Abstract
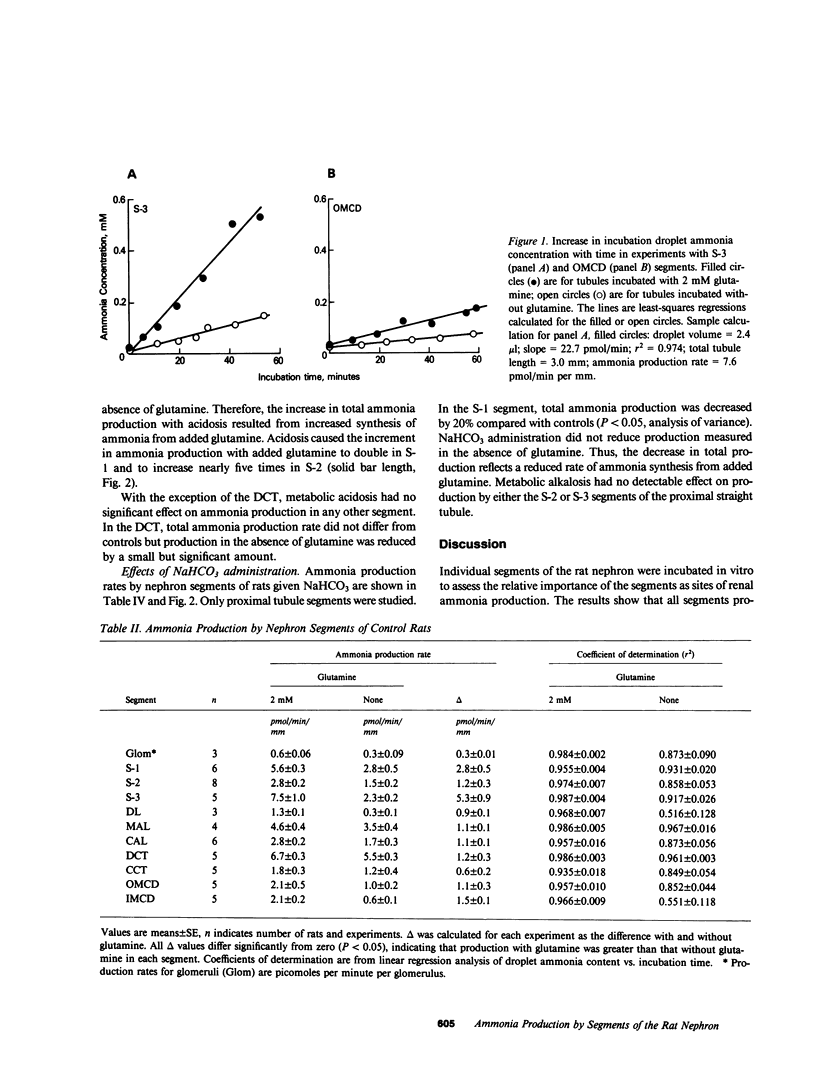
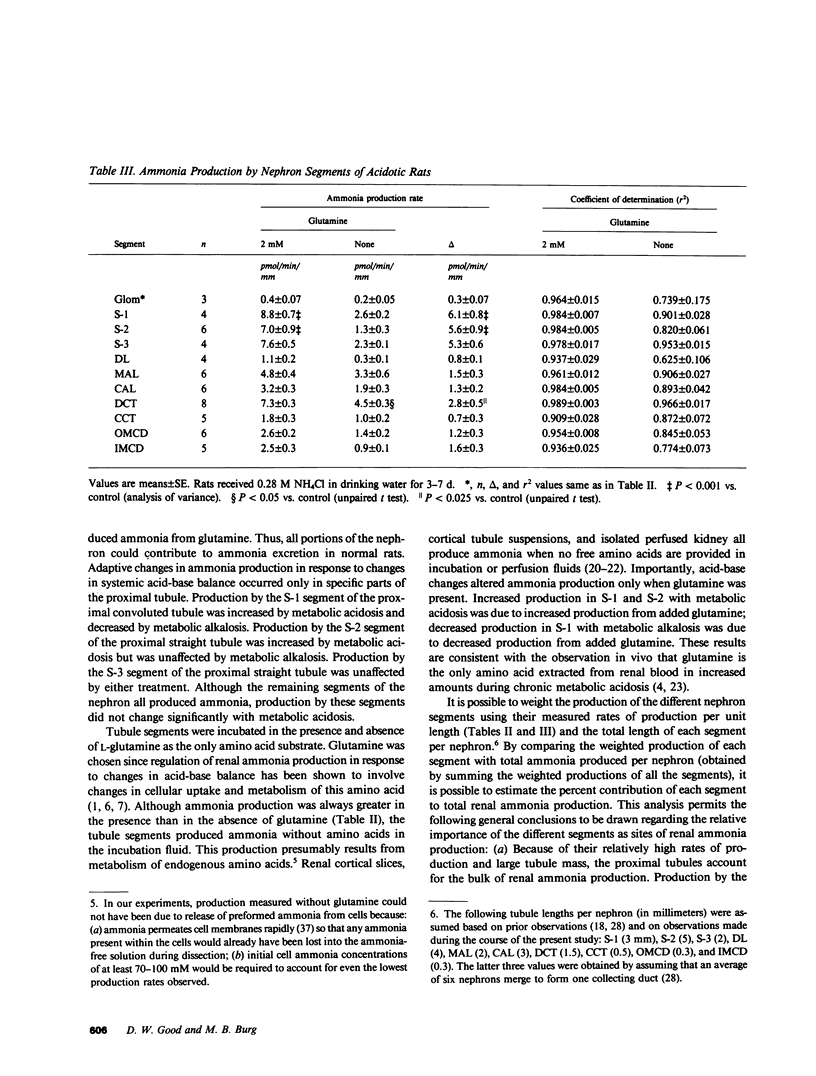
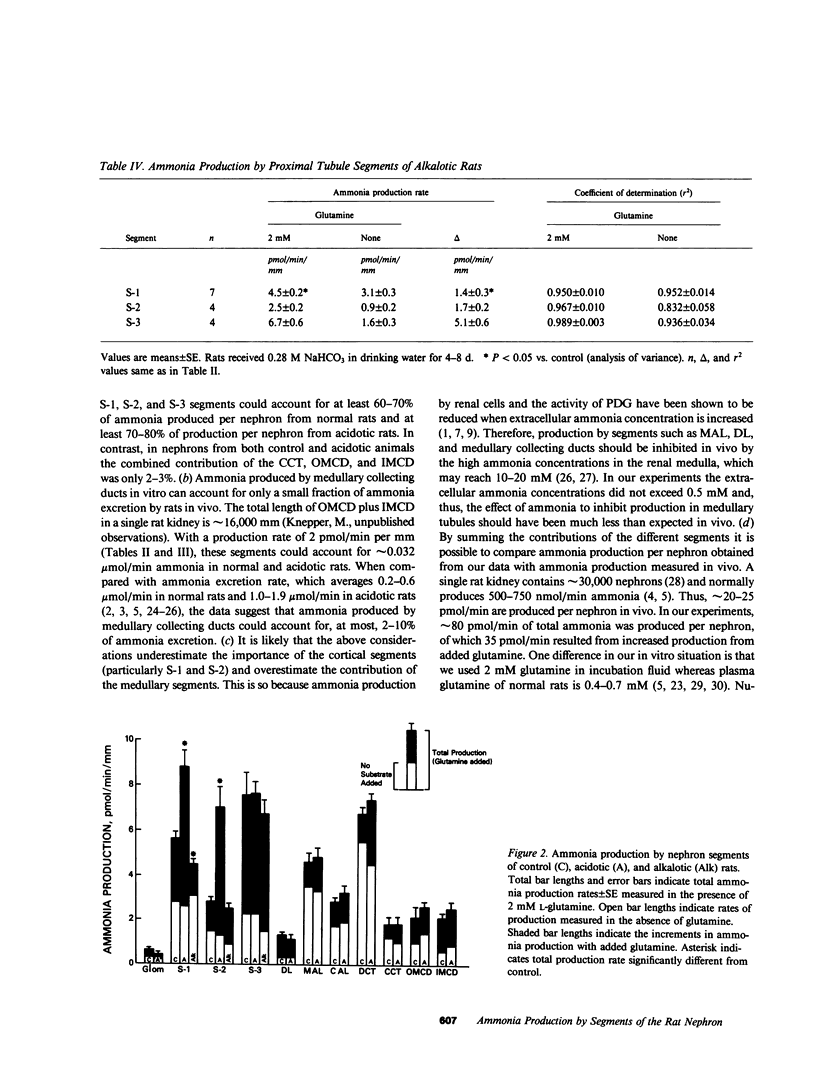
Ammonia production was measured directly in 10 segments of the rat nephron to determine the relative importance of the segments as sites of renal ammonia production. Tubules were microdissected from normal rats and rats drinking 0.28 M NH4Cl or 0.28 M NaHCO3 for 3-8 d. The segments were incubated in vitro with and without 2 mM glutamine. Ammonia concentrations in the incubation fluid were measured by microfluorometry to determine ammonia production rates. All segments produced ammonia from glutamine. In normal rats, production with glutamine was highest (greater than 5 pmol/min per mm) in the proximal convoluted (S-1), proximal straight (S-3), and distal convoluted tubules, and lowest (less than or equal to 2) in cortical and medullary collecting ducts and thin descending limbs. Metabolic acidosis increased production by 60% in the S-1 segment of the proximal convoluted tubule and by 150% in the S-2 segment of the proximal straight tubule without significant effect in any other segment. Bicarbonate loading decreased production by S-1 but had no effect on S-2 or S-3. Thus, acid-base changes altered production only in specific segments of the proximal tubule. We infer that the bulk of ammonia production occurs in the proximal tubules and that production by collecting ducts can account for only a few percent of renal ammonia production and excretion in the rat.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban R. S., Soltoff S. P., Storey J. M., Mandel L. J. Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol. 1980 Jan;238(1):F50–F59. doi: 10.1152/ajprenal.1980.238.1.F50. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct. J Clin Invest. 1982 Jul;70(1):1–12. doi: 10.1172/JCI110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch H. B., Chan A. W., Alvey T. R., Lowry O. H. Localization of glutamine accumulation and tubular reabsorption in rat nephron. Kidney Int. 1978 Nov;14(5):406–413. doi: 10.1038/ki.1978.145. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Narins R. G., Chu C., Fagioli S., Choi S., McCarthy W., Lowry O. H. Distribution along the rat nephron of three enzymes of gluconeogenesis in acidosis and starvation. Am J Physiol. 1978 Sep;235(3):F246–F253. doi: 10.1152/ajprenal.1978.235.3.F246. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. Glutamate and glutamine distribution in the rat nephron in acidosis and alkalosis. Am J Physiol. 1973 Apr;224(4):884–889. doi: 10.1152/ajplegacy.1973.224.4.884. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLABMAN S., KOSE R. M., GIEBISCH G. Micropuncture study of ammonia excretion in the rat. Am J Physiol. 1963 Jul;205:127–132. doi: 10.1152/ajplegacy.1963.205.1.127. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Schooler J. M. Regulation of ammonia production in the rat kidney. Adv Enzyme Regul. 1967;5:71–86. doi: 10.1016/0065-2571(67)90009-x. [DOI] [PubMed] [Google Scholar]
- Good D. W., Vurek G. G. Picomole quantitation of ammonia by flow-through fluorometry. Anal Biochem. 1983 Apr 1;130(1):199–202. doi: 10.1016/0003-2697(83)90670-x. [DOI] [PubMed] [Google Scholar]
- Graber M. L., Bengele H. H., Mroz E., Lechene C., Alexander E. A. Acute metabolic acidosis augments collecting duct acidification rate in the rat. Am J Physiol. 1981 Dec;241(6):F669–F676. doi: 10.1152/ajprenal.1981.241.6.F669. [DOI] [PubMed] [Google Scholar]
- HAYES C. P., Jr, MAYSON J. S., OWEN E. E., ROBINSON R. R. A MICROPUNCTURE EVALUATION OF RENAL AMMONIA EXCRETION IN THE RAT. Am J Physiol. 1964 Jul;207:77–83. doi: 10.1152/ajplegacy.1964.207.1.77. [DOI] [PubMed] [Google Scholar]
- Hems D. A. Metabolism of glutamine and glutamic acid by isolated perfused kidneys of normal and acidotic rats. Biochem J. 1972 Dec;130(3):671–680. doi: 10.1042/bj1300671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey R. P., Rankin B. B., Curthoys N. P. Acute acidosis and renal arteriovenous differences of glutamine in normal and adrenalectomized rats. Am J Physiol. 1980 Mar;238(3):F199–F204. doi: 10.1152/ajprenal.1980.238.3.F199. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., HIMMELHOCH S. R. Histochemical localization of glutaminase I activity in kidney. Am J Physiol. 1961 Nov;201:786–790. doi: 10.1152/ajplegacy.1961.201.5.786. [DOI] [PubMed] [Google Scholar]
- Kamm D. E., Strope G. L. The effects of acidosis and alkalosis on the metabolism of glutamine and glutamate in renal cortex slices. J Clin Invest. 1972 May;51(5):1251–1263. doi: 10.1172/JCI106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD E., ORLOFF J. Regulation of ammonia excretion in the rat. Am J Physiol. 1955 Jul;182(1):131–138. doi: 10.1152/ajplegacy.1955.182.1.131. [DOI] [PubMed] [Google Scholar]
- Lowry M., Ross B. D. Activation of oxoglutarate dehydrogenase in the kidney in response to acute acidosis. Biochem J. 1980 Sep 15;190(3):771–780. doi: 10.1042/bj1900771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the segmentation of the proximal tubule in the rat kidney. Comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res. 1966 Oct;16(3):239–258. doi: 10.1016/s0022-5320(66)80060-6. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, ORLOFF J. The effect of the administration of sodium bicarbonate and ammonium chloride on the excretion and production of ammonia; the absence of alterations in the activity of renal ammonia-producing enzymes in the dog. J Clin Invest. 1959 Feb;38(2):366–372. doi: 10.1172/JCI103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON J. R. Ammonia formation by surviving kidney slices without specific substrates. J Physiol. 1954 Apr 28;124(1):1–7. doi: 10.1113/jphysiol.1954.sp005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sajo I. M., Goldstein M. B., Sonnenberg H., Stinebaugh B. J., Wilson D. R., Halperin M. L. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981 Sep;20(3):353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Guder W. G. Sites of enzyme activity along the nephron. Kidney Int. 1976 Mar;9(3):233–242. doi: 10.1038/ki.1976.26. [DOI] [PubMed] [Google Scholar]
- Sonnenberg H., Cheema-Dhadli S., Goldstein M. B., Stinebaugh B. J., Wilson D. R., Halperin M. L. Ammonia addition into the medullary collecting duct of the rat. Kidney Int. 1981 Feb;19(2):281–287. doi: 10.1038/ki.1981.18. [DOI] [PubMed] [Google Scholar]
- Squires E. J., Hall D. E., Brosnan J. T. Arteriovenous differences for amino acids and lactate across kidneys of normal and acidotic rats. Biochem J. 1976 Oct 15;160(1):125–128. doi: 10.1042/bj1600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Vinay P., Allignet E., Pichette C., Watford M., Lemieux G., Gougoux A. Changes in renal metabolite profile and ammoniagenesis during acute and chronic metabolic acidosis in dog and rat. Kidney Int. 1980 Mar;17(3):312–325. doi: 10.1038/ki.1980.37. [DOI] [PubMed] [Google Scholar]
- Vinay P., Lemieux G., Gougoux A., Lemieux C. Response of the rat and dog kidney to H+ concentration in vitro--a comparative study with slices and tubules. Int J Biochem. 1980;12(1-2):89–98. doi: 10.1016/0020-711x(80)90048-8. [DOI] [PubMed] [Google Scholar]
- WEISS M. B., LONGLEY J. B. Renal glutaminase I distribution and ammonia excretion in the rat. Am J Physiol. 1960 Feb;198:223–226. doi: 10.1152/ajplegacy.1960.198.2.223. [DOI] [PubMed] [Google Scholar]


