Abstract
By using a combination of a heterologous antiserum to GPIb/glycocalicin and a radiolabeled monoclonal antibody to GPIb/glycocalicin, we were able to develop a sensitive and specific radioimmunoelectrophoretic assay that can distinguish small amounts of glycocalicin from GPIb. Normal plasmas were found to contain glycocalicin, even in samples treated with protease inhibitors and centrifuged extensively to remove platelets and platelet fragments. Confirmation that the plasma antigen had a relative molecular weight similar or identical to glycocalicin was obtained from studies employing gel chromatography and affinity chromatography. An immunoradiometric assay was developed to quantify plasma glycocalicin, and normal plasma was found to contain approximately 1-3 micrograms/ml. The plasma of a patient with severe thrombocytopenia due to aplastic anemia had less than 12.5% of the normal level of glycocalicin, whereas two patients with thrombocytopenia due to diseases of increased platelet destruction (idiopathic thrombocytopenic purpura and hemolytic-uremic syndrome) had normal levels. Thus, there appears to be ongoing catabolism of platelet GPIb in vivo, and we postulate that the plasma level of glycocalicin reflects a complex function of factors, including platelet count, platelet turnover, and the site of platelet destruction.
Full text
PDF

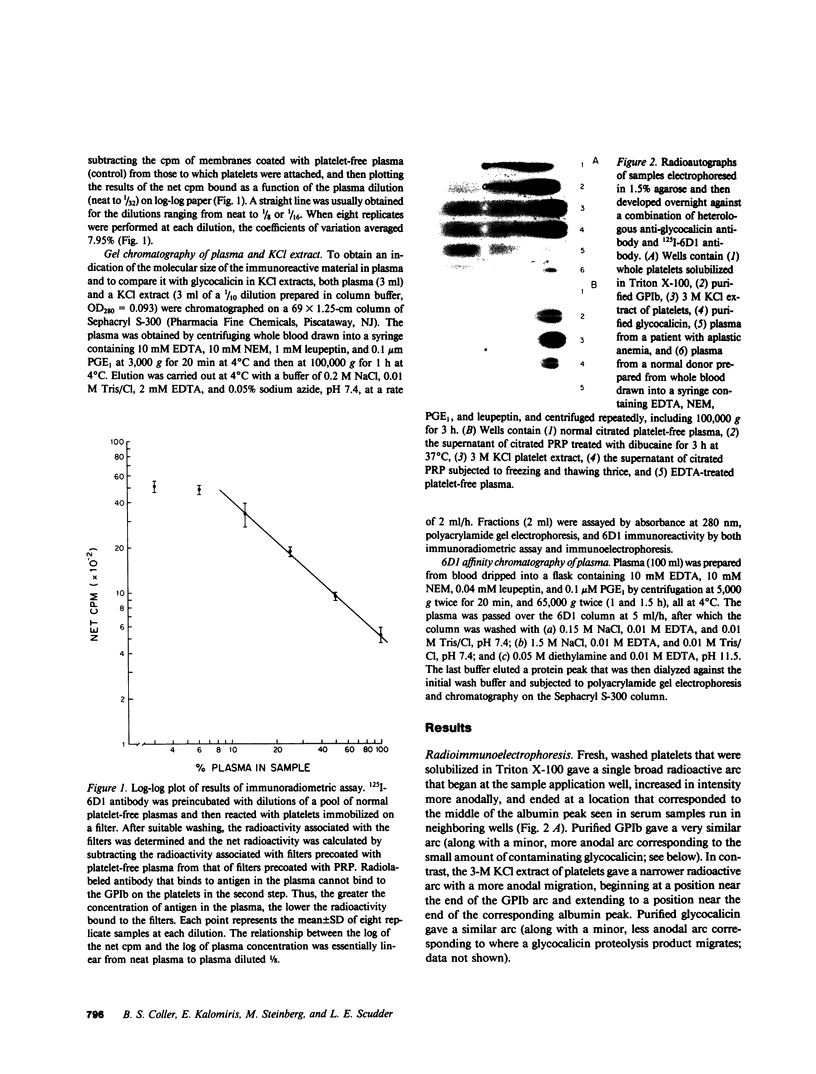

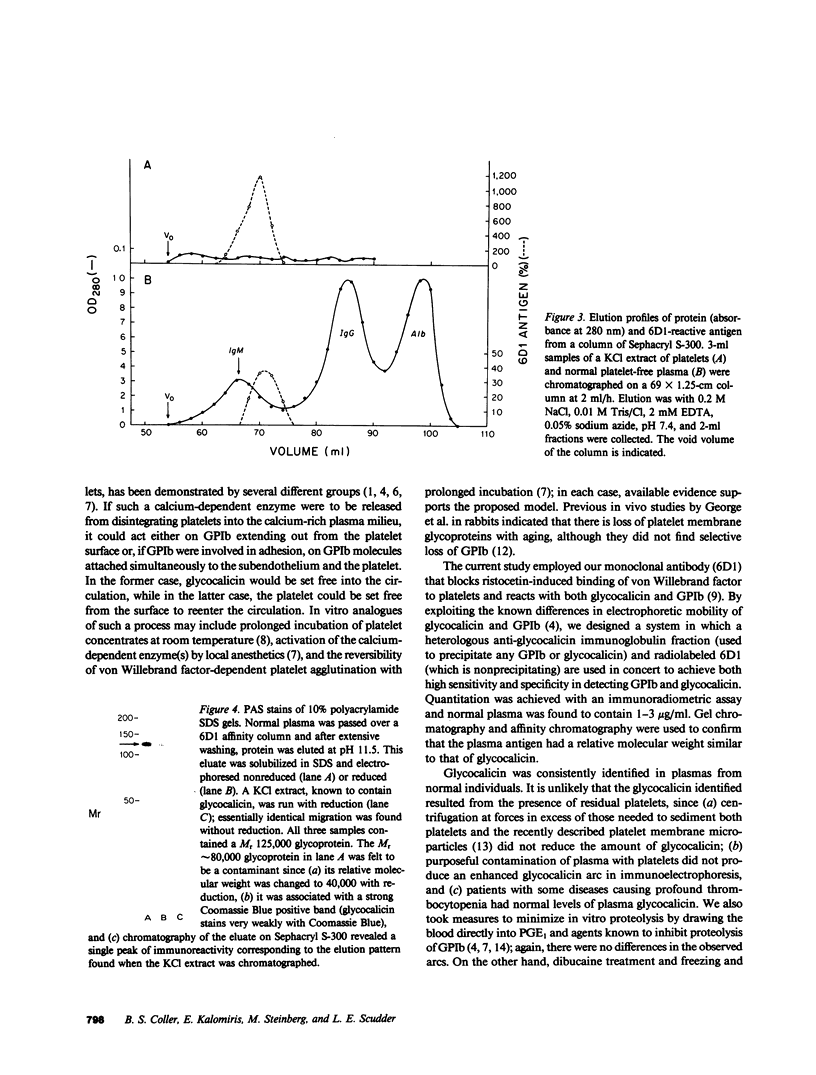

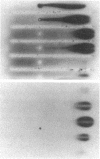
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clemetson K. J., Naim H. Y., Lüscher E. F. Relationship between glycocalicin and glycoprotein Ib of human platelets. Proc Natl Acad Sci U S A. 1981 May;78(5):2712–2716. doi: 10.1073/pnas.78.5.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S. Effects of tertiary amine local anesthetics on von Willebrand factor-dependent platelet function: alteration of membrane reactivity and degradation of GPIb by a calcium-dependent protease(s). Blood. 1982 Sep;60(3):731–743. [PubMed] [Google Scholar]
- George J. N., Lewis P. C., Sears D. A. Studies on platelet plasma membranes. II. Characterization of surface proteins of rabbit platelets in vitro and during circulation in vivo using diazotized (125i)-diiodosulfanilic acid as a label. J Lab Clin Med. 1976 Aug;88(2):247–260. [PubMed] [Google Scholar]
- George J. N. Platelet membrane glycoproteins: alteration during storage of human platelet concentration. Thromb Res. 1976 May;8(5):719–724. doi: 10.1016/0049-3848(76)90253-x. [DOI] [PubMed] [Google Scholar]
- George J. N., Thoi L. L., McManus L. M., Reimann T. A. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982 Oct;60(4):834–840. [PubMed] [Google Scholar]
- Nurden A. T., Kunicki T. J., Dupuis D., Soria C., Caen J. P. Specific protein and glycoprotein deficiencies in platelets isolated from two patients with the gray platelet syndrome. Blood. 1982 Apr;59(4):709–718. [PubMed] [Google Scholar]
- Okumura I., Lombart C., Jamieson G. A. Platelet glycocalicin. II. Purification and characterization. J Biol Chem. 1976 Oct 10;251(19):5950–5955. [PubMed] [Google Scholar]
- Phillips D. R. An evaluation of membrane glycoproteins in platelet adhesion and aggregation. Prog Hemost Thromb. 1980;5:81–109. [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Filion-Myklebust C., Stabaek T. Platelet glycocalicin. Its membrane association and solubilization in aqueous media. Biochim Biophys Acta. 1980 Apr 10;597(2):235–246. doi: 10.1016/0005-2736(80)90102-9. [DOI] [PubMed] [Google Scholar]
- Solum N. O., Olsen T. M., Gogstad G. O., Hagen I., Brosstad F. Demonstration of a new glycoprotein Ib-related component in platelet extracts prepared in the presence of leupeptin. Biochim Biophys Acta. 1983 Mar 23;729(1):53–61. doi: 10.1016/0005-2736(83)90455-8. [DOI] [PubMed] [Google Scholar]