Abstract
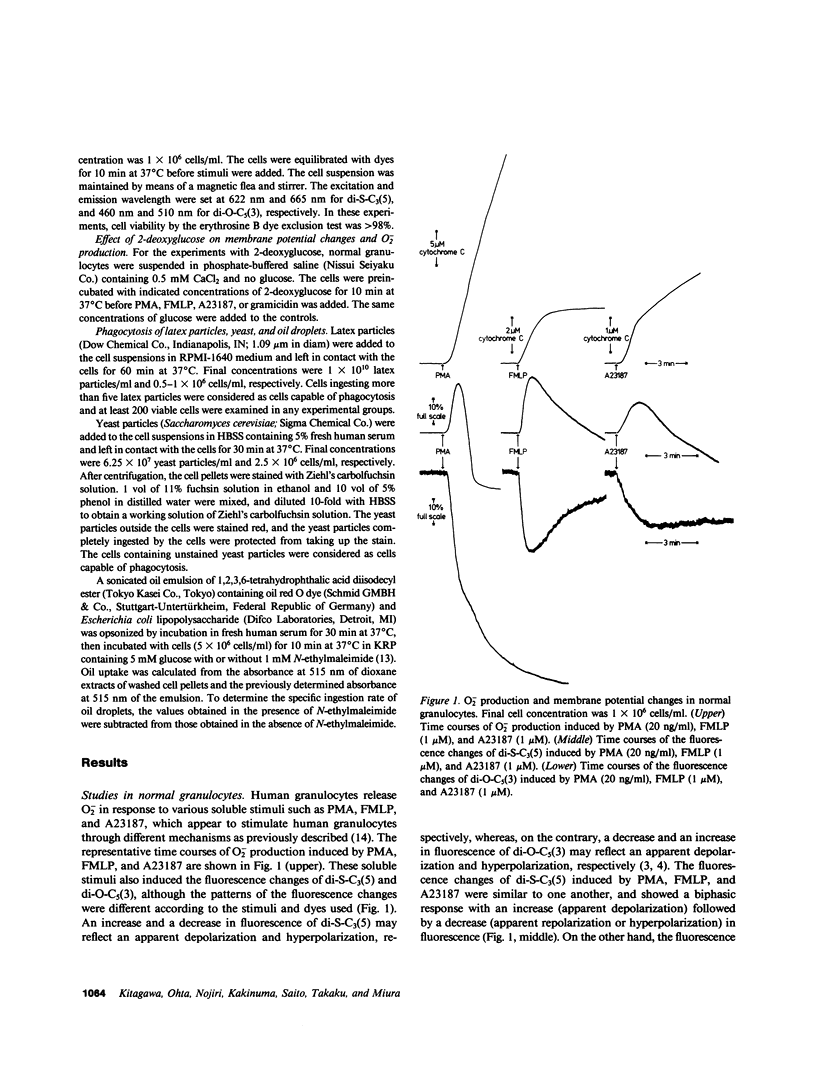
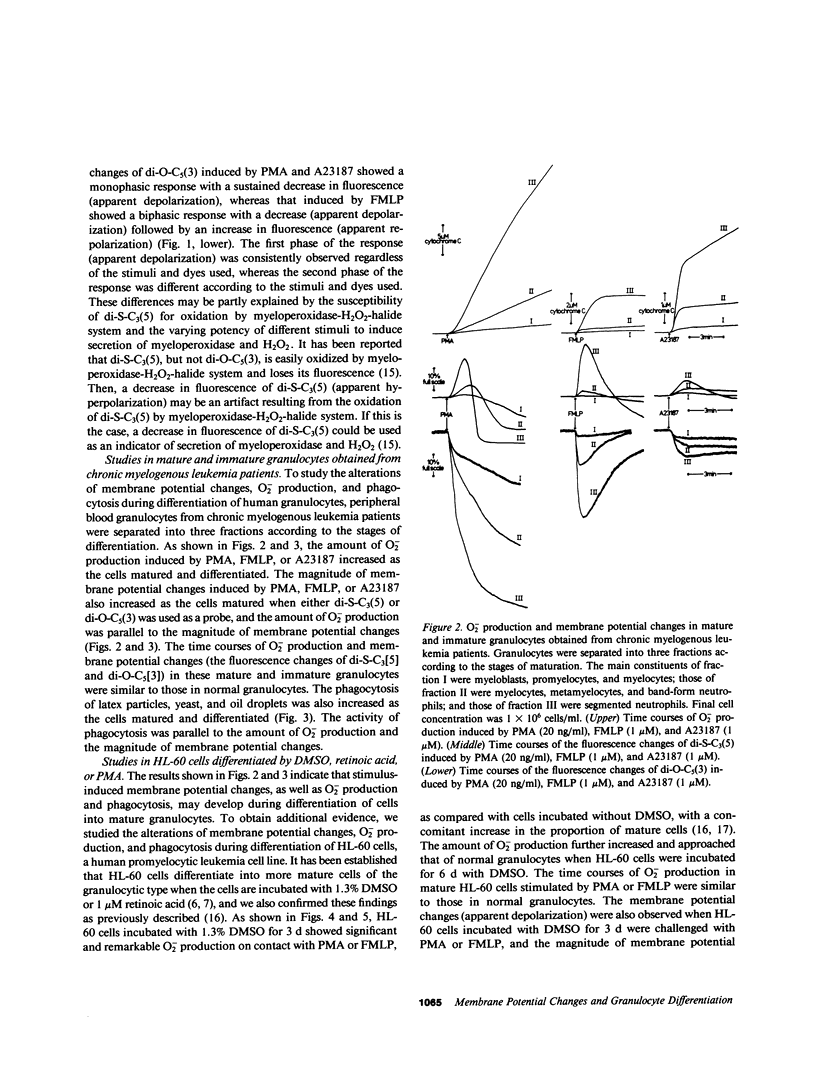
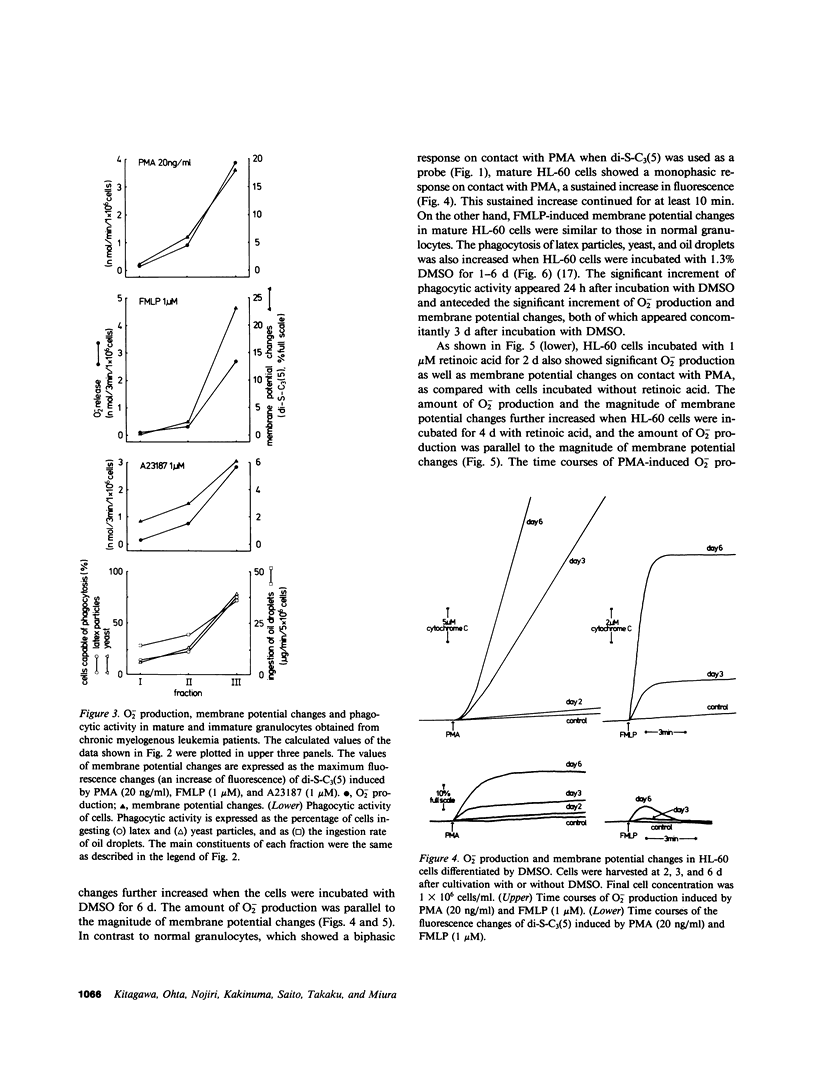
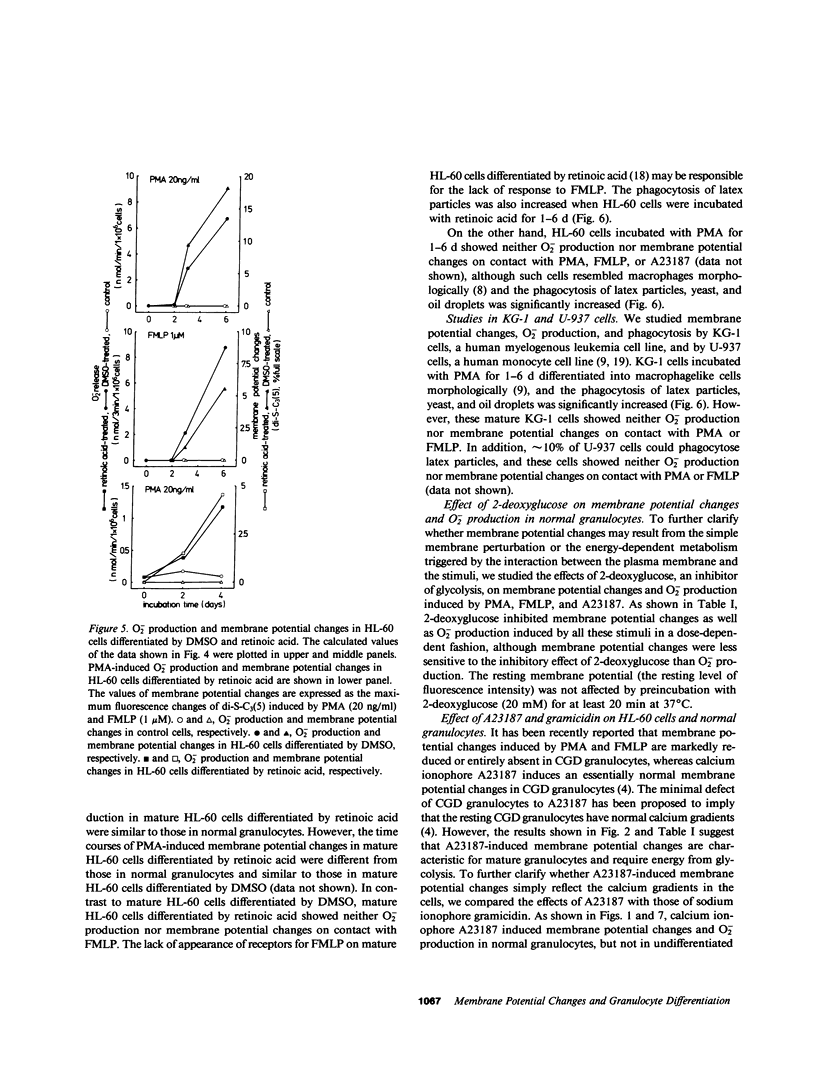
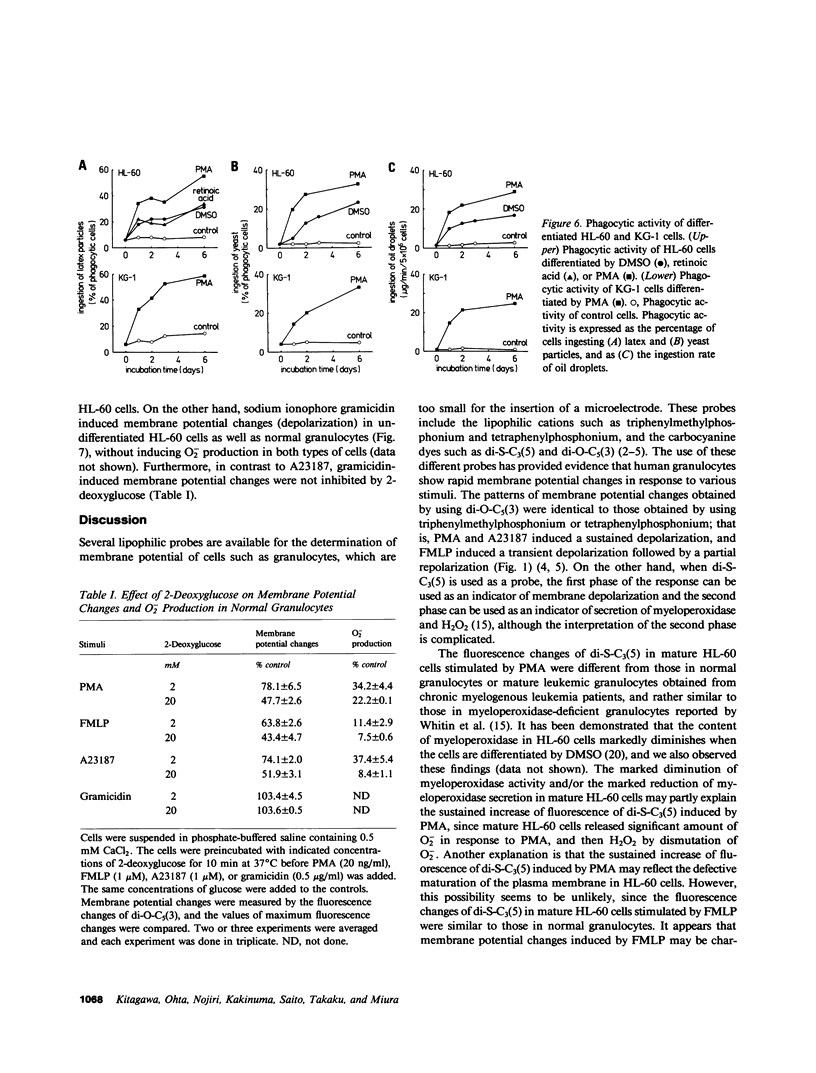
The alterations of stimulus-induced membrane potential changes, superoxide (O2-)-producing capacity and phagocytic activity during differentiation of human granulocytes were investigated in the human leukemia cell lines HL-60 and KG-1 differentiating in vitro and in human leukemic granulocytes obtained from chronic myelogenous leukemia patients. HL-60 cells incubated with dimethyl sulfoxide or with retinoic acid showed progressively increasing O2- production as well as membrane potential changes (depolarization) on contact with phorbol myristate acetate or the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine, with a concomitant increase in the proportion of mature cells of the granulocytic type. Phagocytosis of latex particles, yeast, and oil droplets appeared 24 h after incubation with dimethyl sulfoxide and anteceded the increment of O2- production and membrane potential changes, both of which appeared concomitantly 3 d after incubation with dimethyl sulfoxide. Similar findings were observed when immature and mature granulocytes obtained from chronic myelogenous leukemia patients were stimulated by phorbol ester, the chemotactic peptide, or calcium ionophore A23187, and the amount of O2- production was parallel to the magnitude of membrane potential changes. HL-60 and KG-1 cells incubated for 1-6 d with phorbol myristate acetate showed neither O2- production nor membrane potential changes on contact with phorbol ester, chemotactic peptide, or A23187, although such cells resembled macrophages morphologically, and their phagocytic activity was significantly increased. O2- production and membrane potential changes in normal granulocytes induced by phorbol ester, chemotactic peptide and A23187 were inhibited by 2-deoxyglucose. These findings indicate that the O2--producing system and the system provoking membrane potential changes may develop concomitantly as human granulocytes mature and differentiate, and that the development of these systems and of phagocytic activity may be independently regulated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Peters W. A. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981 Mar 10;256(5):2321–2323. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K., Gallin J. I. Interaction of chemotactic factors with human macrophages. Induction of transmembrane potential changes. J Cell Biol. 1977 Oct;75(1):277–289. doi: 10.1083/jcb.75.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F. Effect of microtubule-disrupting agents on superoxide production in human polymorphonuclear leukocytes. Biochim Biophys Acta. 1982 Dec 17;719(3):589–598. doi: 10.1016/0304-4165(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. A comparison of the superoxide-releasing response in human polymorphonuclear leukocytes and monocytes. J Immunol. 1980 Jul;125(1):359–364. [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Evidence that proteases are involved in superoxide production by human polymorphonuclear leukocytes and monocytes. J Clin Invest. 1980 Jan;65(1):74–81. doi: 10.1172/JCI109662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Serine protease inhibitors inhibit superoxide production by human polymorphonuclear leukocytes and monocytes stimulated by various surface active agents. FEBS Lett. 1979 Nov 15;107(2):331–334. doi: 10.1016/0014-5793(79)80401-9. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Bar-Eli M., Territo M. C. Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res. 1981 Mar;41(3):919–926. [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola C., Romeo D. Calcium movement and membrane potential changes in the early phase of neutrophil activation by phorbol myristate acetate: a study with ion-selective electrodes. J Cell Biol. 1982 Apr;93(1):129–134. doi: 10.1083/jcb.93.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Baker R. D., Hansen S. L., Duncan R. A., Greenberger J. S. Functionally deficient differentiation of HL-60 promyelocytic leukemia cells induced by phorbol myristate acetate. Cancer Res. 1981 May;41(5):1861–1865. [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979 Aug;82(2):315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojiri N., Takaku F., Tetsuka T., Saito M. Stimulation of sialidase activity during cell differentiation of human promyelocytic leukemia cell line HL-60. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1239–1246. doi: 10.1016/0006-291x(82)91383-3. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T. Induction of differentiation in a human promyelocytic leukemic cell line (HL-60). Production of granule proteins. Exp Cell Res. 1981 Jan;131(1):225–230. doi: 10.1016/0014-4827(81)90422-5. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Cross A. R., Jones O. T., Segal A. W. Development of cytochrome b and an active oxidase system in association with maturation of a human promyelocytic (HL-60) cell line. J Cell Biol. 1982 Dec;95(3):720–726. doi: 10.1083/jcb.95.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature. 1978 Nov 30;276(5687):515–517. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz K. M., Zhen Y. S., August J. T. Dexamethasone synergistically induces chemotactic peptide receptor expression in HL-60 cells. Blood. 1982 Mar;59(3):586–593. [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Alper C. A., Rosen F. S. Serum-dependent phagocytosis of paraffin oil emulsified with bacterial lipopolysaccharide. J Exp Med. 1973 Mar 1;137(3):690–705. doi: 10.1084/jem.137.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Whitin J. C., Clark R. A., Simons E. R., Cohen H. J. Effects of the myeloperoxidase system on fluorescent probes of granulocyte membrane potential. J Biol Chem. 1981 Sep 10;256(17):8904–8906. [PubMed] [Google Scholar]
- Williams J. A. Electrical correlates of secretion in endocrine and exocrine cells. Fed Proc. 1981 Feb;40(2):128–134. [PubMed] [Google Scholar]


