Abstract
A significant portion of the world’s population suffers from sporadic Alzheimer’s disease (AD) with available present therapies limited to symptomatic care that does not alter disease progression. Over the next decade, advancing age of the global population will dramatically increase the incidence of AD and severely impact health care resources, necessitating novel, safe, and efficacious strategies for AD. The mammalian target of rapamycin (mTOR) and its protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) offer exciting and unique avenues of intervention for AD through the oversight of programmed cell death pathways of apoptosis, autophagy, and necroptosis. mTOR modulates multi-faceted signal transduction pathways that involve phosphoinositide 3-kinase (PI 3-K), protein kinase B (Akt), hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex, proline-rich Akt substrate 40 kDa (PRAS40), and p70 ribosomal S6 kinase (p70S6K) and can interface with the neuroprotective pathways of growth factors, sirtuins, wingless, fork-head transcription factors, and glycogen synthase kinase-3β. With the ability of mTOR to broadly impact cellular function, clinical strategies for AD that implement mTOR must achieve parallel objectives of protecting neuronal, vascular, and immune cell survival in conjunction with preserving networks that determine memory and cognitive function.
Keywords: Alzheimer’s disease, amyloid, apoptosis, autophagy, mammalian target of rapamycin (mTOR), mTORC1, mTORC2, necroptosis, oxidative stress, rapamycin
The present landscape for Alzheimer’s disease
Alzheimer’s disease (AD) affects a significant portion of the world’s population. Familial cases of AD account for less than 2% of all presentations of AD (1). In contrast, the sporadic form of AD accounts for the remaining majority of the cases for this neurodegenerative disorder. Patients with familial AD have an autosomal dominant form of a mutated amyloid precursor protein (APP) gene as well as mutations in the presenilin 1 or 2 genes (2). Familial AD, which has been reported in approximately 200 families worldwide, can result from variable single-gene mutations on chromosomes 1, 14, and 21, with mutations on chromosome 1 resulting in altered presenilin 2, mutations on chromosome 14 leading to altered presenilin 1, and mutations on chromosome 21 resulting in altered APP. Age of onset and clinical presentation in familial AD is usually prior to reaching 55 years old (3). However, approximately 10% of the present global population over the age of 65 are affected with sporadic AD. In this group of individuals, the ε 4 allele of the apolipoprotein E (APOE) gene is also associated with an increased risk of late-onset AD. These estimates of those suffering from AD may rise to more than 30 million individuals afflicted with AD over the next 15 to 20 years. Currently more than 5 million individuals are diagnosed with AD, and 3.5 million are under treatment at an annual cost of 3.8 billion US dollars. The annual market size for AD is expected to exceed 11 billion US dollars by the year 2021.
Etiology for sporadic AD is considered to be multifactorial with underlying pathologies suspected to include central nervous system (CNS) toxicity of β-amyloid (Aβ), tau, oxidative stress, cerebrovascular disease, cellular metabolism dysfunction, xenobiotic injury, glutamate release, mitochondrial damage, and acetylcholine loss (4). Available present therapies for AD that consist of acetylcholinesterase inhibitors and behavior modification with antidepressants and antipsychotics are symptomatic and do not alter disease progression. Given the progressive increase in life-span and advancing age of the world’s population, it is highly anticipated that the presentation of AD will dramatically increase worldwide and further strain health care resources unless novel, safe, and effective therapies for this disorder can be developed. Some emerging therapies that are under development employ monoclonal antibodies against Aβ, prevention of Aβ aggregation, activation of nicotinic receptors, enhancement of cytokine and growth factor signal transduction, modulation of serotonin receptors, blockade of tau aggregation, and the application of metal chelators (5,6). Yet, what may be one of the most exciting and unique avenues being pursued for the treatment of AD involves the mammalian target of rapamycin (mTOR) and its multi-faceted cellular pathways (7).
mTOR signaling
The target of rapamycin (TOR) and the genes TOR1 and TOR2 that encode two isoforms in yeast, Tor1 and Tor2, were first identified in Saccharomyces cerevisiae. Rapamycin is a macrolide antibiotic from Streptomyces hygroscopicus that inhibits TOR activity. Through the use of rapamycin-resistant TOR mutants in yeast, the yeast genes TOR1 and TOR2 were isolated (8). In mammals, mTOR is encoded by a single gene FRAP1, is a 289-kDa serine/threonine protein kinase, and controls multiple functions throughout the body that involve gene transcription and protein formation, cytoskeleton composition, metabolism, development, survival, and senescence (7). mTOR is sometimes referred to by other terms such as the mechanistic target of rapamycin and FK506-binding protein 12-rapamycin complex-associated protein 1. The N-terminal portion of mTOR contains at least a 20 HEAT (Huntingtin, Elongation factor 3, A subunit of Protein phosphatase-2A, and TOR1) repeat. This region promotes binding with two regulatory proteins, Raptor (regulatory-associated protein of mTOR) and Rictor (rapamycin-insensitive companion of mTOR).
mTOR is an essential component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2), and it is the association of mTOR with either Raptor (component of mTORC1) or Rictor (component of mTORC2) that determines with which protein complex mTOR associates (Figure 1). mTORC1 is more sensitive to the inhibitory effects of rapamycin than mTORC2 (9). Rapamycin blocks mTORC1 activity by binding to immunophilin FK506-binding protein 12 (FKBP12) that attaches to FKBP12-rapamycin-binding domain (FRB) at the C-terminal of mTOR to prevent the phosphorylation of mTOR. Long-term administration of rapamycin also can inhibit mTORC2 which may result from the disruption of the assembly of mTORC2 (9). As noted above, mTORC1 contains Raptor. Phosphorylation of Raptor through several pathways that can include the protein Ras homologue enriched in brain (Rheb) activates mTORC1 and allows it to bind to its complex constituents. Rheb phosphorylates Raptor residue serine863 and other residues that include serine859, serine855, serine877, serine696, and threonine706. mTORC1 activity is reduced if serine863 remains unphosphorylated (10). Once it is active, mTOR can also modulate Raptor activity that can be blocked by rapamycin (10).
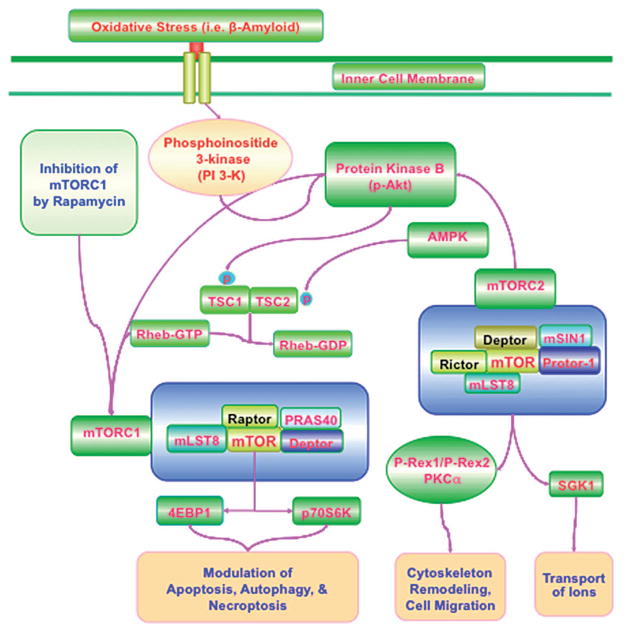
Figure 1.
mTOR signaling in Alzheimer’s Disease. Activation of phosphoinositide 3-kinase (PI 3-K) following oxidative stress with mediators such as Aβ leads to phosphorylation and activation of protein kinase B (Akt). mTORC1 is composed of Raptor, the proline-rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mLST8/GβL (mammalian lethal with Sec13 protein 8, termed mLST8). mTORC1 is more sensitive to the inhibitory effects of rapamycin than mTORC2. mTORC2 is composed of mLST8, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1). Akt can activate mTORC1 through phosphorylating TSC2 and disrupting the interaction between TSC2 and TSC1. TSC2 can function as a GTPase-activating protein (GAP) converting a small G protein Ras homologue enriched in brain (Rheb-GTP) to the inactive GDP-bound form (Rheb-GDP). AMPK phosphorylates TSC2 to lead to increased GAP activity to turn Rheb-GTP into Rheb-GDP and thus inhibits the activity of mTORC1. mTORC2 activates Akt to enhance cell survival and relies upon PKCα for cytoskeleton remodeling. mTORC2 also phosphorylates and activates SGK1 that can control ion transport. mTORC2 through Akt phosphorylates P-Rex1 and P-Rex2 to foster Rac activation and cell migration. mLST8 can promote mTOR kinase activity through p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1). Ultimately, apoptosis, autophagy, and necroptosis can be controlled by mTOR signaling such that during inhibition of mTOR with rapamycin, autophagy and necroptosis can be initiated. mTORC1 activation usually blocks apoptosis.
In addition to Raptor, mTORC1 is composed of the proline-rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mLST8/GβL (mammalian lethal with Sec13 protein 8, termed mLST8). PRAS40 can competitively block the binding of mTORC1 to Raptor (11). Inhibitory phosphorylation of PRAS40 by protein kinase B (Akt) promotes the activity of mTORC1. Phosphorylation of PRAS40 dissociates it from Raptor, promotes the binding of PRAS40 to the cytoplasmic docking protein 14-3-3, and fosters the activity of mTORC1. Deptor also can inhibit mTORC1 by binding to the FAT (FKBP-associated protein, Ataxia-telangiectasia, and Transactivation/transformation domain-associated protein) domain of mTOR. Without Deptor, the activity of Akt, mTORC1, and mTORC2 are enhanced. In contrast to PRAS40 and Deptor, mLST8, which is a component of mTORC1 and mTORC2, promotes mTOR kinase activity and is known to control insulin signaling through the transcription factor FoxO3; it is necessary for the phosphorylation of Akt and protein kinase C-α (PKCα) and is required for the association between Rictor and mTOR (12).
Interestingly, mLST8 promotes mTOR kinase activity through p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) (13), two critical signaling targets of mTORC1. mTORC1 relies upon p70S6K to promote cell growth, mRNA biogenesis, and the translation of ribosomal proteins (14). In the nervous system, amino-acids control cortical function through the mTOR and p70S6K pathways. Both glutamate and leucine rely upon p70S6K to modulate synaptic signaling (15) and food intake (16). However, when 4EBP1 is hypophosphorylated, it prevents protein translation by binding to eukaryotic translation initiation factor 4 epsilon (eIF4E) through eIF4 gamma (eIF4G), a protein that translocates mRNA to the ribosome. mTORC1 phosphorylation of 4EBP1 blocks this activity and leads to the dissociation of 4EBP1 from eIF4E, allowing eIF4G to begin mRNA translation. In addition, binding of 4EBP1 and p70S6K to Raptor can be prevented during activation of PRAS40.
mTOR signaling is intimately connected to the pathways of phosphoinositide 3-kinase (PI 3-K), Akt, and AMP activated protein kinase (AMPK) (Figure 1) (5). For example, mTORC1 activity is controlled by Akt modulating hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex, an inhibitor of mTORC1 (7,17). Although several regulatory phosphorylation sites are known to exist for TSC1, the TSC1/TSC2 complex is believed to be controlled primarily though the phosphorylation of TSC2 by Akt, extracellular signal-regulated kinases (ERKs), activating protein p90 ribosomal S6 kinase 1 (RSK1), AMPK, and glycogen synthase kinase-3β (GSK-3β). TSC2 functions as a GTPase-activating protein (GAP) converting a small G protein Ras homologue enriched in brain (Rheb-GTP) to the inactive GDP-bound form (Rheb-GDP). If Rheb-GTP is active, Rheb-GTP can then associate with Raptor to regulate the binding of 4EBP1 to mTORC1 and increase mTORC1 activity. Akt phosphorylates TSC2 on multiple sites that leads to the destabilization of TSC2 and disruption of its interaction with TSC1. Phosphorylation of TSC2 (serine939, serine981, and threonine1462) results in the binding of TSC2 to protein 14-3-3, disruption of the TSC1/TSC2 complex, and then activation of Rheb and mTORC1. Interestingly, it appears that a limited reduction in TSC2 activity is necessary for cellular protection against Aβ to allow for mTOR activation, since complete knock-down of TSC2 can limit cellular protection (18).
As noted, AMPK also is a vital pathway to control TSC2. AMPK phosphorylates TSC2 to lead to increased GAP activity to turn Rheb-GTP into Rheb-GDP and thus inhibits the activity of mTORC1 (Figure 1). AMPK also controls TSC1/2 activity through RTP801 (REDD1/product of the Ddit4 gene). During hypoxia, AMPK activity can increase REDD1 expression to suppress mTORC1 activity by releasing TSC2 from its inhibitory binding to protein 14-3-3 (19). AMPK activation has been shown to suppress Aβ production (20), regulate tau phosphorylation (21), reduce myocardial infarct size in experimental models of diabetes (22), prevent oxidative stress that may lead to hypertension (23), be required for proper metabolic function of cells (24), and may suppress adipocyte differentiation, lipid accumulation, and obesity (25). However, it should be noted that excessive AMPK activation may lead to aberrant Aβ production (18,20), cardiac dysfunction (26), and cardiac hypertrophy (27).
In regards to the mTOR complex associated with Rictor, mTORC2 has similar components of mTORC1 that include mLST8 and Deptor, but also has additional components that are the mammalian stress-activated protein kinase interacting protein (mSIN1) and the protein observed with Rictor-1 (Protor-1) (7,17). Rictor and mSIN1 form the structural basis of mTORC2. mTORC2 utilizes Rictor to activate and phosphorylate Akt at Ser473, facilitating threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) (7,17). mSIN1 is required for mTORC2 to activate Akt. The kinase domain of mTOR also has been shown to phosphorylate mSIN1, preventing the lysosomal degradation of mSIN1. Protor-1 is a Rictor-binding subunit of mTORC2 and may function to activate serum and glucocorticoid-induced protein kinase 1 (SGK1). Absence of Protor-1 in animal models reduces the hydrophobic motif phosphorylation of SGK1 and its substrate N-Myc downregulated gene 1 in the kidney (NRDG1) (28).
mTORC2 has multiple targets to control cellular survival, ion transport, cell migration, and cytoskeleton remodeling (Figure 1). mTORC2 activates Akt to enhance cell survival and uses PKCα for cytoskeleton remodeling. mTORC2 also phosphorylates and activates SGK1, is a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases, and is activated by growth factors to control ion transport. mTORC2 controls cell migration through the activation of the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling. Expression of the active forms of the Rho GTPases with mTORC2 fosters cell-to-cell contact and decreases remodeling of the actin cytoskeleton. mTORC2 through Akt phosphorylates P-Rex1 and P-Rex2 to foster Rac activation and cell migration. For the TSC1/TSC2 complex, unlike mTORC1, TSC1/TSC2 promotes the activity of mTORC2 through the N-terminal region of TSC2 and the C-terminal region of Rictor (29). Studies have shown that absence of the TSC1/TSC2 complex leads to the loss of mTORC2 kinase activity in vitro (29).
Oxidative stress and pathways of programmed cell death
During oxidative stress, reactive oxygen species (ROS) are formed through superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite (4). Oxidative stress can subsequently damage cells through the loss of DNA integrity, mitochondrial injury, the misfolding of proteins, and lipid peroxidation. Several cellular antioxidant systems such as catalase, superoxide dismutase, glutathione peroxidase, and vitamins C, D, E, and K may limit the production of ROS and reduce ROS to less than toxic levels (5). Oxidative stress impacts multiple neurodegenerative disorders, especially those involving cognitive loss such as AD. Abnormalities in impaired insulin signaling and abnormal cellular metabolism as a result of oxidative stress may both initiate and promote the progression of AD (Figure 1). Impairment in sirtuin signaling during oxidative stress that can affect neuronal as well as vascular function also may contribute to AD (30). The presence of oxidative stress may incite aberrant cell cycle re-entry that can lead to neuronal death during AD (31,32). Reduction in ROS levels in models of AD have led to reduced toxicity of Aβ (33), further suggesting that oxidative stress is a critical component in the pathology of AD. In other models of AD, agents that reduce levels of oxidative stress in conjunction with reduction in Aβ expression resulted in significantly improved cognitive function (34).
Intimately tied to oxidative stress cell injury are the pathways of programmed cell death that involve apoptosis, autophagy, and necroptosis (Figure 1) (35). Apoptosis consists of both an early phase that involves the loss of plasma membrane lipid asymmetry and a later phase that leads to genomic DNA degradation. The loss of asymmetry of membrane phosphatidylserine (PS) distribution is an early component of apoptosis that can be reversible, but, once initiated, genomic DNA degradation usually is not reversible (5). Externalization of PS residues and the onset of genomic DNA degradation are considered to be the outcomes of a series of activation of nucleases and proteases that occur during apoptosis. The early phase of apoptosis with membrane PS externalization alerts inflammatory cells to engulf and remove injured cells. As a result, prevention of membrane PS externalization is necessary to prevent the loss of functional cells that are temporarily injured and expressing membrane PS residues.
Apoptotic DNA fragmentation (36) and caspase activation (37) have been demonstrated in the brains of patients with AD. ‘Pro-apoptotic’ signaling pathways such as Bim also have been identified in vulnerable regions of AD patients that involve the entorhinal cortex (38). In animal models of AD, therapies directed against apoptosis improve cellular survival (18,39) and enhance cognitive function (40).
Several studies support the premise that activation of mTOR may be necessary to prevent apoptotic cell death (Table I). Loss of mTOR activity during oxidative stress can lead to neuronal cell loss (41), and Aβ may inhibit mTOR activity (42). During sepsis, neurons are protected against sepsis with application of the cytokine erythropoietin (EPO) and activation of mTOR (43). Activation of mTOR also is necessary for EPO to protect cerebral microglia that can support neuronal function during oxidative stress and Aβ toxicity (44,45). Prevention of cortical injury during ischemic preconditioning (46) or during permanent cerebral ischemia requires mTOR activation (47).
Table I.
mTOR signaling in Alzheimer’s disease (AD).
| Target pathways | Biological outcome |
|---|---|
| Apoptosis | mTOR functions with multiple pathways that include PI 3-K, Akt, forkhead transcription factors, PRAS40, and p70S6K to prevent apoptotic cellular death in AD. |
| Autophagy | mTOR phosphorylates the mammalian homologue Atg13 and the mammalian Atg1 homologues ULK1 and ULK2 to block autophagy. In some models, mTOR inhibition that results in the induction of autophagy leads to improved cognitive function in animal models of AD. Yet, other models suggest that inflammatory stressors accompanied by autophagy activation may precipitate the pathology of AD. |
| Necroptosis | In animal models that exhibit components of AD, inhibitors of necroptosis prevent neuronal cell death and improve memory function, suggesting that necroptosis also contributes to AD pathology. |
| Memory formation | mTOR fosters dendritic protein synthesis in hippocampal neurons and is necessary for memory formation and consolidation. Reduction in mTOR signaling occurs in patients with AD. Loss of mTOR signaling in animal models of AD leads to impaired long-term potentiation and synaptic plasticity. |
| Neuronal injury | Loss of mTOR in conjunction with other pathways, such as RB1CC1, in AD patients leads to neuronal apoptosis and neuronal atrophy. Cellular protection against the toxicity of Aβ also may require mTOR modulation in conjunction with TSC2, PRAS40, BAD, and Bcl-xL. |
| Amyloid (Aβ) | Increased mTORC1 activity may be necessary to decrease BACE1 and reduce Aβ generation in AD. However, other studies suggest that inhibition of mTOR can enhance Aβ clearance in cell lines and animal models of AD. Repression of Aβ production also may require inhibition of Akt and mTOR with the concomitant activation of AMPK. |
mTOR functions in conjunction with several pathways that involve phosphoinositide 3-kinase (PI 3-K), Akt, forkhead transcription factors, proline-rich Akt substrate 40 kDa (PRAS40), and p70S6K to prevent apoptotic cellular death. In the nervous and vascular systems, activation through the PI 3-K and Akt pathways prevent neuronal injury, protect vascular cells, and block injury to immune cells (48–50). During Aβ toxicity to cells, activation of Akt limits cell injury and prevents the detrimental effects of Aβ (51,52). Pathways of PI 3-K and Akt also are critical for agents such as growth factors to foster neuronal survival and block neurodegeneration, especially during Aβ exposure. EPO activates Akt through its phosphorylation of serine473 (53,54). EPO relies upon Akt activation during oxidative stress to maintain the integrity of cerebral vascular cells through silent mating type information regulator 2 homolog 1 (SIRT1) cell longevity pathways (55). EPO can prevent cell injury through Akt during Aβ exposure and also utilizes pathways that activate mTOR to enhance cell survival (45,56). Proteins associated with mTOR, such as ubiquilin-1, also may be involved with AD. In addition, proteins such as ubiquilin-1 can interact with mTOR without affecting its kinase activity (57). Expression of ubiquilin-1 has been shown to be upregulated during neurofibrillary tangle changes in the hippocampus of patients with AD (58).
These protective pathways of EPO, Akt, and mTOR also rely upon the modulation of forkhead transcription factors. Mammalian forkhead transcription factors of the O class (FoxO1, FoxO3, FoxO4, and FoxO6) oversee multiple cellular functions that include growth, cell cycle regulation, metabolism, and survival (59). Akt prevents apoptotic cell death by phosphorylating FoxO proteins, promoting the sequestration of FoxO proteins by 14-3-3 in the cytoplasm, and ultimately blocking their transcription. In relation to neuronal and immune cell survival, inhibition of forkhead transcription factor activity protects against growth factor deprivation, oxidative stress, and Aβ toxicity (60,61). EPO through Akt can phosphorylate and inhibit FoxO proteins, activate mTOR, and lead to increased cellular survival by blocking apoptotic cell death (62,63). Additional studies further support the ability of mTOR to control forkhead protein activity especially in cellular metabolism, demonstrating that mTOR activation can subsequently lead to forkhead transcription factor repression (64).
mTOR signaling also is tightly linked to PRAS40 and p70S6K through Akt to block apoptotic cell death. Following phosphorylation of PRAS40 by Akt, PRAS40 can dissociate from mTORC1 to promote mTOR activation and prevent apoptosis. PRAS40 appears to be a central component in modulating cell survival since knock-down of PRAS40 can independently protect cells against Aβ toxicity (65) and inhibit caspase 3 activation (66). Under some circumstances, studies suggest that over-expression of PRAS40 that simultaneously activates Akt and mTOR but blocks forkhead transcription factor activity can lead to neuronal protection during ischemic induced oxidative stress (67). Yet without these parameters and over-expression, PRAS40 activity appears to be detrimental with loss of mTOR activity and also leads to metabolic cellular dysfunction (68,69).
Through Akt and mTOR activation, p70S6K can promote cellular protection during adverse cellular conditions. Trophic factor protection against Aβ toxicity in cortical neurons utilizes Akt, mTOR and p70S6K activation (42). In addition, neuronal cell injury (70) or microglial cell loss (18,45,65) during Aβ exposure is believed to be a result of the secondary to the loss of p70S6K signaling. In other models of oxidative stress, p70S6K is necessary to provide growth factor neuroprotection (44,45,66) and progenitor cell induction of neovascularization in ischemic tissue (71), attenuate cortical injury from focal ischemia (72), and reduce insulin resistance (73).
Autophagy is another and independent pathway of programmed cell death that is in contrast to apoptosis (Table I). Autophagy allows cells to recycle cytoplasmic components and discard defective organelles for tissue remodeling. Three categories of autophagy exist termed microautophagy, chaperone-mediated autophagy, and macroautophagy (35,74). Microautophagy involves the sequestration of cytoplasmic components by invagination of the lysosomal membrane for digestion. Cytosolic chaperones transport cytoplasmic components across lysosomal membranes during chaperone-mediated autophagy. Macroautophagy, which is the most prominent of the three categories, consists of the sequestration of cytoplasmic proteins and organelles into autophagosomes that ultimately fuse with lysosomes for degradation and are then recycled for future cellular processes. Several autophagic genes modulate autophagic processes. Thirty-three autophagic related genes (Atg) in yeast have been identified. Of this family of genes, Atg1 and Atg13 (also termed Apg13) are associated with the PI 3-K, Akt, and mTOR cascade. Atg13 is phosphorylated through a Tor-dependent mechanism resulting in its release from Atg1 and a reduction in Atg1 activity. During starvation and Tor inhibition, Atg13 is dephosphorylated and activates Atg1 to result in autophagosome formation. In mammals, a similar oversight of autophagy through mTOR also exists. Two mammalian homologues of Atg1, UNC-51-like kinase 1 (ULK1) and ULK2, have been identified. Mammalian Atg13 binds to ULK1, ULK2, and FIP200 (focal adhesion kinase family interacting protein of 200 kDa) to activate ULKs and facilitate the phosphorylation of FIP200 by ULKs. mTOR phosphorylates the mammalian homologue Atg13 and the mammalian Atg1 homologues ULK1 and ULK2 to block autophagy. In most cases, mTOR activation can block autophagy through inhibition of the ULK– Atg13–FIP200 complex by phosphorylating Atg13 and ULKs. In the absence of mTOR activity, dephosphorylation of ULKs and Atg13 ensues, leading to the induction of autophagy.
For neurodegenerative disorders that are either acute or chronic in presentation, autophagy through mTOR inhibition usually can be protective and promote cellular survival. In neonatal models of ischemia (75) or during excitotoxicity (76), inhibition of mTOR activity with induction of autophagy can be neuroprotective. Trophic factor neuronal protection during cerebral ischemia also may be mediated through the inhibition of mTOR and the induction of autophagy (77). In clinical cases of Lewy body disease and Parkinson’s disease dementia, pathological specimens demonstrated markers of reduced autophagic activity and increased mTOR activity (78). In models of Huntington’s disease (79) or prion protein disease (80), induction of autophagy with mTOR blockade leads to cytoprotection. Inhibition of mTOR signaling with the induction of autophagy also leads to neural tissue protection and functional improvement in models of spinal cord injury (81).
However, other studies demonstrate that mTOR activation with a reduction in autophagy may be necessary to achieve cellular protection and survival in the nervous system. For example, in dopamine neurons exposed to oxidative stress, neuronal cell death was potentiated during the induction of autophagy and the inhibition of mTOR and p70S6K activity, while, in contrast, neurons were protected during oxidative stress with the blockade of autophagy and enhancement of mTOR activity (82). In Purkinje neurons, trophic factor withdrawal leads to neuronal cell death through the accumulation of autophagic vesicle accumulation. Application of trophic factors such as insulin growth factor-1 prevents neuronal injury and blocks the induction of autophagy (83). Models of ischemic stroke also demonstrate that reduction in autophagy leads to the reduction in infarct size and the protection of cerebral neurons (84). During traumatic spinal cord injury in rats, activation of mTOR with reduction in autophagy also leads to improved cell survival of motor neurons and improved functional capabilities (85). In tri-cultures of neurons, astrocytes, and microglia that were exposed to inflammatory stressors and Aβ to provide a model of AD, cell survival was reduced with increased injury during the induction of autophagy (86), suggesting in this model that inflammatory stressors accompanied by autophagy may precipitate the pathology of AD.
It should be noted that the pathways of apoptosis and autophagy do not occur in isolation and may influence each other. For example, some agents designed to inhibit cancer cell growth have been shown to lead to autophagy initially, but later initiate apoptotic pathways that promote cell death, illustrating that autophagy in some cells may modulate an adaptive response over apoptosis (87). In other tumor cells such as in leukemia, targeting mTORC1 and mTORC2 complexes together to overcome leukemic cell resistance may require the induction of apoptosis with the repression of autophagy (88). Under other circumstances such as during oxidative stress in neurons, WISP1, a member of the CCN family of proteins and component of the wingless pathway Wnt1, can prevent cell death primarily through inhibition of apoptotic pathways in neurons with a minimal contribution in a subset of neurons that also requires the inhibition of autophagy (89).
In addition to apoptosis and autophagy, necroptosis is another pathway of programmed cell death relevant to neurodegenerative disorders (Table I). In animal models of aluminum exposure that exhibit components of AD, inhibitors of necroptosis prevented neuronal cell death and improved memory function (90), suggesting that necroptosis in addition to apoptosis and autophagy contributes to neurodegenerative disorders and should be considered a potential target for AD. As a regulated necrotic cell death pathway, necroptosis is controlled by receptor-interacting protein (RIP-1 and RIP-3) kinases and cylindromatosis (turban tumor syndrome) (CYLD). However, these pathways may have a complex relationship since recent work also suggests that RIP-1 kinase activity may be necessary to block RIP-3-mediated necroptosis during the early postnatal period (91). Pathways such as Akt and mTOR are involved with necroptotic cell death. Inhibition of mTOR in acute lymphoblastic leukemia leads to autophagy-dependent cell loss with features that are consistent with necroptosis (92). In human carcinoma cell lines, agents that can repress cell cycle progression have been demonstrated to utilize mechanisms of necroptosis (93). Glioblastoma cell proliferation also has been linked to a number of mechanisms that include inhibition of Akt, mTORC1 and mTORC2, cell cycle block at G2-M, and the initiation of necroptosis and autophagy (94).
mTOR in memory and Alzheimer’s disease
Several studies suggest that mTOR signaling pathways play a vital role in memory formation and protection of neuronal pathways mediating cognitive function that are relevant to AD (17). Survival of newborn neurons and development of dendritic density in mice have been shown to be impaired by Aβ through the loss of Akt, mTOR, and p70S6K activity (95). Dendritic protein synthesis in hippocampal neurons also is fostered through mTOR activity (96). In animal models of remote and global cerebral ischemic reperfusion injury, activation rather than inhibition of mTOR activity was found to be protective of networks controlling memory in the hippocampus (46). In addition, Aβ exposure in neuronal cultures has been shown to lead to disruption in PI 3-K, Akt, and mTOR signaling that may precipitate cognitive loss during AD (42). mTOR can regulate protein synthesis in neurons and has been shown to be necessary for the formation of long-term memory in the amygdala (97). mTOR also is necessary for long-term memory formation and consolidation. Loss of mTOR impairs the reconsolidation phase of traumatic memory (98). In patients with AD, a decrease in mTOR activity in peripheral lymphocytes appears to correlate with the progression of AD (99). Loss of mTOR signaling also has been shown to impair long-term potentiation and synaptic plasticity in animal models of AD that can be restored through the upregulation of mTOR signaling (100).
Given the studies that illustrate the critical role of mTOR in memory formation and maintenance, the observations that a reduction in mTOR signaling occurs in patients with AD, and the studies that demonstrate the detrimental effects of Aβ exposure on mTOR, it is conceivable to consider that a minimum level of activity of the PI 3-K, Akt, and mTOR pathways may be required to prevent the onset and progression of AD. In fact, inhibition of mTOR activity may ultimately lead to neuronal atrophy in AD. Loss of retinoblastoma-1 (RB1) inducible coiled-coil 1 (RB1CC1) tumor suppressor has been observed in the brains of AD patients. In these patients, RB1CC1 appears to be necessary for neurite growth and to maintain mTOR signaling, since reduced expression of RB1CC1 leads to loss of mTOR activity, neuronal apoptosis, and neuronal atrophy (101). Recent work also has illustrated that increased Rheb GTPase activity that is known to increase mTORC1 activity may be necessary to regulate the β-site amyloid precursor protein (APP)-cleaving enzyme 1 (β-secretase, BACE1) that promotes Aβ accumulation in AD, since over-expression of Rheb depletes BACE1 and is able to reduce Aβ generation (102).
However, the degree of mTOR activity to achieve a therapeutic benefit during AD is not entirely clear, and several studies also support the premise that inhibition of mTOR signaling is a viable avenue for the treatment of AD. In the temporal cortex of patients with AD, increased levels of phosphorylation for Akt, mTOR, and tau have been reported to suggest that aberrant and increased mTOR signaling may contribute to the pathology of AD (103,104). As part of the mTOR signaling cascade, phosphorylated p70S6K has been demonstrated to co-localize with increased hyperphosphorylated tau deposition in the brains of AD patients, and it is suggested that p70S6K may foster the accumulation of hyperphosphorylated tau (105). Temsirolimus, an inhibitor of mTOR and approved by the US Federal Drug Administration (FDA) for the treatment of renal cell carcinoma, has been shown to enhance Aβ clearance in cell lines and animal models of AD and improve spatial learning through the induction of autophagy (40). In other animal models of AD, long-term inhibition of mTOR with rapamycin decreased levels of Aβ, slowed the progression of cognitive deficits (106), and inhibited tau phosphorylation (107,108). Additional studies suggest that by using agents that can suppress BACE1 expression through the inhibition of Akt and mTOR as well as through the activation of AMPK, Aβ deposition and senile plaque formation are decreased (109).
Future directions and strategies for mTOR and Alzheimer’s disease
Sporadic AD is a syndrome that may result from multiple etiologies that lead to cognitive loss and widespread neuronal, vascular, and immune cell impairment in the nervous system. With more than 30 million individuals estimated to suffer from AD over the next 20 years and current annual costs to the US economy alone estimated at approximately 4 billion US dollars, significant emphasis has been placed on the development of novel therapeutic strategies that can either abort or at least slow progression of AD. Current therapies for AD are extremely limited and, at best, offer symptomatic relief but do not alter the progression of the disease. Multiple emerging therapies are under consideration, but none may offer the promise to target improvement in both cognitive function and nervous system cell survival as mTOR and its signaling pathways.
Interestingly, mTOR has been demonstrated to be instrumental in a number of cellular protective pathways that involve sirtuins (30), wingless pathways (18,65), forkhead transcription factors (59,64,67), GSK-3β (110), and growth factors (48,66,77). Furthermore, mTOR is intimately linked to the pathways of PI 3-K and Akt that control programmed cell death during neurodegenerative disorders especially in AD (Table I). Yet, the pathways of apoptosis, autophagy, and necroptosis can have a complex relationship that may produce varied clinical outcomes that involve mTOR. Memory function may not be dependent upon only apoptosis or autophagic cell survival, but also require the activation of necroptosis (90). In a similar fashion, cellular proliferation may require both necroptosis and autophagy as well as mTOR signaling (94). In contrast under other parameters, proper cell growth may necessitate autophagy and apoptosis that progress at different time periods (87), while other cell survival scenarios support the need for the activity of apoptosis but the inhibition of autophagy (88).
These observations suggest that multiple pathways of the PI 3-K, Akt, and mTOR cascade in combination or independently may influence biological function and therapeutic outcome. In some circumstances, specific targeting of individual components of the cascade may offer the best outcome. For example, rodent models of epilepsy indicate that specific targeting of mTORC1 in the developing brain may block the detrimental effects of neonatal seizures (111). In other studies evaluating mitochondrial injury and apoptotic cell death following serum deprivation, mTORC2 was considered to have greater efficacy in improving cell survival than mTORC1 (112). However, additional work supports a broader targeting approach (113), since entities such as nervous system tumors can develop resistance to agents that inhibit mTOR signaling or express an abnormal increased basal activity of the PI 3-K, Akt, mTOR axis requiring targeting of multiple components (114). In clinical studies with chronic myelogenous leukemia (88) and colorectal cancer metastases (115), targeting both mTORC1 and mTORC2 pathways has been advocated. In models of AD, repression of Aβ production may require inhibition of Akt and mTOR with the concomitant activation of AMPK (109). Cellular protection against the toxicity of Aβ also may require mTOR modulation in conjunction with TSC2, PRAS40, BAD, and Bcl-xL (Table I) (18,45,65).
Yet, whether single or multiple components of the mTOR pathway are targeted, caution must be exercised in neurodegenerative disorders, since PI 3-K, Akt, and mTOR are proliferative pathways and may lead to unchecked cell growth and tumorigenesis (116,117). Therefore, it may come as no surprise that rapamycin (sirolimus) and rapamycin derivative compounds (‘rapalogs’) have been developed as anti-tumor agents and are currently approved by the FDA for the treatment of central nervous system and neuroendocrine tumors (17). Yet, the clinical use of rapamycin or its derivative compounds to inhibit mTOR can present additional complications. These agents can result in side effects that include oral and respiratory infections, stomatitis, hypercholesterolemia, leukopenia, hypertriglyceridemia, and immunosuppression (118,119). As a result, the focused development of new agents that can target mTOR or specific components of the mTOR cascade with precision could be particularly advantageous for developing treatments for neurodegenerative disorders and AD (7,120).
In particular for the development of strategies for AD, temporal targeting of the mTOR pathway may be a vital consideration especially in regards to early onset versus chronic progression of AD. Recent clinical phase III trials for AD designed to reduce Aβ load with immunomodulators bapineuzumab or with solanezumab (LY2062430) resulted in limited or absent clinical success believed to be a result of not instituting therapy prior to significant clinical disease presentation or progression. This concept may have support with the development of therapies that target mTOR. In animal models of temporal lobe epilepsy, early inhibition of mTOR with rapamycin rather than late rapamycin administration following epileptic activity achieved significantly greater protection against neuronal death and the loss of neurogenesis (121). Furthermore, in animal models of AD, early inhibition of the mTOR pathway fostered a higher tolerance against Aβ injury (122), suggesting that modulation of mTOR pathways may have a temporal variable that can ultimately impact clinical outcome.
Critical to the several considerations presented in developing strategies with mTOR for AD is the understanding that the level of activity of mTOR may significantly affect the clinical response. Although some studies suggest that inhibition of mTOR in models of AD can lead to a reduction in BACE1 (109), reduced senile plaque formation, and diminished Aβ levels (106), other work indicates the importance of mTOR activation to preserve cognitive function (Table I). During oxidative stress injury, mTOR activation protects neuronal networks controlling memory in the hippocampus (46). Activity of mTOR may be required for long-term memory formation (97) and the reconsolidation phase of traumatic memory (98). In addition, loss of mTOR activity can impair long-term potentiation and synaptic plasticity in animal models of AD (100). It is therefore vital in the overall clinical design of therapeutic targets for AD that focuses upon mTOR signaling that modulation of mTOR activity achieves the goals of reducing or eliminating the accumulation of potential neurotoxins such as Aβ, but in parallel also preserves or restores memory and cognitive function for individuals suffering from neurodegenerative disorders, such as AD.
Key messages.
Alzheimer’s disease (AD) affects a significant portion of the global population and will dramatically increase over the next 15–20 years, necessitating the development of novel, safe, and effective therapies for AD.
The mammalian target of rapamycin (mTOR) and its multi-faceted cellular pathways offers one of the most exciting and unique pathways for the development of therapeutic strategies against AD.
Given the ability of mTOR pathways to broadly impact cellular function in the nervous system, it is vital for the clinical design of therapy that targets mTOR for AD to achieve the parallel goals of protecting neuronal, vascular, and immune cell survival in conjunction with maintaining and preserving memory and cognitive function.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Declaration of interest: The authors declare no conflicts of interest.
References
- 1.Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer’s disease and cognitive impairment. Oxid Med Cell Longev. 2009;2 :279–89. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filley CM, Rollins YD, Anderson CA, Arciniegas DB, Howard KL, Murrell JR, et al. The genetics of very early onset Alzheimer disease. Cogn Behav Neurol. 2007;20:149–56. doi: 10.1097/WNN.0b013e318145a8c8. [DOI] [PubMed] [Google Scholar]
- 3.Agis-Torres A, Solhuber M, Fernandez M, Sanchez-Montero JM. Multi-target-directed ligands and other therapeutic strategies in the search of a real solution for Alzheimer’s disease. Curr Neuropharmacol. 2014;12:2–36. doi: 10.2174/1570159X113116660047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. Int J Mol Sci. 2013;13:13830–66. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno S, Iijima R, Ogishima S, Kikuchi M, Matsuoka Y, Ghosh S, et al. AlzPathway: a comprehensive map of signaling pathways of Alzheimer’s disease. BMC Syst Biol. 2012;6:52. doi: 10.1186/1752-0509-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Lawrence JC, Jr, Sturgill TW, Harris TE. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem. 2009;284:14693–7. doi: 10.1074/jbc.C109.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 14.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–26. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 15.Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–4. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- 16.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 17.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99:128–48. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10:29–38. doi: 10.2174/156720213804806007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–51. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Z, Li B, Li K, Zhao B. Down-regulation of amyloid-beta through AMPK activation by inhibitors of GSK-3beta in SH-SY5Y and SH-SY5Y-AbetaPP695 cells. J Alzheimers Dis. 2012;29:89–98. doi: 10.3233/JAD-2012-111649. [DOI] [PubMed] [Google Scholar]
- 21.Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011;118:460–74. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- 22.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123–34. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng PW, Ho WY, Su YT, Lu PJ, Chen BZ, Cheng WH, et al. Resveratrol decrease fructose-induced oxidative stress mediated by NADPH oxidase via an AMPK-dependent mechanism. Br J Pharmacol. 2014;171:2739–50. doi: 10.1111/bph.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jessen N, Koh HJ, Folmes CD, Wagg C, Fujii N, Lofgren B, et al. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta. 2010;1802:593–600. doi: 10.1016/j.bbadis.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J Agric Food Chem. 2012;60:1094–101. doi: 10.1021/jf204862d. [DOI] [PubMed] [Google Scholar]
- 26.Aragno M, Mastrocola R, Ghe C, Arnoletti E, Bassino E, Alloatti G, et al. Obestatin induced recovery of myocardial dysfunction in type 1 diabetic rats: underlying mechanisms. Cardiovasc Diabetol. 2012;11:129. doi: 10.1186/1475-2840-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011;286 :18465–73. doi: 10.1074/jbc.M110.200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436:169–79. doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167–78. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folch J, Junyent F, Verdaguer E, Auladell C, Pizarro JG, Beas-Zarate C, et al. Role of cell cycle re-entry in neurons: a common apoptotic mechanism of neuronal cell death. Neurotox Res. 2012;22:195–207. doi: 10.1007/s12640-011-9277-4. [DOI] [PubMed] [Google Scholar]
- 33.Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1-42)-induced rat model of Alzheimer’s disease. Free Radic Res. 2014;48:146–58. doi: 10.3109/10715762.2013.857018. [DOI] [PubMed] [Google Scholar]
- 34.Wang CM, Liu MY, Wang F, Wei MJ, Wang S, Wu CF, et al. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol Biochem Behav. 2013;106:57–67. doi: 10.1016/j.pbb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203–14. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broe M, Shepherd CE, Milward EA, Halliday GM. Relationship between DNA fragmentation, morphological changes and neuronal loss in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2001;101:616–24. doi: 10.1007/s004010000337. [DOI] [PubMed] [Google Scholar]
- 37.Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am J Pathol. 2008;173:1488–95. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, Greene LA. Bim is elevated in Alzheimer’s disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci. 2007;27:893–900. doi: 10.1523/JNEUROSCI.3524-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lourhmati A, Buniatian GH, Paul C, Verleysdonk S, Buecheler R, Buadze M, et al. Age-dependent astroglial vulnerability to hypoxia and glutamate: the role for erythropoietin. PLoS One. 2013;8:e77182. doi: 10.1371/journal.pone.0077182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L, et al. Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol Res. 2014;81C:54–63. doi: 10.1016/j.phrs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab Invest. 2010;90:762–73. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen TJ, Wang DC, Chen SS. Amyloid-beta interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor-induced Arc expression in rat cortical neurons. J Neurosci Res. 2009;87:2297–307. doi: 10.1002/jnr.22057. [DOI] [PubMed] [Google Scholar]
- 43.Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385:125–32. doi: 10.1007/s11010-013-1821-5. [DOI] [PubMed] [Google Scholar]
- 44.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270–85. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4:187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zare Mehrjerdi F, Aboutaleb N, Habibey R, Ajami M, Soleimani M, Arabian M, et al. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res. 2013;1526:94–101. doi: 10.1016/j.brainres.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404:941–5. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 48.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–50. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317–29. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uranga RM, Katz S, Salvador GA. Enhanced phosphatidylinositol 3-kinase (PI3K)/Akt signaling has pleiotropic targets in hippocampal neurons exposed to iron-induced oxidative stress. J Biol Chem. 2013;288:19773–84. doi: 10.1074/jbc.M113.457622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zara S, De Colli M, Rapino M, Pacella S, Nasuti C, Sozio P, et al. Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer’s disease rat model. Gerontology. 2013;59:250–60. doi: 10.1159/000346445. [DOI] [PubMed] [Google Scholar]
- 53.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 54.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220–35. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011;47:145–53. doi: 10.1016/j.mcn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Wu S, Mikhailov A, Kallo-Hosein H, Hara K, Yonezawa K, Avruch J. Characterization of ubiquilin 1, an mTOR-interacting protein. Biochim Biophys Acta. 2002;1542:41–56. doi: 10.1016/s0167-4889(01)00164-1. [DOI] [PubMed] [Google Scholar]
- 58.Mizukami K, Abrahamson EE, Mi Z, Ishikawa M, Watanabe K, Kinoshita S, et al. Immunohistochemical analysis of ubiquilin-1 in the human hippocampus: association with neurofibrillary tangle pathology. Neuropathology. 2014;34:11–18. doi: 10.1111/neup.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong YK, Lee S, Park SH, Lee JH, Han SY, Kim ST, et al. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer’s disease model. Biochem Biophys Res Commun. 2012;419:49–53. doi: 10.1016/j.bbrc.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 61.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamorro ME, Wenker SD, Vota DM, Vittori DC, Nesse AB. Signaling pathways of cell proliferation are involved in the differential effect of erythropoietin and its carbamylated derivative. Biochim Biophys Acta. 2013;1833:1960–8. doi: 10.1016/j.bbamcr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp Dermatol. 2013;22:311–15. doi: 10.1111/exd.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 inducible signaling pathway protein 1 (WISP1) Targets PRAS40 to govern beta-amyloid apoptotic injury of microglia. Curr Neurovasc Res. 2012;9:239–49. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS One. 2012;7:e45456. doi: 10.1371/journal.pone.0045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong X, Xie R, Zhang H, Gu L, Xie W, Cheng M, et al. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis. 2014;66:43–52. doi: 10.1016/j.nbd.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das F, Dey N, Venkatesan B, Kasinath BS, Ghosh-Choudhury N, Choudhury GG. High glucose upregulation of early-onset Parkinson’s disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal. 2011;23:1311–19. doi: 10.1016/j.cellsig.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hao J, Li F, Liu W, Liu Q, Liu S, Li H, et al. Phosphorylation of PRAS40-Thr246 involves in renal lipid accumulation of diabetes. J Cell Physiol. 2014;229:1069–77. doi: 10.1002/jcp.24533. [DOI] [PubMed] [Google Scholar]
- 70.Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, et al. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem. 2005;94:215–25. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- 71.Huang H, Huang F, Huang JP. Transplantation of bone marrow derived endothelial progenitor cells overexpressing Delta-like-4 enhances functional neovascularization in ischemic myocardium. Mol Med Rep. 2013;8:1556–62. doi: 10.3892/mmr.2013.1657. [DOI] [PubMed] [Google Scholar]
- 72.Koh PO. Ferulic acid attenuates focal cerebral ischemia-induced decreases in p70S6 kinase and S6 phosphorylation. Neurosci Lett. 2013;555:7–11. doi: 10.1016/j.neulet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, Pende M. The combined deletion of S6K1 and Akt2 deteriorates glycaemic control in high fat diet. Mol Cell Biol. 2012;32:4001–11. doi: 10.1128/MCB.00514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61:272–80. doi: 10.2337/db11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):30–4. doi: 10.3109/14767058.2012.663176. [DOI] [PubMed] [Google Scholar]
- 76.Kulbe JR, Mulcahy Levy JM, Coultrap SJ, Thorburn A, Bayern KU. Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res. 2014;1542:12–19. doi: 10.1016/j.brainres.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen A, Xiong LJ, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep. 2013;8:1011–16. doi: 10.3892/mmr.2013.1628. [DOI] [PubMed] [Google Scholar]
- 78.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012;318:33–42. doi: 10.1016/j.yexcr.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Kang HS, et al. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res. 2012;73:99–105. doi: 10.1016/j.neures.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29:946–56. doi: 10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- 82.Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010;112:366–76. doi: 10.1111/j.1471-4159.2009.06463.x. [DOI] [PubMed] [Google Scholar]
- 83.Bains M, Zaegel V, Mize-Berge J, Heidenreich KA. IGF-I stimulates Rab7-RILP interaction during neuronal autophagy. Neurosci Lett. 2011;488:112–17. doi: 10.1016/j.neulet.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin B, Liang H, Chen Y, Chu K, Huang L, Fang L, et al. EGB1212 post-treatment ameliorates hippocampal CA1 neuronal death and memory impairment induced by transient global cerebral ischemia/reperfusion. Am J Chin Med. 2013;41:1329–41. doi: 10.1142/S0192415X13500894. [DOI] [PubMed] [Google Scholar]
- 85.Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Francois A, Terro F, Janet T, Bilan AR, Paccalin M, Page G. Involvement of interleukin-1beta in the autophagic process of microglia: relevance to Alzheimer’s disease. J Neuroinflammation. 2013;10:151. doi: 10.1186/1742-2094-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol. 2012;83:16–26. doi: 10.1016/j.bcp.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–74. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012;9:89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qinli Z, Meiqing L, Xia J, Li X, Weili G, Xiuliang J, et al. Necrostatin-1 inhibits the degeneration of neural cells induced by aluminum exposure. Restor Neurol Neurosci. 2013;31:543–55. doi: 10.3233/RNN-120304. [DOI] [PubMed] [Google Scholar]
- 91.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–23. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Werneck MB, Hottz E, Bozza PT, Viola JP. Cyclosporin A inhibits colon cancer cell growth independently of the calcineurin pathway. Cell Cycle. 2012;11:3997–4008. doi: 10.4161/cc.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther. 2014;13:596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li L, Xu B, Zhu Y, Chen L, Sokabe M. DHEA prevents Abeta25–35-impaired survival of newborn neurons in the dentate gyrus through a modulation of PI3K-Akt-mTOR signaling. Neuropharmacology. 2010;59:323–33. doi: 10.1016/j.neuropharm.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–15. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- 97.Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–83. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, Carret-Rebillat AS, et al. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:320–6. doi: 10.1159/000095562. [DOI] [PubMed] [Google Scholar]
- 100.Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chano T, Okabe H, Hulette CM. RB1CC1 insufficiency causes neuronal atrophy through mTOR signaling alteration and involved in the pathology of Alzheimer’s diseases. Brain Res. 2007;1168:97–105. doi: 10.1016/j.brainres.2007.06.075. [DOI] [PubMed] [Google Scholar]
- 102.Shahani N, Pryor W, Swarnkar S, Kholodilov N, Thinakaran G, Burke RE, et al. Rheb GTPase regulates beta-secretase levels and amyloid beta generation. J Biol Chem. 2014;289:5799–808. doi: 10.1074/jbc.M113.532713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–17. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 104.Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: implication for Alzheimer disease. J Biol Chem. 2013;288:15556–70. doi: 10.1074/jbc.M112.435123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell. 2013;12:370–80. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y, Su Y, Wang J, Sun S, Wang T, Qiao X, et al. Rapamycin decreases tau phosphorylation at Ser214 through regulation of cAMP-dependent kinase. Neurochem Int. 2013;62:458–67. doi: 10.1016/j.neuint.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 109.Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, et al. Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both beta-amyloid production and clearance. J Neurosci. 2013;33:13138–49. doi: 10.1523/JNEUROSCI.4790-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–72. [PMC free article] [PubMed] [Google Scholar]
- 111.Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, et al. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang Z, Baykal AT, Gao H, Quezada HC, Zhang H, Bereczki E, et al. mTor Is a signaling hub in cell survival: a mass-spectrometry-based proteomics investigation. J Proteome Res. 2014 Apr 14; doi: 10.1021/pr500192g. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 113.Barrett D, Brown VI, Grupp SA, Teachey DT. Targeting the PI3K/AKT/mTOR Signaling axis in children with hematologic malignancies. Paediatr Drugs. 2012;14:299–316. doi: 10.2165/11594740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jin N, Jiang T, Rosen DM, Nelkin BD, Ball DW. Dual inhibition of mitogen-activated protein kinase kinase and mammalian target of rapamycin in differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:4107–12. doi: 10.1210/jc.2009-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maiese K. Therapeutic targets for cancer: current concepts with PI 3-K, Akt, & mTOR. Indian J Med Res. 2013;137:243–6. [PMC free article] [PubMed] [Google Scholar]
- 117.Wang S, Wu J, Nie SD, Bereczki E, Pei JJ. Dysregulated mTOR-dependent signaling in neurodegeneration or carcinogenesis: implication for Alzheimer’s disease and brain tumors. J Alzheimers Dis. 2013;37:495–505. doi: 10.3233/JAD-130641. [DOI] [PubMed] [Google Scholar]
- 118.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 119.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–12. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 120.Curatolo P, Moavero R. mTOR inhibitors as a new therapeutic option for epilepsy. Expert review of neurotherapeutics. 2013;13:627–38. doi: 10.1586/ern.13.49. [DOI] [PubMed] [Google Scholar]
- 121.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723–36. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]