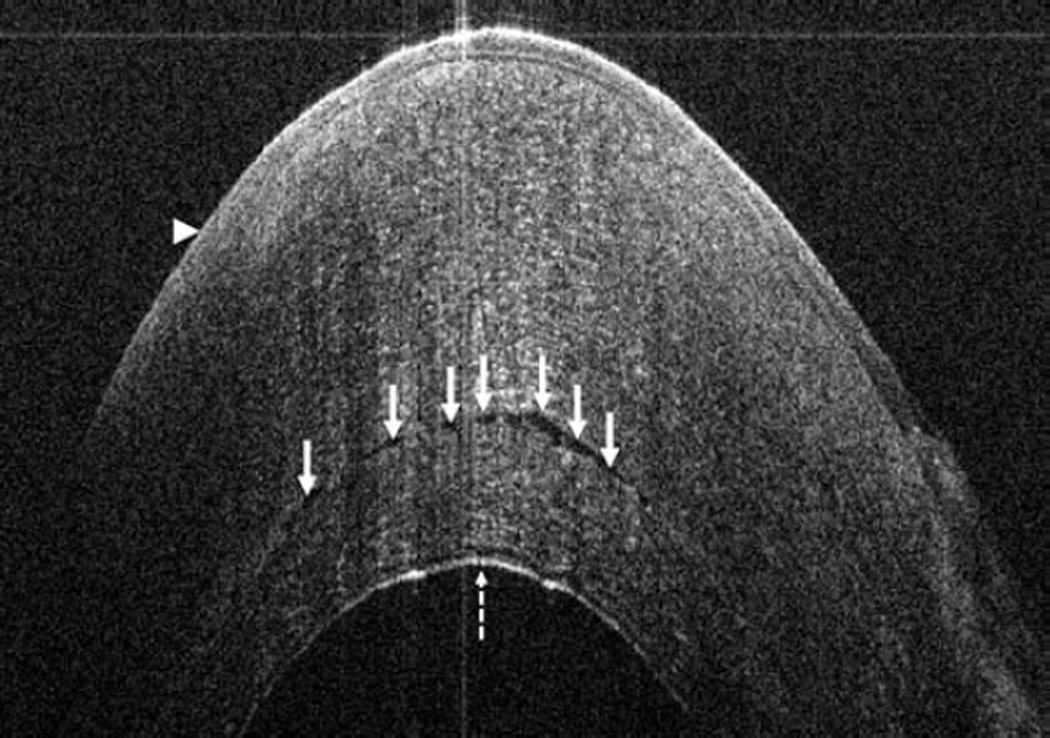
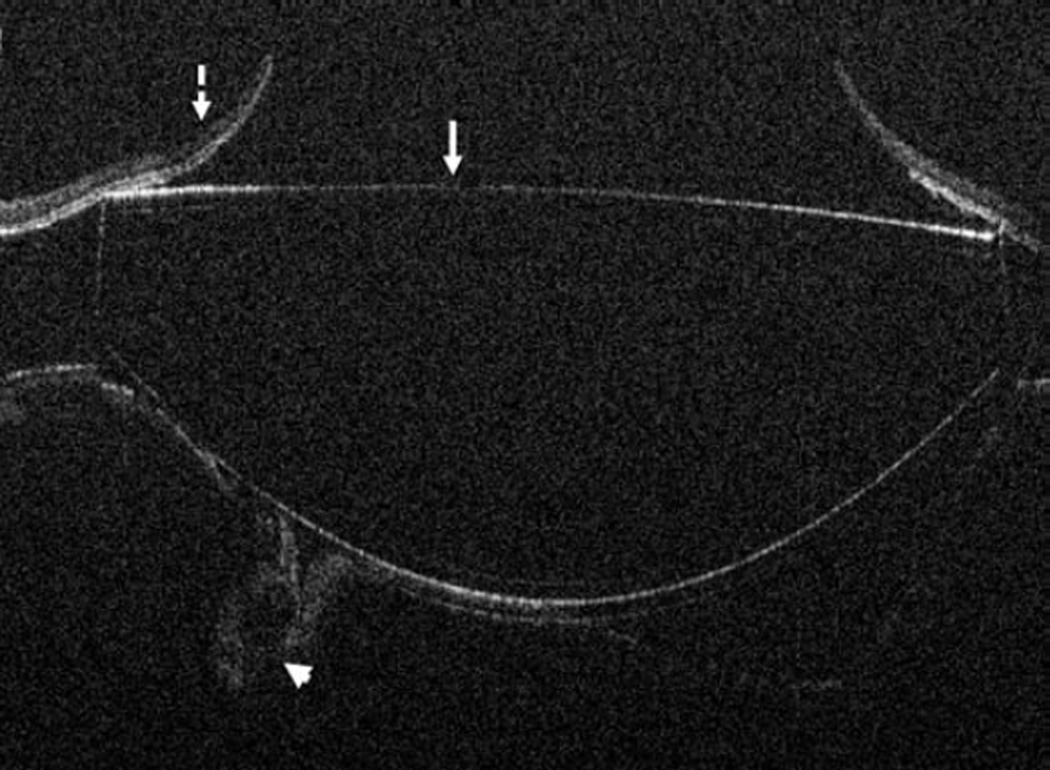
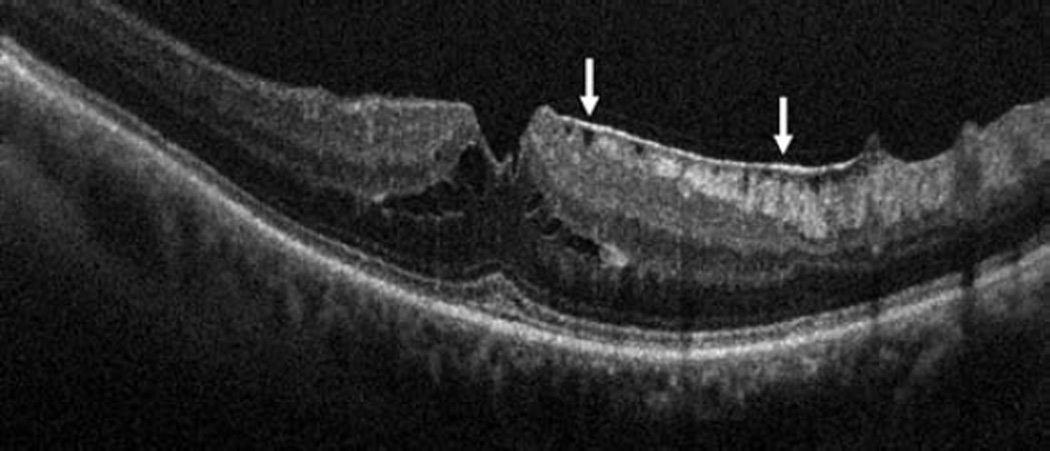
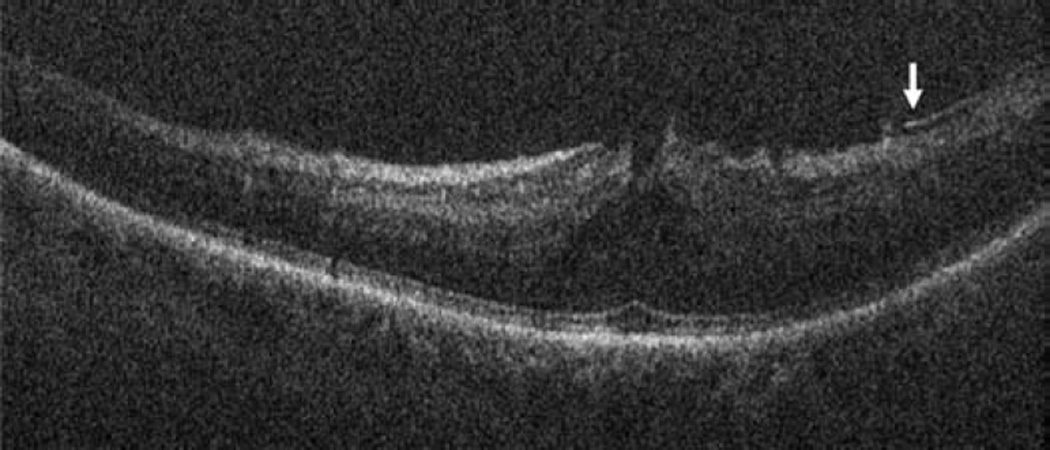
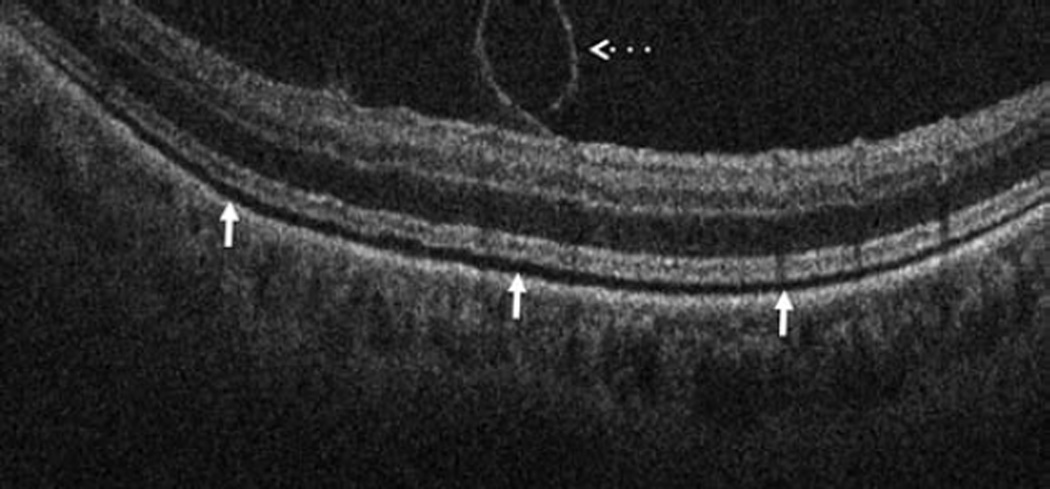
In the posterior segment group, 256 eyes were included. Of these eyes, 87 were pseudophakic, 47 were phakic, 109 underwent concurrent phacoemulsification, and 13 were aphakic. The most common indications for posterior segment surgery were epiretinal membrane (ERM, 35%), full-thickness macular hole (FTMH, 23%), rhegmatogenous retinal detachment (RD, 17%), proliferative diabetic retinopathy (PDR, 13%), and vitreous hemorrhage (VH, 3%)(Table 1). Nearly all eyes (253 of 256) underwent vitrectomy. Fifteen of 256 eyes underwent combined scleral buckle and vitrectomy procedures. For epiretinal membrane cases, intraoperative OCT was able to visualize areas of successful membrane peeling and reveal any residual membranes (Figure 4). Additionally, alterations in the outer retinal architecture were noted with observation of the expansion between the ellipsoid zone and the retinal pigment epithelium (RPE) after peeling. Similarly in FTMH cases, changes in hole architecture were frequently seen and identification of residual internal limiting membrane as well as expansion of the ellipsoid zone to RPE height was noted after membrane peeling (Figure 5). intraoperative OCT imaging in retinal detachment surgery identified variable amounts of residual subretinal fluid following perfluorocarbon liquid placement (Figure 6). Utilizing intraoperative OCT imaging during surgical intervention for vitreomacular traction, confirmation of release of traction was feasible and identification of newly formed FTMH occurred in select cases (Figure 7).
Figure 4.
Epiretinal membrane surgery and intraoperative optical coherence tomography. Preincision intraoperative optical coherence tomography (OCT) B-scan reveals epiretinal membrane (ERM, arrows, top left) and attached posterior hyaloid attached (arrows) at the optic nerve (Bottom left). Intraoperative OCT B-scan following membrane peeling and hyaloid elevation identifies residual ERM (arrow, top right) and confirms hyaloid release from the optic nerve with minimal residual hyaloid elements (arrows, bottom right).
Figure 5.
Macular hole surgery and intraoperative optical coherence tomography. Preincision intraoperative optical coherence tomography (OCT) B-scan provides visualization of baseline ellipsoid zone to retinal pigment epithelium (EZ-RPE) distance (solid arrows) and full-thickness macular hole (arrowhead, top). Following internal limiting membrane (ILM) peeling, intraoperative OCT reveals focal full-thickness retinal elevation (arrowhead) and generalized expansion of the EZ-RPE distance (solid arrows, middle). Extrafoveal B-scan in same eye following ILM peeling identifies residual curled ILM (dashed arrow) and similar expansion of the EZ-RPE distance (solid arrow, bottom).
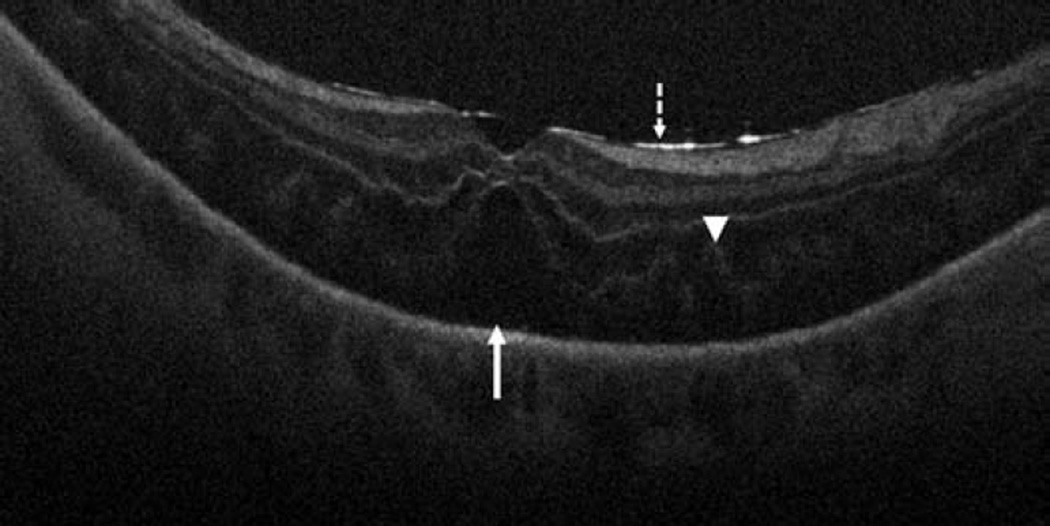
Figure 6.
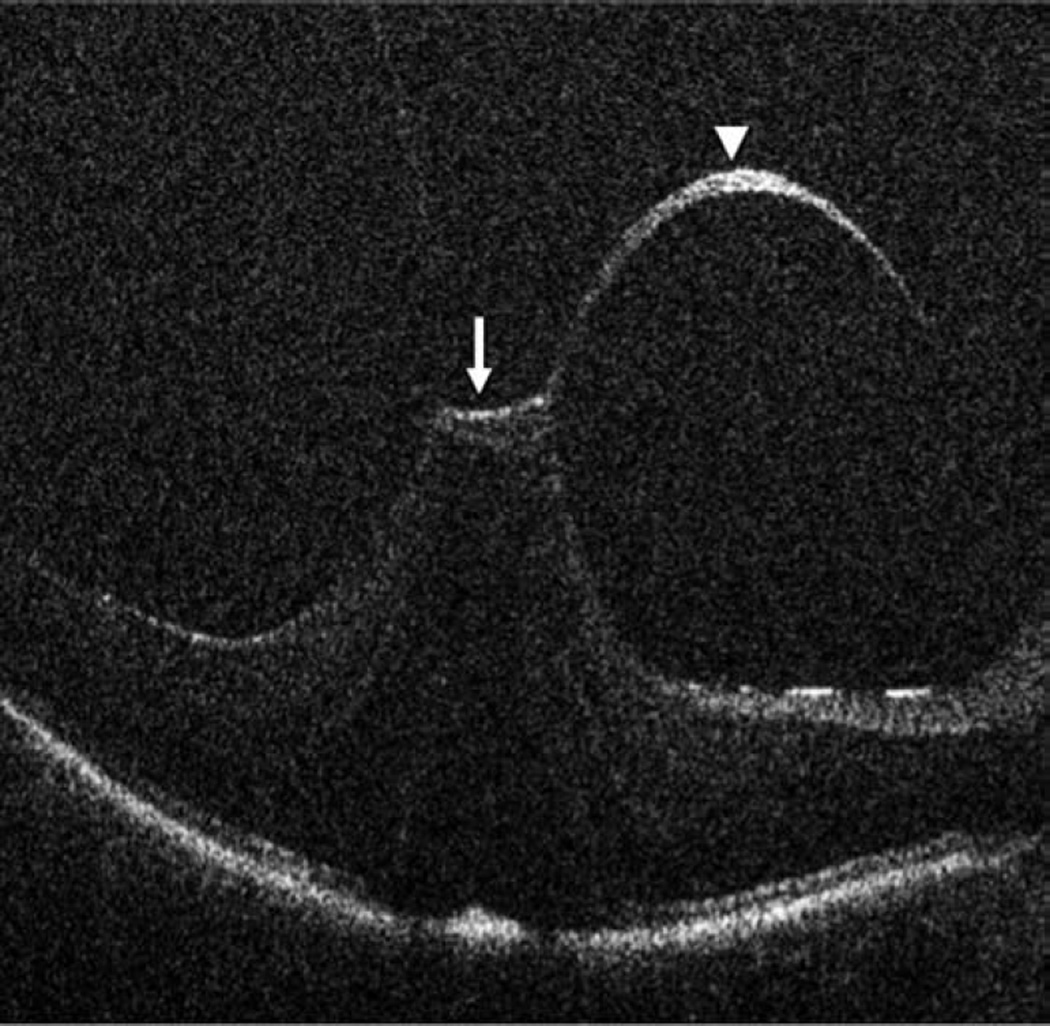
Retinal detachment and intraoperative optical coherence tomography. B-scan image following perfluorocarbon liquid tamponade reveals persistent subretinal fluid (solid arrow), outer retinal corrugations (arrowhead), and the hyperreflective signal of the retina/perfluorocarbon liquid interface (dashed arrow)
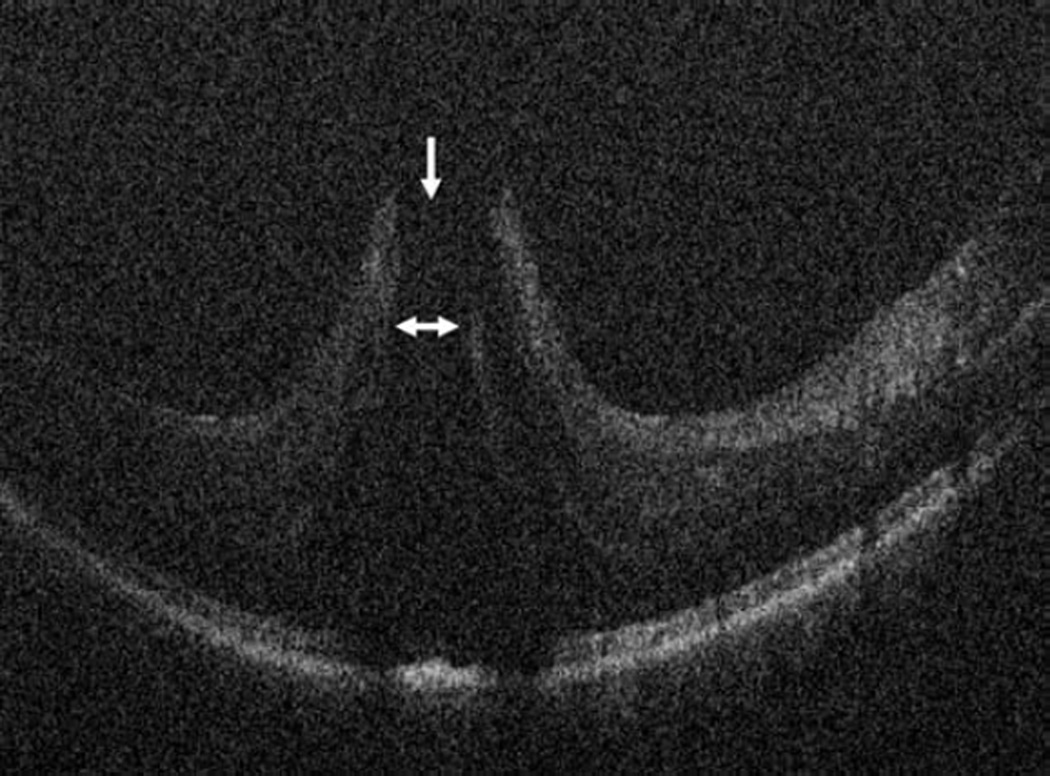
Figure 4.
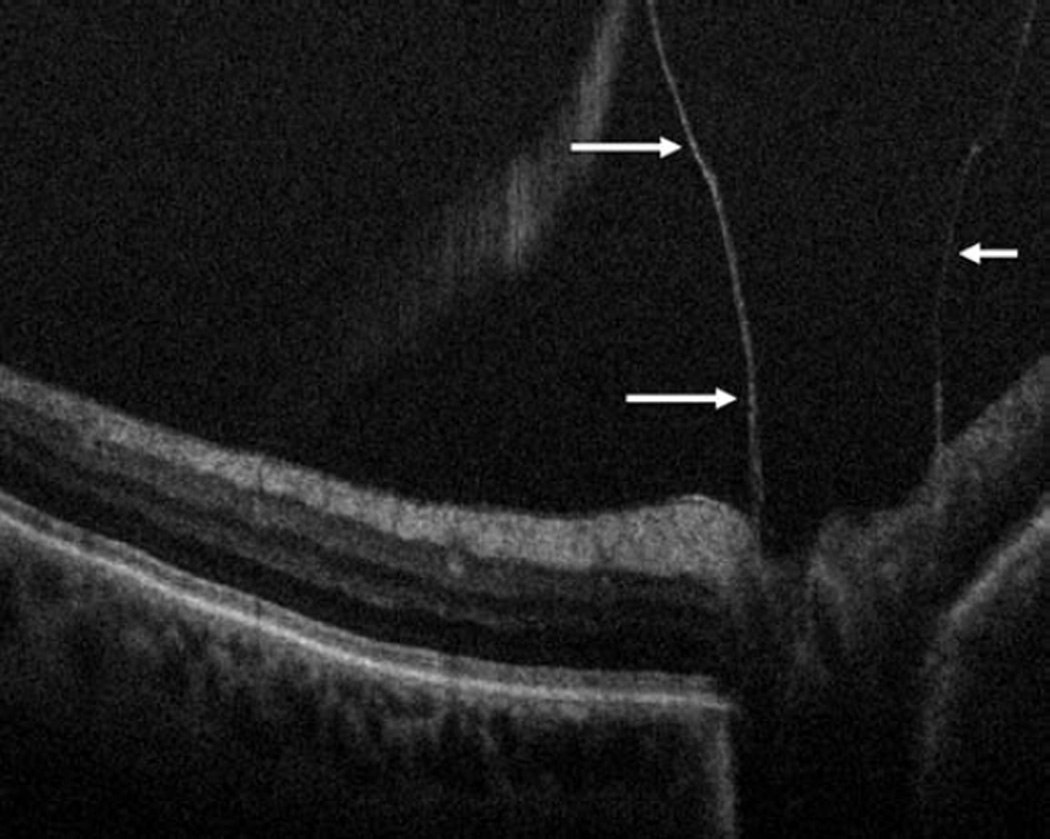
Vitreomacular traction intraoperative optical coherence tomography. Preincision intraoperative optical coherence tomography (OCT) B-scan revealing prominent foveal traction (arrow) and partially separated posterior hyaloid (arrowhead, top). Intraoperative OCT B-scan following hyaloid elevation reveals resolution of the traction with resultant occult full-thickness macular hole (arrows, bottom).
Impact of Intraoperative OCT on Surgical Decision-making: Retinal Membrane Peeling
In 146 vitreoretinal procedures involving membrane peeling, surgeon feedback forms were obtained regarding the impact of intraoperative OCT on decision-making. In 63/146 (43%), intraoperative OCT was reported to have impacted the surgeon’s understanding of the surgical configuration and/or the surgical procedure. The most common reported impact of the intraoperative OCT was related to the completeness of the membrane peel. Following initial membrane peel and prior to intraoperative OCT scanning, the surgeon felt the membrane peel was complete in 91/146 (62%), in 5/146 (3%) cases they thought there was residual membrane that needed peeling, and in 51/146 (35%) cases they were unsure regarding completion of membrane peeling. In 10/81 (13%) cases where the surgeon believed all surgical objectives had been met and the membrane peeling was thought to be complete, intraoperative OCT revealed residual membranes that resulted in additional peeling. Following initial peeling, if the surgeon believed that there were additional membranes that required peeling, 2/5 (40%) cases revealed completion of surgical objectives. In the 51 cases where the surgeon was unsure if membrane peeling was complete, intraoperative OCT gave the definitive answer in 48 cases (94%). Overall, the intraoperative OCT gave an answer that definitively reversed surgical decision-making in 12/96 (13%) cases. The intraoperative OCT information following membrane peeling gave information that impacted the surgeon’s understanding of the completion of surgical objectives and potentially impacted surgical decision-making in 60/146 (41%) cases.
Time Implications of Intraoperative OCT Imaging
Based on surgeon preference, the scan protocol utilized, and the number of scan sessions per surgery, the number of actual scans performed varied from case to case. Overall, the median number of scan sessions per case was 2 (range: 1–5 sessions) with 6 median total scans (range: 1–24 scans) (Table 2). For anterior segment surgery, the median number of scan sessions was 2 (range: 1–4) with a median number of actual scans of 4 (range: 1–14). For posterior segment surgery, the median number of scan sessions was 2 (range: 1–5) with a median number of actual scans of 10 (range: 1–24) (Table 2).
Table 2.
Time Implications for Performing Intraoperative Optical Coherence Tomography During Ophthalmic Surgery with a Microscope Mounted System.
| Overall | Anterior | Posterior | |
|---|---|---|---|
| Scan sessions, median (range) | 2 (1–5) | 2 (1–4) | 2 (1–5) |
| Total scans, median (range) | 6 (1–24) | 4 (1–14) | 10 (1–24) |
| Time to 1st image after pausing surgery, median (range), minutes | 1.7 (0.3–12.4) | 1.6 (0.3–12.4) | 1.7 (0.6–7.1) |
| Total duration surgery paused for intraoperative OCT imaging, median (range), minutes | 4.9 (0.4–26.7) | 3.2 (0.4–17.8) | 6.6 (1.0–26.7) |
| Median duration surgery paused for intraoperative OCT imaging per scan session | 2.8 minutes | 1.9 minutes | 3.4 minutes |
The overall median time to obtain the first image after pausing surgery was 1.7 minutes (range: 0.3 to 12.4). During anterior segment surgery, the median time for initial image acquisition was 1.6 minutes (range: 0.3 to 12.4); and in posterior segment surgery the median time was 1.7 minutes (range 0.6 to 7.1). As noted above, there was significant variation between number of actual scan sessions and also number of scans resulting in varied total duration of halting surgery. The overall median time surgery was paused to perform intraoperative OCT imaging was 4.9 minutes (range: 0.4 – 26.7) with a median time per scanning session of 2.8 minutes. For anterior segment surgery, the median total time surgery was paused to perform imaging was 3.2 minutes (range: 0.4–17.8 minutes) with a median time of per scanning session of 1.9 minutes. For posterior segment surgery, the overall median time surgery was paused to perform imaging was 6.6 minutes (range: 1.0–26.7 minutes) with a median time of per scanning session of 3.4 minutes (Table 2).
Adverse Events
No adverse events were identified specifically related to the intraoperative OCT scanning procedure intraoperatively. Intraoperative complications were noted related to the surgical procedure in 9 cases. Postoperative day 1 adverse events were noted in 132 of 531 cases. The most common postoperative day 1 adverse events in both groups included corneal epithelial defects and abnormal intraocular pressure values. Corneal epithelial defects were noted in 67 eyes (34 anterior, 33 posterior). The vast majority (33 of 34) of the anterior segment cases were in eyes that may be expected to have an epithelial defect based on the procedure being performed (e.g., DALK, DSAEK, keratectomy, penetrating keratoplasty). In posterior segment cases, the majority of epithelial defects were in cases with more complex preoperative diagnoses (20 of 33), including retinal detachment and proliferative diabetic retinopathy that had longer surgical times. Elevated intraocular pressure (defined as >24 mmHg) was seen in 45 eyes (39 posterior, 6 anterior). Hypotony (defined as < 7 mmHg) was noted in 8 eyes, all after posterior segment surgery. One serious adverse event (culture-negative presumed endophthalmitis) was noted during the course of the study, which occurred in a subject that underwent cataract surgery and who had uneventful intraoperative OCT imaging during the procedure.
Discussion
In much the same way that OCT has been a disruptive and transformational technology in ophthalmology clinics, the introduction of OCT into the operating room has the potential to be a paradigm-shifting technology for ophthalmic surgery. Numerous small retrospective case series have documented significant architectural alterations during ophthalmic surgical procedures utilizing intraoperative OCT.2–12
In the clinic environment, OCT has been integrated into the clinical work-flow to allow for rapid testing and patient throughput. In order to successfully integrate OCT into the surgical environment, similar work-flow metrics and ease of use for the surgeon is critical for widespread adoption. In the current climate of cost control and enhanced patient value, introducing OCT into ophthalmic surgery must be able to minimally increase cost (e.g., increased surgical time) while enhancing quality of care (e.g., improved clinical care) and subsequently increasing the value of our surgical approach to patients.
In this multi-surgeon, prospective case series, intraoperative OCT was clearly feasible in the vast majority of cases successfully being imaged with the intraoperative OCT system. A wide-variety of cases was included in this study with a preponderance of lamellar keratoplasty and macular vitreoretinal procedures. One of the most important questions related to intraoperative OCT is related to the impact on patient care and surgeon decision-making. In this study, surgeons reported that intraoperative OCT potentially altered surgeon understanding and decision-making in 40% of cases in lamellar keratoplasty. When considering only those cases where the intraoperative OCT demonstrated a finding disparate from the surgeon’s assessment and definitively changed surgical management, 9% of cases resulted in alterations to surgical care for lamellar keratoplasty. For posterior segment surgery, intraoperative OCT potentially enhanced/altered surgeon’s understanding of the completeness of peel status and potentially impacted management in 40% of cases. If considering only those cases where intraoperative OCT information was discordant with the assumption of the surgeon and directly impacted surgical care for membrane peeling, 8% of cases were altered based on the intraoperative OCT findings. In addition, the confirmation of the surgeon’s impressions may have important implications to surgeon management as well (e.g., knowing when to stop peeling).
Previous studies have not specifically commented on the time burden of performing intraoperative OCT. The actual impact on overall surgical time was generally minimal. The median time to obtain the first image after pausing surgery was 1.7 minutes and on average the surgery was paused for approximately 5 minutes to obtain the scans. This was slightly longer for vitreoretinal procedures compared to anterior segment surgeries. Two potential reasons for the increased time interval was the increased number of scans (e.g., 10 vs 2 mean scans per session) in the posterior segment intraoperative OCT imaging protocols and the slightly more challenging aiming procedure for posterior segment OCT with the SD-OCT probe.
When considering the time required for intraoperative OCT in the PIONEER study, a few factors should be considered. In all of these cases, a research coordinator was present in the operating room to facilitate imaging efficiency. This may result in a shorter duration during each scan session compared to a surgeon using the intraoperative OCT system without assistance. For this study, a standardized image acquisition protocol was developed for the various procedures. For example, during imaging for vitreoretinal procedures, 5 scans per scan session were typically obtained under the research protocol. However, in “real world” use, a single scan might be the only thing required to give the surgeon the feedback needed, which could result in shorter imaging duration. In this study, a microscope mounted intraoperative OCT system was utilized which may impact time for imaging. In the future, systems that are integrated into the optics of the microscope would not necessitate halting surgery for obtaining images, potentially drastically reducing the time required for intraoperative OCT imaging.6, 13–15
The physical approach to surgical integration may take many forms. Currently, handheld or portable systems are the only commercially available system in the United States. In this report, a modified approach to the handheld system was utilized through a microscope-mounted system. This afforded the surgeon the stability of the microscope, enhancing axial stability while allowing for foot-pedal control of X-Y-Z translational movement. However, many shortcomings still exist in the integrative potential of this system. This system does not allow for true “real-time” intraoperative OCT: the visualization of tissue-instrument interactions with OCT.16–18 Surgical maneuvers had to be stopped and the OCT device had to be moved into position to obtain a scan. OCT scans were reviewed on a monitor that was in close proximity to the surgical bed. More recent second-generation microscope integrated prototypes have been described that include heads-up display systems for immediate surgeon intraoperative OCT feedback, including the Carl Zeiss RESCAN 700 and the Cole Eye Institute integrated prototype system.15, 19 Integrating the OCT system into the microscope would eliminate many of these barriers through creating a system that was par focal to the surgeon’s view. Research prototypes have been described that allow for visualization of surgical maneuvers, though significant limitations exist due to the lack of OCT compatibility of current surgical instruments. In Europe, two microscope-integrated systems have CE approval: one released by Haag-Streit Surgical with OPMedT and the previously mentioned Carl Zeiss Meditec RESCAN 700, based on the Lumera 700 microscope platform.
As this was a surgical case series, it was expected for baseline level of adverse events to occur related to the surgical procedure. In this series, corneal epithelial defects and intraocular pressure abnormalities were the most frequently observed adverse events. Although OCT is a noncontact modality, introducing a nonsterile device into the proximity of the surgical field raises the concern of potential infection risk. In PIONEER, a single case of culture-negative presumed endophthalmitis occurred out of 531 surgical procedures. This is an area of interest in intraoperative OCT that requires continued surveillance and research. Although this study was quite large relative to published literature on intraoperative OCT, the sample size still limits the ability to capture rare adverse events.
This study does have limitations that should be acknowledged. It was not masked or randomized. Surgeons knew they would be using the intraoperative OCT during the case and this may have resulted in a bias towards reducing intervention aggressiveness knowing that the intraoperative OCT was available. This bias must be considered when interpreting the feedback results. Additionally, the specific impact of intraoperative OCT on the requirement for membrane staining was not examined in this study. This preliminary report does not include functional outcomes orientations. Analysis is ongoing for presenting disease-specific functional outcomes and their association with intraoperative OCT findings but they are beyond the scope of this report.
The PIONEER study represents the largest prospective study to date to assess the feasibility, safety and utility of intraoperative OCT across ophthalmic surgery. This study suggests that intraoperative OCT can be performed for numerous surgical procedures in an efficient fashion with minimal impact on surgical workflow. Perhaps even more importantly, the prospective surgeon feedback results suggest that there may be significant utility to the use of intraoperative OCT during both anterior and posterior segment surgery. Ultimately, future research is needed with randomized controlled trial to better elucidate the clinical impact on surgical outcomes and intraoperative decision-making. Additionally, integrative solutions research is needed to better understand the optimal platform for utilizing intraoperative OCT. These are active areas of research that are currently being explored.
ACKNOWLEDGEMENTS
Funding and support: National Institutes of Health/National Eye Institute K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, WJD, SKS); Research to Prevent Blindness (PKK)
Biography

Justis P. Ehlers, MD
Biosketch
Justis P. Ehlers, MD, is an Assistant Professor of Ophthalmology and a vitreoretinal surgeon at the Cleveland Clinic Cole Eye Institute. He completed his training at the Wills Eye Hospital in Philadelphia, PA and at the Duke Eye Center in Durham, NC. Dr. Ehlers’ research laboratory focuses on translational optical coherence tomography (OCT) technology with a particular focus on microscope integrated OCT systems, OCT-compatible surgical instruments, and pathology-specific software OCT algorithms and applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
- JPE: Bioptigen (P), Thrombogenics (C,S, R), Synergetics (P), Genentech (R), Leica (C), Carl Zeiss Meditec (C)
- WJD: Carl Zeiss Meditec (R)
- PKK: Carl Zeiss Meditec (C), Topcon (C), Alcon (C), Novartis (C), Bausch and Lomb (C)
- JG: None
- RPS: Carl Zeiss Meditec (C)
- DP: None
- SKS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R); Synergetics (P); Leica (C), Carl Zeiss Meditec (C)
Author Contributions: Design of the study (JPE, WJD, SKS, PKK); Conduct of the study (All authors); Data collection (All authors); Data management (JPE, SKS); Data analysis (All authors); Data Interpretation (JPE, WJD, PKK, JG, RPS, SKS); Preparation of the manuscript (JPE); Review and approval of the manuscript (All authors); Provision of patients (JPE, PKK, WJD, JG, RPS, SKS)
Other Contributors: The authors would like to acknowledge Jamie Reese, RN at Cole Eye Institute, our lead intraoperative OCT research coordinator and the entire intraoperative OCT research team for helping to making this study possible.
REFERENCES
- 1.Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123(12):1715–1720. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 2.Ehlers JP, Kernstine K, Farsiu S, Sarin N, Maldonado R, Toth CA. Analysis of pars plana vitrectomy for optic pit-related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011;129(11):1483–1486. doi: 10.1001/archophthalmol.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33(7):1428–1434. doi: 10.1097/IAE.0b013e31828396b7. [DOI] [PubMed] [Google Scholar]
- 4.Ehlers JP, Tam T, Kaiser PK, Martin DF, Smith GM, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014;34(7):1341–1346. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Xu D, Kaiser PK, Singh RP, Srivastava SK. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014;34(2):213–221. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 6.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 7.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray R, Baranano DE, Fortun JA, et al. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology. 2011;118(11):2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Knecht PB, Kaufmann C, Menke MN, Watson SL, Bosch MM. Use of intraoperative fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am J Ophthalmol. 2010;150(3):360 e2–365 e2. doi: 10.1016/j.ajo.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Lee LB, Srivastava SK. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011;42 doi: 10.3928/15428877-20110804-05. Onlinee71-4. [DOI] [PubMed] [Google Scholar]
- 12.Steven P, Le Blanc C, Velten K, et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 2013;131(9):1135–1142. doi: 10.1001/jamaophthalmol.2013.4672. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3153–3159. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn P, Migacz J, O'Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33(7):1328–1337. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers JP, Tao YK, Srivastava SK. The value of intraoperative optical coherence tomography in vitreoretinal surgery. Curr Opin Ophthalmol. 2014;25(3):221–227. doi: 10.1097/ICU.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013;33(1):232–236. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn P, Migacz J, O'Connell R, Izatt JA, Toth CA. Unprocessed real-time imaging of vitreoretinal surgical maneuvers using a microscope-integrated spectral-domain optical coherence tomography system. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):213–220. doi: 10.1007/s00417-012-2052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao YK, Srivastava SK, Ehlers JP. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers. Biomed Opt Express. 2014;5(6):1877–1885. doi: 10.1364/BOE.5.001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014 doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]