Abstract
The Sleeping Beauty (SB) transposon system has been shown to mediate new gene sequence integration resulting in long-term expression. Here the effectiveness of hyperactive SB100X transposase was tested, and we found that hydrodynamic co-delivery of a firefly luciferase transposon (pT2/CaL) along with SB100X transposase (pCMV-SB100X) resulted in remarkably sustained, high levels of luciferase expression. However, after 4 weeks there was a rapid, animal-by-animal loss of luciferase expression that was not observed in immunodeficient mice. We hypothesized that this sustained, high-level luciferase expression achieved using the SB100X transposase elicits an immune response in pT2/CaL co-administered mice, which was supported by the rapid loss of luciferase expression upon challenge of previously treated animals and in naive animals adoptively transferred with splenocytes from previously treated animals. Specificity of the immune response to luciferase was demonstrated by increased cytokine expression in splenocytes after exposure to luciferase peptide in parallel with MHC I–luciferase peptide tetramer binding. This anti-luciferase immune response observed following continuous, high-level luciferase expression in vivo clearly impacts its use as an in vivo reporter. As both an immunogen and an extremely sensitive reporter, luciferase is also a useful model system for the study of immune responses following in vivo gene transfer and expression.
Introduction
Immune responses present a major challenge to the introduction and expression of new gene sequences in somatic cells and tissues in vivo (Raper et al., 2002, 2003; Muruve, 2004; Manno et al., 2006; Mingozzi and High, 2007; Matrai et al., 2011). Humoral and cellular responses to transgene product (Ohlfest et al., 2005; Liu et al., 2006; Aronovich et al., 2007) as well as to viral vector capsid proteins (Manno et al., 2006) can compromise the maintenance of transgene expression, sometimes leading to complete loss of expression. The use of nonviral vectors can alleviate problems associated with antiviral vector immune responses. However, the effectiveness of nonviral vectors is generally limited by relatively low gene transfer efficiency and short-term gene expression.
Sleeping Beauty (SB) is a transposon vector system that overcomes the limitation of short-term nonviral expression by integrating a gene of interest into the host cell genome. SB was originally reconstructed from an evolutionarily inactive salmonid transposable element (Ivics et al., 1997) and has been tested extensively for stable gene delivery in vitro and in vivo into a wide variety of cell and tissue types (Yant et al., 2000; Montini et al., 2002; Ohlfest et al., 2005; Liu et al., 2006; Aronovich et al., 2007; Wilber et al., 2007; Ni et al., 2008; Izsvak et al., 2010). There have been numerous improvements in the SB system reported (Ivics et al., 1997; Geurts et al., 2003; Yant et al., 2004; Zayed et al., 2004; Baus et al., 2005; Mates et al., 2009), culminating most recently with the generation of hyperactive SB100X, a transposase exhibiting a substantial increase in activity compared with the first-generation SB10 transposase (Mates et al., 2009) or other variants.
In its initial description, SB100X was found to mediate superior efficiencies of gene transfer into mouse liver and into the mouse germline (Mates et al., 2009), and provided the impetus to achieve nonviral gene transfer into human CD34+ hematopoietic stem cells (Mates et al., 2009; Xue et al., 2009). In this study, we found that hyperactive SB100X transposase supported remarkably high levels of sustained transgene expression in the liver after hydrodynamic delivery of a luciferase-encoding SB transposon. Surprisingly, maintenance of such a high level of luciferase expression (above 109p/sec/cm2) for at least 4 weeks resulted in a subsequent dramatic decline in expression in individual animals 1–3 months post-injection, indicative of an immune response. Through a series of adoptive transfer, luciferase peptide response, and MHC I–luciferase peptide tetramer binding experiments, we were able to demonstrate a specific cell-based anti-luciferase immune response in animals hydrodynamically injected with luciferase-encoding transposon plus SB100X encoding plasmid and exhibiting dramatic loss of expression. There was evidence of a threshold effect for immune response induction, where high levels of luciferase expression elicited an immune response, while moderate levels of luciferase expression in animals injected with luciferase transposon alone did not induce immunity and may have tolerized animals to high levels of sustained luciferase expressed upon subsequent injection of luciferase transposon plus SB100X encoding plasmid. These results have significant implications in the development of the SB transposon system for in vivo gene delivery and for the use of luciferase as an in vivo reporter.
Materials and Methods
Plasmids
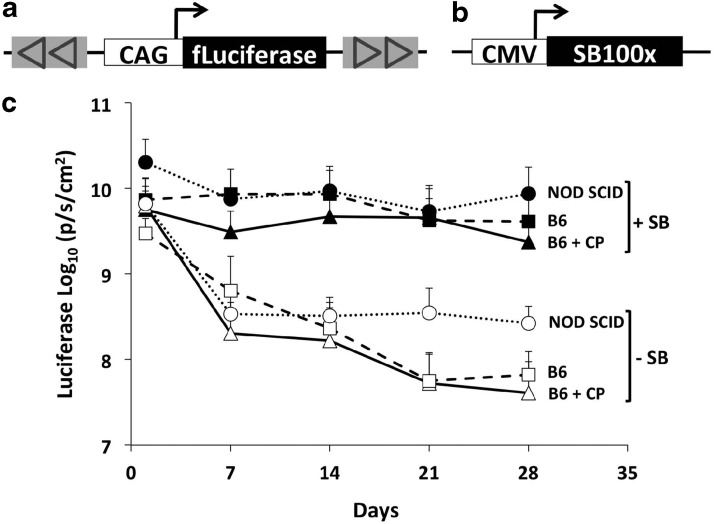
Transposon plasmid pT2/CaL (Fig. 1a), containing the CAG promoter regulating firefly luciferase, was previously described (Belur et al., 2008). Plasmid pCMV-SB100X (Fig. 1b), encoding hyperactive SB100X transposase under transcriptional regulation of the cytomegalovirus (CMV) early promoter, was kindly provided by Dr. Zsuszanna Isvak (Mates et al., 2009).
FIG. 1.
Long-term, high-level luciferase expression mediated by hyperactive transposase SB100X. (a) Transposon plasmid pT2/CaL contains the CAG promoter regulating firefly luciferase flanked by Sleeping Beauty (SB) T2 IR/DRs. (b) pCMV-SB100X; expression of hyperactive SB100X is regulated by the cytomegalovirus (CMV) early promoter. (c) Time course of mean luciferase activity after hydrodynamic injection of 5 μg pT2/CaL with or without 0.5 μg pCMV-SB100X (as indicated on the right, +SB or −SB) into NOD.SCID, C57BL/6, or temporarily immunosuppressed C57BL/6 mice. Data are expressed as mean (n=5) p/sec/cm2±SD. Immunosuppression was achieved by intraperitoneal (i.p.) injection of 120 mg/kg cyclophosphamide (CP) daily for 4 days beginning the day before hydrodynamic injection.
Animals
C57BL/6NCr and NOD.SCID/NCr mice (National Cancer Institute, Fredrick, VA) were housed under specific pathogen-free conditions and given food and water ad libitum. All animal procedures were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Hydrodynamic injection
Mice were administered 0.03 ml analgesic cocktail consisting of 8 mg/ml ketamine (Phoenix Scientific, St. Joseph, MO), 0.01 mg/ml butorphanol tartrate (Fort Dodge Animal Health, Fort Dodge, IA), and 0.1 mg/ml acepromazine maleate (Phoenix Scientific) in 0.9% sodium chloride by intraperitoneal (i.p.) injection. Plasmid DNA was diluted in lactated Ringer's solution to 10% volume per animal weight. As previously described, the plasmid DNA solution was infused through the lateral tail vein of the mouse in less than 10 sec (Liu et al., 1999; Zhang et al., 1999; Bell et al., 2007).
Immunosuppression
Cyclophosphamide (CP; Sigma-Aldrich Co., St Louis, MO) was administered at a dose of 120 mg/kg by i.p. injection on the day before, on the day of, and daily for 2 days after hydrodynamic injection. For continued immunosuppression, CP was delivered weekly.
In vivo bioluminescence imaging
Mice were anesthetized with 0.2 ml of ketamine cocktail i.p., and then given 0.1 ml of 28.5 mg/ml D-luciferin (Gold Biotechnology, St. Louis, MO) i.p. Animals were then imaged for a duration of 0.5 sec to 5 min using the Xenogen IVIS imaging system (Xenogen Corp., Alameda, CA). Assessed luciferase activity levels are expressed in photons emitted per second per square centimeter, denoted as p/sec/cm2.
Adoptive transfer
Test animals were sacrificed by CO2 asphyxiation. Spleens were harvested and disrupted using a syringe plunger, and then single-cell suspensions of splenocytes were injected into naïve mice by lateral tail vein injection (one donor animal per recipient animal).
Intracellular cytokine staining
Following CO2 asphyxiation, animals were perfused with 0.9% sodium chloride and then the liver and spleen were harvested and disrupted into single-cell suspensions. Liver lymphocytes were isolated on a 44–67% percoll gradient. Red blood cells in splenocyte suspensions were lysed using ACK lysis buffer (Life Technologies, Grand Island, NY). Cell suspensions were stimulated with 1 μg/ml luciferase peptide (LMYRFEEEL) (Limberis et al., 2009) plus 1 μg/ml GolgiPlug for 4 hr at 37°C. The cells were stained with anti-CD8α-Pacific Blue antibody, and then stained intracellularly for cytokine expression in response to luciferase peptide exposure using the Cytofix/Cytoperm kit (BD Pharmingen, San Jose, CA) according to manufacturer's instructions. The following antibodies were used: anti-IFNγ-APC, anti-TNFα-FITC, and anti-IL-2-PE. An LSRII instrument was used for flow cytometry. CellQuest Pro (BD Biosciences, San Jose, CA) was used for data collection and FlowJo (Tree Star Inc., Ashland, OR) software for analysis.
MHC I–luciferase peptide tetramer assays
Liver and spleen cell suspensions were stained with anti-CD8α-Pacific Blue, anti-CD11a-FITC, and anti-PD-1-PE-Cy7. At the same time, the cell suspensions were exposed to Kb LMYRFEEEL tetramer-PE, a CD8α-specific luciferase tetramer, kindly provided by Dr. Roberto Calcedo and Dr. James M. Wilson, University of Pennsylvania (Altman et al., 1996; Sims et al., 2010). Absolute lymphocyte count was determined using PKH26 Reference Microbeads (Sigma-Aldrich Co.). Flow cytometry was conducted as described above.
Quantitative polymerase chain reaction
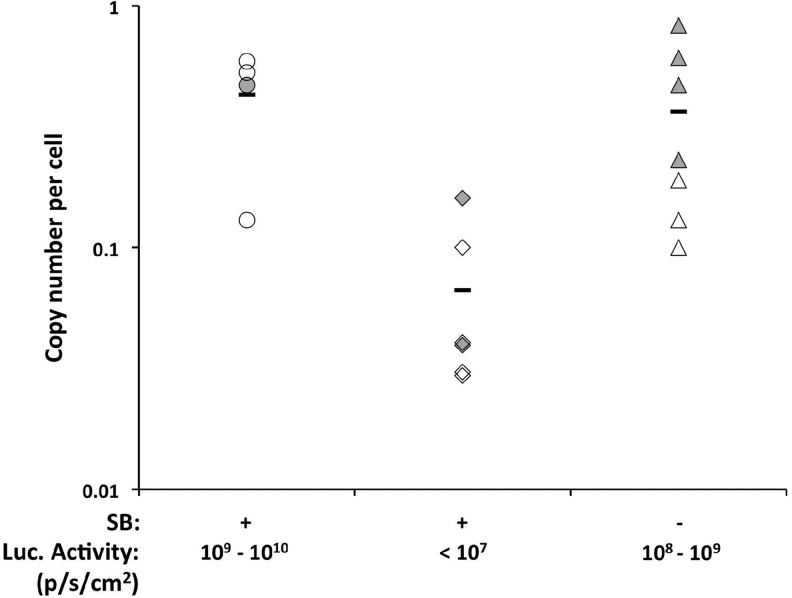
Total DNA was extracted from tissues using phenol-chloroform and from cell suspensions using the 5 PRIME ArchivePure DNA Purification: Cell and Tissue kit (Thermo Fisher Scientific Inc., Waltham, MA). Luciferase sequences in DNA extracts were quantified by real-time PCR using the Applied Biosystems TaqMan Gene Expression Assays kit (Life Technologies) and an Eppendorf thermocycler realplex. DNA extracted from mouse CT26 cells containing a single luciferase gene copy (Hsu et al., article in preparation) was diluted into parental CT26 cell DNA to generate copy number standards. Results are expressed as luciferase sequence copies per genome equivalent.
Statistics
Statistical analysis was performed using Prism 4 (www.graphpad.com). An unpaired t-test was used to evaluate the significance of differences among test groups.
Results
Immune response-associated loss of luciferase activity after high-level, long-term gene expression mediated by the SB transposon system
The SB transposon system has been tested extensively for stable gene transfer and long-term expression after hydrodynamic delivery in mice (Aronovich et al., 2007; Wilber et al., 2007; Bell et al., 2010). Using an SB transposon encoding the firefly luciferase gene (pT2/CaL; Fig. 1a), we previously reported expression levels above 109 p/sec/cm2 1 day following hydrodynamic injection that subsequently fell to about 107 p/sec/cm2 by 14 days postinfusion (Podetz-Pedersen et al., 2010). In the current study, we sought to test the relative effectiveness of hyperactive SB100X transposase (Mates et al., 2009) by hydrodynamic injection of 5 μg pT2/CaL with and without 0.5 μg pCMV-SB100X (Fig. 1b). To test for the effect of a potential immune response, injections were carried out in normal C57BL/6 (“B6”) mice, in B6 mice temporarily immunosuppressed by cyclophosphamide administration, and in immunodeficient NOD.SCID mice, subsequently evaluating the animals for luciferase expression by in vivo bioluminescence imaging. All mice exhibited high levels of luciferase expression (>1.5×108 and up to 3.8×1010 p/sec/cm2) 1 day postinfusion, and as previously observed there was a rapid loss of luciferase expression in animals administered pT2/CaL alone (Fig. 1c). Surprisingly, all animals co-infused with pCMV-SB100X maintained high levels of luciferase expression (>109 p/sec/cm2), without diminution from day 1 levels, which lasted for at least 4 weeks. Such high-level retention of luciferase expression after hydrodynamic plasmid delivery in mice has not been previously observed in our laboratory or elsewhere.
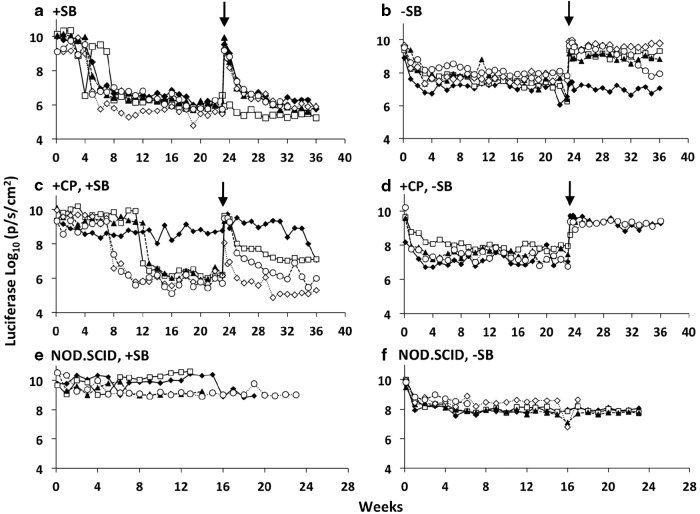
Each of the NOD.SCID animals treated with pT2/CaL plus pCMV-SB100X maintained a high level of luciferase expression for over 3 months (Fig. 2e). However, for most of the B6 mice treated with pT2/CaL plus SB100X, there was a sudden, precipitous loss of expression (to around 106 p/sec/cm2) that occurred, animal by animal, 4–7 weeks post-injection (Fig. 2a). This level of expression was 10- to 100-fold lower than that observed in animals injected with pT2/CaL alone. A similar effect was observed in four of the five CP-immunosuppressed animals hydrodynamically injected with pT2/CaL plus pCMV-SB100X, except that the sudden loss of luciferase expression was delayed until between 8 and 13 weeks post-injection (Fig. 2c). The remaining fifth animal had a sustained (out to 3 months) level of luciferase expression that was less than 109 p/sec/cm2. B6 mice that received pT2/CaL alone had sustained luciferase expression levels of around 108 p/sec/cm2 for 21 weeks (Fig. 2b and d), similar to the luciferase expression profiles seen in the NOD.SCID mice infused with pT2/CaL alone (Fig. 2f).
FIG. 2.
Sustained, high-level luciferase expression and resulting immune response. Luciferase activities (p/sec/cm2) are plotted for each of the individual animals from the same groups described in Fig. 1, shown here over the entire 9-month time course of the experiment. (a) C57BL/6 co-injected with pT2/CaL plus SB transposase-encoding plasmid pCMV-SB100X; (b) C57BL/6 injected with pT2/CaL alone; (c) CP immunosuppressed C57BL/6 co-injected with pT2/CaL plus pCMV-SB100X; (d) CP immunosuppressed C57BL/6 injected with pT2/CaL alone; (e) NOD.SCID co-injected with pT2/CaL plus pCMV-SB100X; (f) NOD.SCID injected with pT2/CaL alone. Each line represents an individual mouse, and each point indicates a specific luciferase reading. The black vertical arrow indicates the time at which C57BL/6 mice were challenged with 5 μg pT2/CaL plus 0.5 μg pCMV-SB100X by hydrodynamic injection.
These results indicated the likelihood of an immune response bringing about the loss of luciferase expression when there is sustained, high-level expression (above 109 p/sec/cm2) after hydrodynamic injection. To test this possibility, all B6 mice were challenged by hydrodynamic injection with 5 μg pT2/CaL plus 0.5 μg SB100X at week 23 after the initial infusion (arrow in Fig. 2a–d). All animals exhibited an initial burst of luciferase expression 1 day post-injection, but expression levels in the mice originally treated with SB100X fell off dramatically to around 107 p/sec/cm2 in less than 2 weeks (Fig. 2a). A similar effect was observed in animals originally immunosuppressed with CP (Fig. 2c), where luciferase expression levels in 3 out of 4 animals dropped to 107 p/sec/cm2 in 3 weeks or less. This rapid decline in luciferase expression immediately post-challenge is indicative of an immune response in mice that were initially infused with pT2/CaL plus pCMV-SB100X. In stark contrast, animals originally treated with luciferase transposon alone either with (Fig. 2d) or without (Fig. 2b) temporary CP immunosuppression showed high-level luciferase expression (3–4×109p/sec/cm2) immediately post-challenge that remained undiminished for the remaining 13 weeks in this experiment (i.e., far exceeding the time at which loss of expression was observed for naïve animals administered pT2/CaL plus pCMV-100X; shown in Fig. 2a). One possible explanation for these results is the induction of tolerance (or at least nonresponsiveness of immune cells) in animals expressing a moderate level of luciferase (∼108 p/sec/cm2) after the initial injection of pT2/CaL alone.
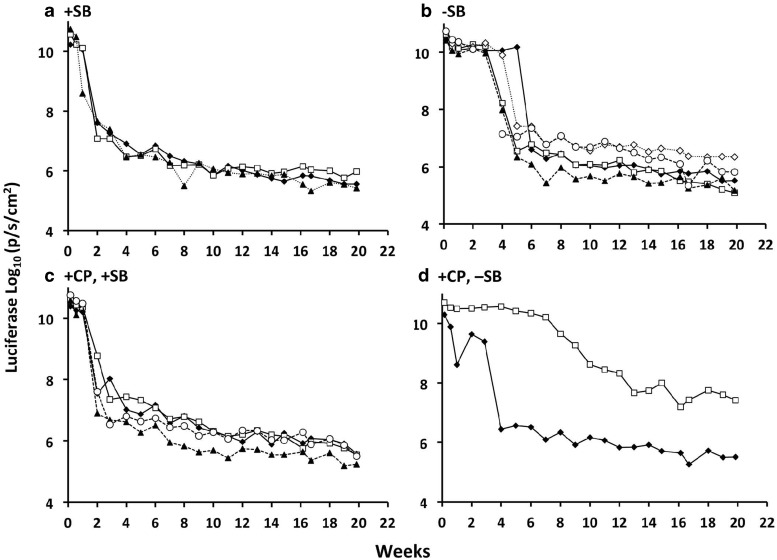
To further test for immune response, splenocytes were collected from all previously treated B6 mice and then adoptively transferred into naïve mice. The recipient animals were challenged the following day with 5 μg pT2/CaL plus 0.5 μg SB100X and then monitored for luciferase expression by in vivo bioluminescence imaging. All animals that received splenocytes from mice originally injected with pT2/CaL plus pCMV-SB100X displayed a rapid decline in luciferase expression from around 1010 p/sec/cm2 to∼1.7×108 p/sec/cm2 (+CP; Fig. 3a) and ∼3.7×107 p/sec/cm2 (−CP; Fig. 3c) in 14 days, and then a slower decline to less than 106 p/sec/cm2 by the end of the experiment at week 20. This dramatic decline is consistent with an immune response that was adoptively transferred in the splenocyte cell population, including memory cells. Animals that received splenocytes from mice originally treated with pT2/CaL alone exhibited sustained luciferase expression at levels above 1010 p/sec/cm2 for 4–6 weeks, but then one by one in five of the six animals there was a rapid drop in luciferase expression to around 106 p/sec/cm2 (Fig. 3b and d). These luciferase expression profiles are similar to those of animals originally injected with 5 μg pT2/CaL+0.5 μg SB100X in Fig. 2a, indicating a primary immune response in these animals. The adoptive transfer experiments thus provided evidence for an immune response, possibly against luciferase, causing the rapid decline in luciferase expression in animals hydrodynamically injected with pT2/CaL plus pCMV-SB100X (i.e., in Fig. 2a and c). However, there was no evidence for adoptive transfer of cells conferring tolerance from animals initially injected with pT2/CaL alone and expressing moderate (∼108 p/sec/cm2) levels of luciferase (i.e., in Fig. 2b and d).
FIG. 3.
Immune reactivity following adoptive transfer of splenocytes from previously treated immunocompetent animals. Splenocytes were harvested from each of the animals depicted in Fig. 2a–d and infused into naïve C57BL/6 mice. One day later, the recipient mice were hydrodynamically injected with 5 μg pT2/CaL plus 0.5 μg pCMV-SB100X. Each subfigure is titled according to the source of splenocytes used for each of the injections, that is, the original C57BL/6 treatment groups described in Fig. 1 and expression detailed for individual mice in Fig. 2. (a) C57BL/6 co-injected with pT2/CaL plus pCMV-SB100X (Fig. 2a). (b) C57BL/6 injected with pT2/CaL alone (Fig. 2b). (c) CP immunosuppressed C57BL/6 co-injected with pT2/CaL plus pCMV-SB100X (Fig. 2c). (d) CP immunosuppressed C57BL/6 injected with pT2/CaL alone (Fig. 2d). Each line represents an individual mouse. Luciferase expression (log10 of p/sec/cm2) is plotted over a period of 20 weeks.
Luciferase expression following multiple hydrodynamic injections
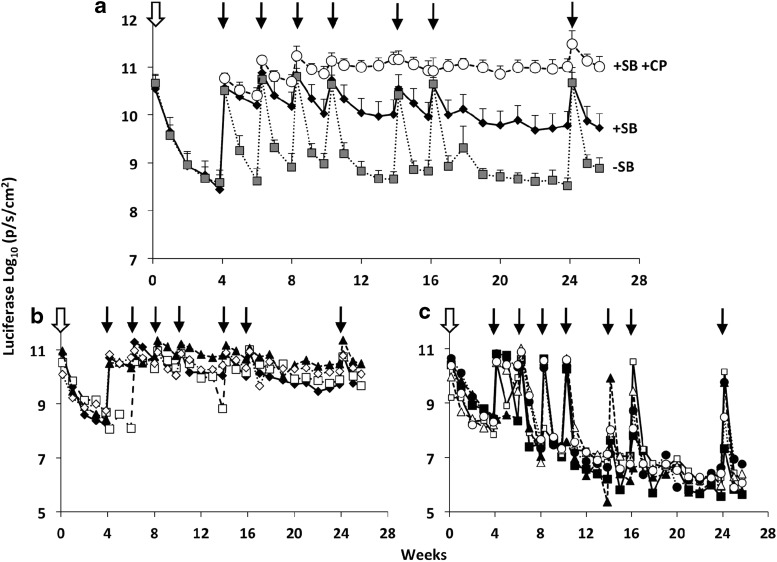
As described above, mice treated with pT2/CaL alone expressed sustained, moderate levels of luciferase (∼108 p/sec/cm2; Fig. 2b and d), but then undiminished, high levels of luciferase (>109 p/sec/cm2) postchallenge with pT2/CaL plus pCMV-SB100X. We hypothesized that these animals had become tolerized by sustained, moderate-level expression of luciferase. To more extensively test this induced tolerance, we designed an experiment in which similarly “tolerized” animals were challenged by multiple injections of pCMV-SB100X plus pT2/CaL transposon DNA. C57BL/6 mice were injected hydrodynamically with 5 μg pT2/CaL alone as a means of tolerizing the animals, and then starting on day 28 the mice were challenged with six rounds of hydrodynamic injection using 5 μg pT2/CaL plus 0.5 μg pCMV-SB100X over a 12-week period (+SB in Fig. 4a). A control group omitting pCMV-SB100X transposase was included (−SB in Fig. 4a), as well as an untolerized group (i.e., no initial pT2/CaL injection) that instead received continuous CP immunosuppression during repeated challenge with 5 μg pT2/CaL plus 0.5 μg SB100X starting on day 28. Control animals multiply treated with pT2/CaL alone expressed peak luciferase levels of around 1010 p/sec/cm2 after each hydrodynamic injection that consistently fell to a baseline of around 108 p/sec/cm2 after each infusion (Fig. 4a). The CP immunosuppressed animals expressed an initial mean baseline luciferase level of 5.7×1010 p/sec/cm2, and following subsequent rounds of hydrodynamic injections, there was a step-wise increase in the mean luciferase expression level to around 1×1011 p/sec/cm2 at week 24 (Fig. 4a).
FIG. 4.
Tolerizing effect of luciferase transposon alone against subsequent repetitive dosing with luciferase transposon plus transposase-encoding plasmid. C57BL/6 mice were hydrodynamically injected with 5 μg pT2/CaL alone to achieve tolerization (open arrow) to luciferase. (a) Starting on day 28, these animals were injected about every 2 weeks (black arrows) with 5 μg pT2/CaL with (closed diamonds, “ +SB”) or without (shaded squares, “−SB”) 0.5 μg pCMV-SB100X. A positive control group of CP-treated (cyclophosphamide was administered on days −1, 0, 1, and 2, and then weekly at a dose of 120 mg/kg for immunosuppression) C57BL/6 mice injected with pT2/CaL plus pCMV-SB100X was added at day 28. Data are presented as the mean luciferase activity (p/sec/cm2)±SD for each group. Mice that were tolerized with pT2/CaL alone and subsequently injected repeatedly with pT2/CaL plus pCMV-SB100X are also plotted individually to emphasize (b) those animals maintaining high levels of luciferase expression (>109 p/sec/cm2) and (c) those animals exhibiting a dramatic reduction of luciferase expression (<107 p/sec/cm2).
Interestingly, based on outcome the tolerized animals multiply injected with pT2/CaL plus pCMV-SB100X split into two groups; 4 out of 10 animals maintained high levels of baseline luciferase expression (above 109 p/sec/cm2; Fig. 4b), supporting the establishment of tolerance in these four animals. In the remaining six animals, there was a progressive loss of expression to a baseline below 107 p/sec/cm2 (Fig. 4c), most likely because of the development of an immune response. There was also a 10-fold reduction in luciferase sequences detected by Q-PCR in the livers of animals exhibiting a loss of luciferase expression in comparison with those animals in which luciferase expression was maintained (Fig. 5). Hydrodynamic injection of pT2/CaL alone thus induced a tolerance-like state in some animals that prevented immune response during repeated challenge with pT2/CaL plus pCMV-SB100X. However, in some animals either this tolerance-like state was not induced or it was broken upon challenge with pT2/CaL plus pCMV-SB100X. Alternatively, the difference in levels of sustained luciferase expression shown in Fig. 4b and c could be because of differential potency of cytotoxic T cells generated in different multiply injected animals. A stepwise increase in luciferase expression upon multiple injections with pT2/CaL plus pCMV-SB100X was only observed when animals were immunosuppressed with cyclophosphamide.
FIG. 5.
Effect of anti-luciferase immune response on luciferase gene copy frequency. DNA was extracted from hepatocytes (open symbols) or whole liver (closed symbols) of individual animals depicted in Fig. 4a–c. The frequency of luciferase sequences was determined by real-time quantitative PCR as described in Materials and Methods.
Specific cellular immune response to luciferase
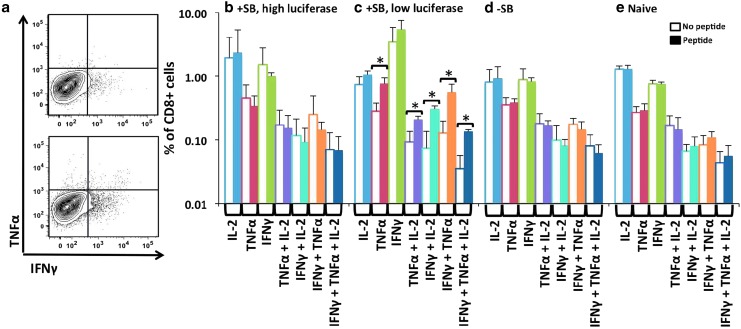
To test for an immune response against luciferase, splenocytes and mononuclear liver cells were harvested and assayed for (1) cytokine expression following stimulation with luciferase peptide by intracellular cytokine staining (ICCS), and (2) MHC I–luciferase peptide tetramer binding with cell surface phenotyping. These experiments were based on previously published results characterizing the dominant, CD8 T cell–specific immunoreactive firefly luciferase epitope in C57BL/6 mice, LMYRFEEEL (Limberis et al., 2009). To achieve an ongoing, robust immune response, the animals that had multiple injections of pT2/CaL with or without pCMV-SB100X depicted in Fig. 4a were given an additional set of injections on week 24 (2 weeks before sacrifice). Splenocytes and mononuclear liver lymphocytes were collected and stimulated with LMYRFEEEL luciferase peptide, and ICCS was performed for IFNγ, TNFα, and IL-2 expression (see Materials and Methods). For splenocytes from naïve animals (Fig. 6e), animals treated with pT2/CaL alone (−SB; Fig. 6d), or animals treated with pT2/CaL plus SB100X expressing greater than 109 p/sec/cm2 luciferase (Fig. 6b), exposure to luciferase peptide had no discernable effect on IFNγ, TNFα, and IL-2 production by CD8+ T cells. However, CD8+ T cells from SB100X-treated mice expressing <107 p/sec/cm2 luciferase responded to luciferase peptide exposure with a significant increase in IFNγ, TNFα, and IL-2 expression in comparison with unstimulated controls (p<0.01 for TNFα +, TNFα+IL-2+, IFNγ+IL-2+, IFNγ+TNFα+, and IFNγ+TNFα+IL-2+ cells; Fig. 6c). Figure 6a shows an example of the difference in IFNγ and TNFα antibody staining of CD8+T cells from a mouse expressing <107 p/sec/cm2 luciferase with and without exposure to luciferase peptide. For the liver lymphocytes from all groups, there was no effect of peptide exposure on cytokine expression (data not shown).
FIG. 6.
Specific luciferase peptide responsiveness by intracellular cytokine staining. Splenocytes were prepared from test animals (individual animals from Fig. 4a, groups −SB and +SB), stimulated with luciferase peptide (LMYRFEEEL), and assayed for responsive cytokine expression by intracellular staining as described in Materials and Methods. (a) ICCS of CD8+ lymphocytes for expression of IFNγ and TNFα after incubation with luciferase peptide (bottom) or without luciferase peptide (top). In (b–e), the percentage of CD8+ T cells staining positive for IL-2, IFNγ, and TNFα expression is shown with (shaded bars) and without (open bars) exposure to luciferase peptide. (b) Splenocytes from animals that had multiple injections of pT2/CaL plus pCMV-SB100X and maintaining high levels (>109 p/sec/cm2) of luciferase expression. (c) Splenocytes from animals that had multiple injections of pT2/CaL plus pCMV-SB100X and exhibiting a dramatic reduction (<107 p/sec/cm2) in luciferase expression. (d) Splenocytes from animals that had multiple injections of pT2/CaL alone. (e) Splenocytes from untreated naive mice. *p<0.01. Color images available online at www.liebertpub.com/hum
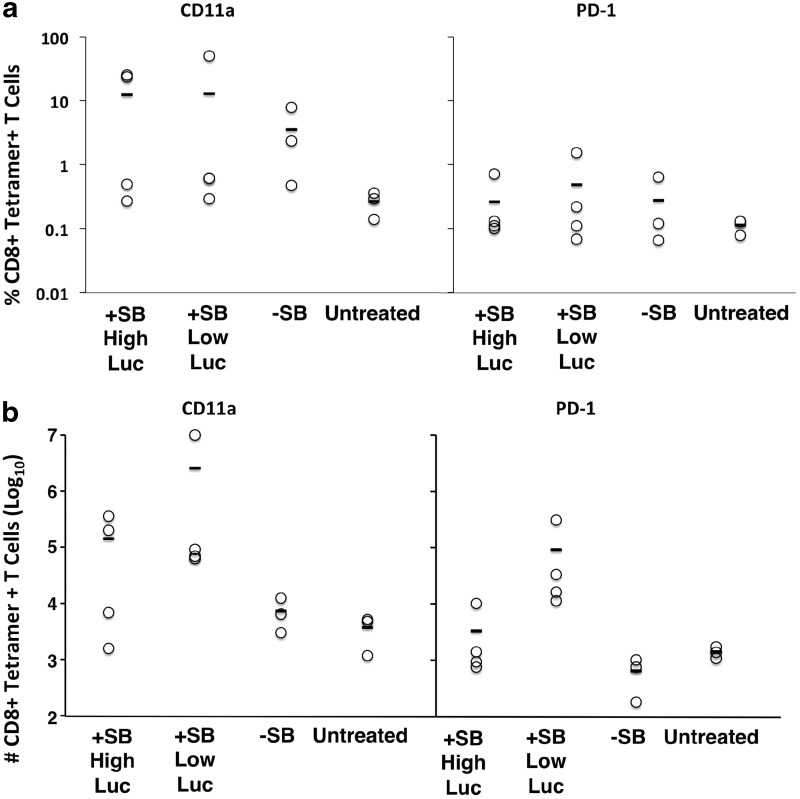
In parallel with ICCS, the same splenocyte and liver lymphocyte preparations were stained with LMYRFEEEL-MHC class I tetramer, costaining for CD8α and T-cell activation markers CD11a and PD-1. Several animals with a large proportion of tetramer-positive CD8+ liver lymphocytes were identified in each of the three treatment groups (Supplementary Figs S1 and S2; Supplementary Data are available online at www.liebertpub.com/hum). The percentage of CD11a+ CD8+ MHC I–luciferase peptide tetramer binding T cells was highest in liver lymphocytes from SB100X-treated mice compared with the other groups (Fig. 7a). SB100X-treated animals in which luciferase expression had fallen below 107 p/sec/cm2 had the highest number of CD11a+ or PD-1+CD8+ MHC I–luciferase peptide tetramer binding T cells (Fig. 7b). SB100X-treated animals expressing high levels of luciferase (above 109 p/sec/cm2) also exhibited an increase in the number of luciferase peptide-binding CD8+ T cells in the liver. The total number of CD8+ T cells extracted from the liver was highest for SB100X-treated animals expressing low levels of luciferase (1.7×107 cells) in comparison, for example, to the naïve mouse group (1.3×106 cells). There was no significant difference in MHC I–luciferase peptide tetramer binding between CD11a+ or PD-1+ splenocytes (data not shown).
FIG. 7.
MHC I–luciferase peptide tetramer binding of CD8+ T cells. Liver lymphocytes were prepared from test animals (same individuals shown in Fig. 6, tested in parallel) and stained with anti-CD8α, anti-CD11a, anti-PD-1, and luciferase peptide–MHC I tetramer as described in Materials and Methods. (a) The percentage of CD8+ MHC I–luciferase peptide tetramer+liver cell populations±SD staining positive for CD11a (left) and PD-1 (right). (b) The mean number±SD of CD8+ cells staining positive for MHC I–luciferase peptide tetramer is shown for CD11a+ (left) and PD-1+(right) cell populations from liver. Treatment groups are detailed in the legend to Fig. 4.
The increase in IFNγ, TNFα, and IL-2 expression observed by intracellular staining of splenocytes after exposure to luciferase peptide plus the specific binding of MHC I–luciferase peptide tetramer to activated liver lymphocytes provides definitive evidence for a cytotoxic T cell response against luciferase in animals infused with pT2/CaL plus SB100X, resulting in the rapid loss of luciferase expression from around 1010 p/sec/cm2 to less than 107 p/sec/cm2.
Discussion
Hydrodynamic delivery of an SB transposon expressing the firefly luciferase gene resulted in high levels (>1010 p/sec/cm2) of long-term reporter gene expression when co-infused with a CMV-regulated plasmid encoding the hyperactive SB100X SB transposase. These results corroborate those reported by Mates et al. (2009), and characterize in greater detail the time course of expression achieved in the liver under these conditions of delivery. Following most infusions, there was less than a 10-fold reduction in the long-term luciferase expression level in comparison with the peak level observed 1 day postinfusion, most likely attributable to the efficiency of the transposition process. These extended, high levels of luciferase expression resulted in an unexpected consequence: an immune response evidenced by a dramatic decrease in luciferase expression to low levels (below 107 p/sec/cm2). This loss of expression was not observed in similarly treated immunodeficient and immunosuppressed animals. Luciferase levels also rapidly plummeted following pT2/CaL plus pCMV-SB100X challenge of immunoresponsive mice and mice adoptively transferred with splenocytes from immunoresponsive animals, indicating a cellular-based immune response. A specific anti-luciferase, CD8 T cell-mediated immune response was characterized by cytokine expression in response to luciferase peptide stimulation, in parallel with MHC I–luciferase peptide tetramer binding.
While these experiments demonstrated an immune response specific to firefly luciferase, this does not exclude the possibility of other antigens contributing the observed loss of expression, including SB transposase. However, a rapid decline in luciferase expression was not observed in animals infused with pT2/CaL alone and subsequently challenged with transposon plus pCMV-SB100X (Fig. 2b and d). Furthermore, the animals depicted in Fig. 2c were immunosuppressed with CP at the time of pCMV-SB100X hydrodynamic delivery and transient SB transposase expression. Bell et al. (2010) showed that the SB protein was undetectable by Western blot by 2 weeks postdelivery and that SB transposase mRNA levels could only support transposition for the first 4 days after hydrodynamic delivery (Bell et al., 2010). We nonetheless observed loss of luciferase expression in the CP immunosuppressed animals, although at a 1-month delay (Fig. 2c), and animals adoptively transferred with splenocytes from the CP-immunosuppressed animals (Fig. 3c) exhibited a loss of luciferase expression that was just as rapid as animals adoptively transferred with splenocytes from animals that were not CP immunosuppressed (Fig. 3a). This argues for an anti-luciferase immune response as the explanation for the observed loss of luciferase activity. Immune responses following hydrodynamic delivery of the SB transposon system have also been reported for other transgenes, such as human factor VIII (Ohlfest et al., 2005) and human alpha-L-iduronidase (IDUA) (Aronovich et al., 2007).
Interestingly, animals hydrodynamically injected with pT2/CaL transposon alone did not exhibit an anti-luciferase immune response when challenged later with transposon plus pCMV-SB100X (Fig. 2). Additionally, several animals injected with pT2/CaL alone maintained a high level of luciferase expression even after multiple subsequent challenges with transposon plus pCMV-SB100X (Fig. 4a and b). We hypothesized that these animals had been tolerized by persistent, moderate-level luciferase expression (107–108 p/sec/cm2). This tolerance-like effect was not adoptively transferred through splenocytes from tolerized animals into naïve mice (Fig. 3). It is possible that in animals expressing a moderate level of luciferase, MHC molecules on antigen presenting cells present luciferase peptide to T cells by engagement of the T-cell receptor, but in the absence of costimulation these T cells become nonfunctional or anergic (Srinivasan and Frauwirth, 2009). We observed that liver lymphocytes from animals treated with multiple rounds of pT2/CaL plus pCMV-SB100X exhibited considerable luciferase-MHC I-tetramer binding to CD8+CD11a+ T cells. The animals sustaining high levels of luciferase expression had a lower amount of luciferase-MHC I-tetramer binding, which is consistent with the presence of nonresponsive T cells, whereas the animals with low luciferase expression (i.e., undergoing an active immune response against luciferase) had a higher number of cells with MHC I–luciferase peptide tetramer binding.
The liver has been reported to have unique immunologic character with a bias toward tolerance (Knolle and Limmer, 2001; Tiegs and Lohse, 2010; Crispe, 2011), owing to its constant exposure to foreign and intestinal antigens (Tiegs and Lohse, 2010). This tolerance bias is exemplified by successful liver transplantation across incompatible MHC barriers without immunosuppression (Doherty and O'Farrelly, 2001). In the gene therapy field, tolerance has been induced by neonatal delivery of the transgene product before gene transfer. Olhfest et al. (2005) were able to phenotypically correct murine hemophilia A using the SB transposon system, but to achieve long-term expression it was necessary to immunotolerize the animals shortly after birth by intravenous injection of human factor VIII protein. Murine hemophilia A was also corrected using the SB transposon system by delivery of polyethyleneimine complexes into neonatal mice, at a time when the animals are immunologically naïve (Liu et al., 2006). Matrai et al. (2009) induced tolerance by gene transfer to the liver of adult mice using integrase-defective lentiviral vectors containing a hepatocyte-specific promoter. Transgene expression was limited to hepatocytes rather than other liver cells, such as liver APCs, thus avoiding immune response to the transgene product (Matrai et al., 2009).
We observed what appears to be a threshold level of luciferase expression necessary for induction of anti-luciferase immune response. Immune response was evident only in those animals that maintained high levels of luciferase expression (above 109 p/sec/cm2), while there was no immune response seen in animals that expressed moderate levels of luciferase (107–108 p/sec/cm2). These results indicate a threshold of around 109 p/sec/cm2 luciferase expression to elicit an anti-luciferase immune response. One mouse in Fig. 2c (indicated by filled diamonds) serves to illustrate this threshold effect. This animal maintained a luciferase expression level of around 109 p/sec/cm2, starting at 1.9×109 p/sec/cm2 and ending at 6×108 p/sec/cm2 before challenge, but this level of expression was insufficient to elicit an immune response. Wilber et al. (2007) also reported high levels of sustained luciferase expression in conjunction with an in vivo selection strategy in FAH-deficient mice using an FAH/luciferase transposon plus SB11 transposase. In that study, the FAH mice may have become tolerized to luciferase, since there was an initial peak of luciferase expression followed by a rapid drop and then a gradual increase in expression to above 109 p/sec/cm2 (Wilber et al., 2007).
Firefly luciferase is an extremely useful reporter, particularly with the development of in vivo bioluminescence imaging techniques (Wilber et al., 2005). Assays for the enzyme are extremely sensitive, providing a broad range of quantifiable detection. The effectiveness of luciferase as an in vivo reporter is clearly impacted by its immunogenicity, as we observed in immunocompetent animals expressing sustained, high levels of the enzyme. Development of antiluciferase immunity could affect its usefulness to track in vivo expression in normal tissues as well as the growth and regression of tumors that have been engineered for luciferase expression. For example, Jeon and coworkers observed growth inhibition of CT26/FLuc (murine CT26 colorectal tumor engineered for stable expression of luciferase) when mice were immunized with naked plasmid DNA expressing luciferase (Jeon et al., 2007; Su et al., 2011). On the other hand, immunodeficient animals are commonly used as a test system for growth of human tumor xenografts, and immunocompetent animals may become tolerized when a minimal luciferase-positive tumor implant increases in size and luciferase expression level over time (Hwang et al., 2011; Mordant et al., 2011; Su et al., 2011). Our observation that a minimum, sustained expression level of at least 109 p/sec/cm2 was necessary to elicit an immune response, and that animals not achieving this level in fact became tolerized, implies that immune response may not be problematic for experiments not achieving this threshold level of expression (Belur et al., 2003, 2011; Wilber et al., 2005; Podetz-Pedersen et al., 2010; Hwang et al., 2011; Mordant et al., 2011; Su et al., 2011).
In conclusion, we found that use of SB100X transposase resulted in surprisingly high levels of long-term luciferase expression after hydrodynamic delivery of luciferase-encoding SB transposon DNA. Interestingly, this high level of sustained luciferase expression induced a CD8 T cell response against luciferase, accompanied by a dramatic reduction in expression. These results illustrate the relationship between high levels of maintained gene expression, mediated by SB100X transposase in this case, and the induction of a cellular immune response as well as induction of tolerance to the gene product in some animals. Luciferase remains an exceptionally useful reporter, and the associated immune response characterized here broadens its potential application. As a system for preclinical testing of immune responses to transgene products, luciferase is benefited by previous characterization of dominant immunogenic epitopes in C57BL/6 and Balb/c mice (Limberis et al., 2009). Conditions that elicit immune response or tolerance, such as those identified in this article, may then serve as a model to address the problem of immune responses to therapeutic transgene products, one of the major challenges currently facing the gene therapy field.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the formative role of Dr. David Schowalter in the inception of this project. This work was supported by the Minnesota Partnership for Biotechnology and Medical Genomics.
Author Disclosure Statement
R.S.M. is a founder and manager of Discovery Genomics, Inc., a company that is commercially developing the Sleeping Beauty transposon system. S.J.R. is a founder and manager of Imanis Life Sciences LLC, a company that is commercially developing reporter gene technologies. For all other authors, no competing financial interests exist.
References
- Altman J.D., Moss P.A., Goulder P.J., et al. (1996). Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–968810254 [Google Scholar]
- Aronovich E.L., Bell J.B., Belur L.R., et al. (2007). Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J. Gene Med. 9, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baus J., Liu L., Heggestad A.D., et al. (2005). Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol. Ther. 12, 1148–1156 [DOI] [PubMed] [Google Scholar]
- Bell J.B., Podetz-Pedersen K.M., Aronovich E.L., et al. (2007). Preferential delivery of the Sleeping Beauty transposon system to livers of mice by hydrodynamic injection. Nat. Protoc. 2, 3153–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J.B., Aronovich E.L., Schreifels J.M., et al. (2010). Duration of expression and activity of Sleeping Beauty transposase in mouse liver following hydrodynamic DNA delivery. Mol. Ther. 18, 1796–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belur L.R., Frandsen J.L., Dupuy A.J., et al. (2003). Gene insertion and long-term expression in lung mediated by the Sleeping Beauty transposon system. Mol. Ther. 8, 501–507 [DOI] [PubMed] [Google Scholar]
- Belur L.R., McIvor R.S., and Wilber A. (2008). Liver-directed gene therapy using the Sleeping Beauty transposon system. Methods Mol. Biol. 434, 267–276 [DOI] [PubMed] [Google Scholar]
- Belur L.R., Podetz-Pedersen K.M., Sorenson B.S., et al. (2011). Inhibition of angiogenesis and suppression of colorectal cancer metastatic to the liver using the Sleeping Beauty transposon system. Mol. Cancer 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I.N. (2011). Liver antigen-presenting cells. J. Hepatol. 54, 357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D.G., and O'Farrelly C. (2001). Dendritic cells: regulators of hepatic immunity or tolerance? J. Hepatol. 34, 156–160 [DOI] [PubMed] [Google Scholar]
- Geurts A.M., Yang Y., Clark K.J., et al. (2003). Gene transfer into genomes of human cells by the Sleeping Beauty transposon system. Mol. Ther. 8, 108–117 [DOI] [PubMed] [Google Scholar]
- Hwang J.E., Shim H.J., Park Y.K., et al. (2011). Intravenous KITENIN shRNA injection suppresses hepatic metastasis and recurrence of colon cancer in an orthotopic mouse model. J. Korean Med. Sci. 26, 1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z., Hackett P.B., Plasterk R.H., and Izsvak Z. (1997). Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91, 501–510 [DOI] [PubMed] [Google Scholar]
- Izsvak Z., Hackett P.B., Cooper L.J., and Ivics Z. (2010). Translating Sleeping Beauty transposition into cellular therapies: victories and challenges. Bioessays 32, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.H., Choi Y., Kang J.H., et al. (2007). Immune response to firefly luciferase as a naked DNA. Cancer Biol. Ther. 6, 781–786 [DOI] [PubMed] [Google Scholar]
- Knolle P.A., and Limmer A. (2001). Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 22, 432–437 [DOI] [PubMed] [Google Scholar]
- Limberis M.P., Bell C.L., and Wilson J.M. (2009). Identification of the murine firefly luciferase-specific CD8 T-cell epitopes. Gene Ther. 16, 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Song Y., and Liu D. (1999). Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 [DOI] [PubMed] [Google Scholar]
- Liu L., Mah C., and Fletcher B.S. (2006). Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted Sleeping Beauty transposon. Mol. Ther. 13, 1006–1015 [DOI] [PubMed] [Google Scholar]
- Manno C.S., Pierce G.F., Arruda V.R., et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 [DOI] [PubMed] [Google Scholar]
- Mates L., Chuah M.K., Belay E., et al. (2009). Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 [DOI] [PubMed] [Google Scholar]
- Matrai J., Cantore A., Bartholomae C.C., et al. (2011). Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 53, 1696–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F., and High K.A. (2007). Immune responses to AAV in clinical trials. Curr. Gene Ther. 7, 316–324 [DOI] [PubMed] [Google Scholar]
- Montini E., Held P.K., Noll M., et al. (2002). In vivo correction of murine tyrosinemia type I by DNA-mediated transposition. Mol. Ther. 6, 759–769 [DOI] [PubMed] [Google Scholar]
- Mordant P., Loriot Y., Lahon B., et al. (2011). Bioluminescent orthotopic mouse models of human localized non-small cell lung cancer: feasibility and identification of circulating tumour cells. PLoS One 6, e26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve D.A. (2004). The innate immune response to adenovirus vectors. Hum. Gene Ther. 15, 1157–1166 [DOI] [PubMed] [Google Scholar]
- Ni J., Clark K.J., Fahrenkrug S.C., and Ekker S.C. (2008). Transposon tools hopping in vertebrates. Brief Funct. Genomic Proteomic 7, 444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlfest J.R., Frandsen J.L., Fritz S., et al. (2005). Phenotypic correction and long-term expression of factor VIII in hemophilic mice by immunotolerization and nonviral gene transfer using the Sleeping Beauty transposon system. Blood 105, 2691–2698 [DOI] [PubMed] [Google Scholar]
- Podetz-Pedersen K.M., Bell J.B., Steele T.W., et al. (2010). Gene expression in lung and liver after intravenous infusion of polyethylenimine complexes of Sleeping Beauty transposons. Hum. Gene Ther. 21, 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper S.E., Yudkoff M., Chirmule N., et al. (2002). A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 13, 163–175 [DOI] [PubMed] [Google Scholar]
- Raper S.E., Chirmule N., Lee F.S., et al. (2003). Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158 [DOI] [PubMed] [Google Scholar]
- Sims S., Willberg C., and Klenerman P. (2010). MHC-peptide tetramers for the analysis of antigen-specific T cells. Expert Rev. Vaccines 9, 765–774 [DOI] [PubMed] [Google Scholar]
- Srinivasan M., and Frauwirth K.A. (2009). Peripheral tolerance in CD8+ T cells. Cytokine 46, 147–159 [DOI] [PubMed] [Google Scholar]
- Su W., Zhou M., Zheng Y., et al. (2011). Bioluminescence reporter gene imaging characterize human embryonic stem cell-derived teratoma formation. J. Cell. Biochem. 112, 840–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G., and Lohse A.W. (2010). Immune tolerance: what is unique about the liver. J. Autoimmun. 34, 1–6 [DOI] [PubMed] [Google Scholar]
- Wilber A., Frandsen J.L., Wangensteen K.J., et al. (2005). Dynamic gene expression after systemic delivery of plasmid DNA as determined by in vivo bioluminescence imaging. Hum. Gene Ther. 16, 1325–1332 [DOI] [PubMed] [Google Scholar]
- Wilber A., Wangensteen K.J., Chen Y., et al. (2007). Messenger RNA as a source of transposase for Sleeping Beauty transposon-mediated correction of hereditary tyrosinemia type I. Mol. Ther. 15, 1280–1287 [DOI] [PubMed] [Google Scholar]
- Xue X., Huang X., Nodland S.E., et al. (2009). Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system. Blood 114, 1319–1330 [DOI] [PubMed] [Google Scholar]
- Yant S.R., Meuse L., Chiu W., et al. (2000). Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat. Genet. 25, 35–41 [DOI] [PubMed] [Google Scholar]
- Yant S.R., Park J., Huang Y., et al. (2004). Mutational analysis of the N-terminal DNA-binding domain of Sleeping Beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells. Mol. Cell Biol. 24, 9239–9247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed H., Izsvak Z., Walisko O., and Ivics Z. (2004). Development of hyperactive Sleeping Beauty transposon vectors by mutational analysis. Mol. Ther. 9, 292–304 [DOI] [PubMed] [Google Scholar]
- Zhang G., Budker V., and Wolff J.A. (1999). High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 10, 1735–1737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.