Abstract
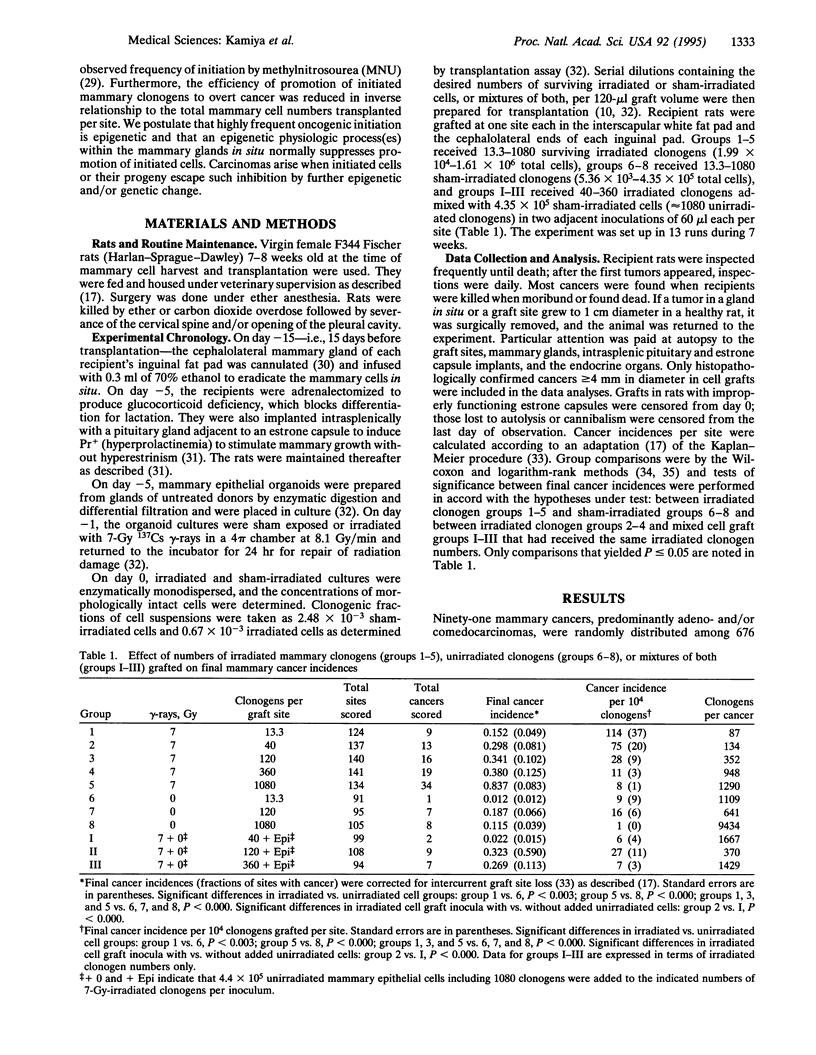
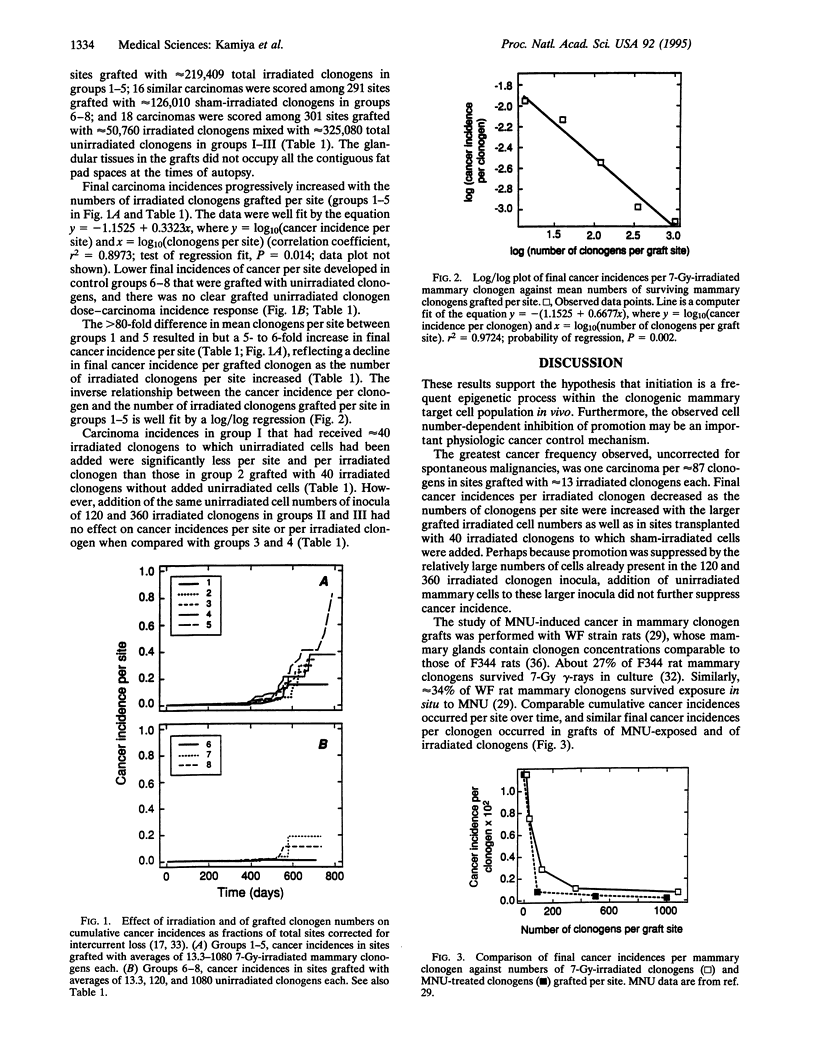
Evidence is presented in support of the hypothesis that cancer development depends on an imbalance between highly frequent epigenetic initiation and suppression of promotion of the initiated cells. When irradiated clonogenic mammary epithelial cells are transplanted and hormonally stimulated, they give rise to clonal glandular structures within which carcinomas may arise. In the current study, the cancer incidence in grafts of approximately 13 7-Gy-irradiated clonogens per site indicated that at least 1 of approximately 95 clonogens was radiogenically initiated. A similar initiation frequency had been seen in grafts of approximately 5 methylnitrosourea (MNU)-treated clonogens. Such initiation is thus far more frequent than specific locus mutations. In sites grafted with larger cell inocula, cancer incidences per clonogen were suppressed inversely as the numbers of irradiated or MNU-treated clonogens per graft increased. Addition of unirradiated cells to small irradiated graft inocula also suppressed progression. Radiation and MNU thus produce quantitatively, and perhaps qualitatively, similar carcinogenesis-related sequelae in mammary clonogens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. M., Ethier S. P., Ullrich R. L. Enhanced in vitro proliferation and in vivo tumorigenic potential of mammary epithelium from BALB/c mice exposed in vivo to gamma-radiation and/or 7,12-dimethylbenz[a]anthracene. Cancer Res. 1987 Aug 15;47(16):4425–4431. [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat Res. 1991 May;257(3):229–306. doi: 10.1016/0165-1110(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Benjamin M. B., Little J. B. X rays induce interallelic homologous recombination at the human thymidine kinase gene. Mol Cell Biol. 1992 Jun;12(6):2730–2738. doi: 10.1128/mcb.12.6.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman D. A., Meyers M., Fukunaga N., Lee S. W. Isolation of x-ray-inducible transcripts from radioresistant human melanoma cells. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7200–7204. doi: 10.1073/pnas.90.15.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton K. H., Tanner M. A., Gould M. N. Assessment of radiogenic cancer initiation frequency per clonogenic rat mammary cell in vivo. Cancer Res. 1986 May;46(5):2390–2395. [PubMed] [Google Scholar]
- Clifton K. H., Yasukawa-Barnes J., Tanner M. A., Haning R. V., Jr Irradiation and prolactin effects on rat mammary carcinogenesis: intrasplenic pituitary and estrone capsule implants. J Natl Cancer Inst. 1985 Jul;75(1):167–175. [PubMed] [Google Scholar]
- Domann F. E., Freitas M. A., Gould M. N., Clifton K. H. Quantifying the frequency of radiogenic thyroid cancer per clonogenic cell in vivo. Radiat Res. 1994 Mar;137(3):330–337. [PubMed] [Google Scholar]
- Domann F. E., Mitchen J. M., Clifton K. H. Restoration of thyroid function after total thyroidectomy and quantitative thyroid cell transplantation. Endocrinology. 1990 Dec;127(6):2673–2678. doi: 10.1210/endo-127-6-2673. [DOI] [PubMed] [Google Scholar]
- Ethier S. P., Ullrich R. L. Detection of ductal dysplasia in mammary outgrowths derived from carcinogen-treated virgin female BALB/c mice. Cancer Res. 1982 May;42(5):1753–1760. [PubMed] [Google Scholar]
- Feinberg A. P., Gehrke C. W., Kuo K. C., Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988 Mar 1;48(5):1159–1161. [PubMed] [Google Scholar]
- Ford J. R., Terzaghi-Howe M. Basal cells are the progenitors of primary tracheal epithelial cell cultures. Exp Cell Res. 1992 Jan;198(1):69–77. doi: 10.1016/0014-4827(92)90150-7. [DOI] [PubMed] [Google Scholar]
- Ford J. R., Terzaghi-Howe M. Characteristics of magnetically separated rat tracheal epithelial cell populations. Am J Physiol. 1992 Nov;263(5 Pt 1):L568–L574. doi: 10.1152/ajplung.1992.263.5.L568. [DOI] [PubMed] [Google Scholar]
- Hardwick J. P., Schlenker R. A., Huberman E. Alteration of the c-mos locus in "normal" tissues from humans exposed to radium. Cancer Res. 1989 May 15;49(10):2668–2673. [PubMed] [Google Scholar]
- Holliday R. Mutations and epimutations in mammalian cells. Mutat Res. 1991 Sep-Oct;250(1-2):351–363. doi: 10.1016/0027-5107(91)90192-q. [DOI] [PubMed] [Google Scholar]
- Kamiya K., Gould M. N., Clifton K. H. Differential control of alveolar and ductal development in grafts of monodispersed rat mammary epithelium. Proc Soc Exp Biol Med. 1991 Mar;196(3):284–292. doi: 10.3181/00379727-196-43190. [DOI] [PubMed] [Google Scholar]
- Kamiya K., Kim N. D., Gould M. N., Clifton K. H. Repair of potentially lethal damage in rat mammary clonogens following irradiation in organoid culture. Int J Radiat Biol. 1991 May;59(5):1207–1216. doi: 10.1080/09553009114551081. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R. Is there a critical target gene for the first step in carcinogenesis? Environ Health Perspect. 1991 Jun;93:199–203. doi: 10.1289/ehp.9193199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R., Little J. B. Evidence that a second event in X-ray-induced oncogenic transformation in vitro occurs during cellular proliferation. Radiat Res. 1984 Aug;99(2):228–248. [PubMed] [Google Scholar]
- Landolph J. R., Heidelberger C. Chemical carcinogens produce mutations to ouabain resistance in transformable C3H/10T1/2 Cl 8 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Feb;76(2):930–934. doi: 10.1073/pnas.76.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., Shepherd F., Gropp T. Enhancement of the tumorigenicity of preneoplastic mammary nodule lines by enzymatic dissociation. J Natl Cancer Inst. 1978 May;60(5):1121–1126. doi: 10.1093/jnci/60.5.1121. [DOI] [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. In vitro malignant transformation by methylcholanthrene of the progeny of single cells derived from C3H mouse prostate. Proc Natl Acad Sci U S A. 1970 Jan;65(1):219–225. doi: 10.1073/pnas.65.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy R. T., Gould M. N., Clifton K. H. Radiogenic initiation of thyroid cancer: a common cellular event. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 May;45(5):419–426. doi: 10.1080/09553008414550621. [DOI] [PubMed] [Google Scholar]
- Parodi S., Brambilla G. Relationships between mutation and transformation frequencies in mammalian cells treated "in vitro" with chemical carcinogens. Mutat Res. 1977;47(1):53–74. doi: 10.1016/0165-1110(77)90017-3. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cellular epigenetics: control of the size, shape, and spatial distribution of transformed foci by interactions between the transformed and nontransformed cells. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1039–1043. doi: 10.1073/pnas.91.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Is somatic mutation the major mechanism of malignant transformation? J Natl Cancer Inst. 1980 May;64(5):995–1000. [PubMed] [Google Scholar]
- SINCLAIR W. K. X-RAY-INDUCED HERITABLE DAMAGE (SMALL-COLONY FORMATION) IN CULTURED MAMMALIAN CELLS. Radiat Res. 1964 Apr;21:584–611. [PubMed] [Google Scholar]
- Shimada Y., Yasukawa-Barnes J., Kim R. Y., Gould M. N., Clifton K. H. Age and radiation sensitivity of rat mammary clonogenic cells. Radiat Res. 1994 Jan;137(1):118–123. [PubMed] [Google Scholar]
- Stanbridge E. J., Der C. J., Doersen C. J., Nishimi R. Y., Peehl D. M., Weissman B. E., Wilkinson J. E. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- Terzaghi-Howe M. Inhibition of carcinogen-altered rat tracheal epithelial cell proliferation by normal epithelial cells in vivo. Carcinogenesis. 1987 Jan;8(1):145–150. doi: 10.1093/carcin/8.1.145. [DOI] [PubMed] [Google Scholar]
- Terzaghi-Howe M. Inhibition of carcinogen-altered rat tracheal epithelial cell proliferation by normal epithelial cells in vivo. Carcinogenesis. 1987 Jan;8(1):145–150. doi: 10.1093/carcin/8.1.145. [DOI] [PubMed] [Google Scholar]
- Terzaghi-Howe M., McKeown C. Inhibition of carcinogen-altered rat tracheal epithelial cells by normal epithelial cell-conditioned medium. Cancer Res. 1986 Feb;46(2):917–921. [PubMed] [Google Scholar]
- Terzaghi M., Nettesheim P. Dynamics of neoplastic development in carcinogen-exposed tracheal mucosa. Cancer Res. 1979 Oct;39(10):4003–4010. [PubMed] [Google Scholar]
- Wang B. C., Kennan W. S., Yasukawa-Barnes J., Lindstrom M. J., Gould M. N. Carcinoma induction following direct in situ transfer of v-Ha-ras into rat mammary epithelial cells using replication-defective retrovirus vectors. Cancer Res. 1991 May 15;51(10):2642–2648. [PubMed] [Google Scholar]
- Watanabe H., Tanner M. A., Domann F. E., Gould M. N., Clifton K. H. Inhibition of carcinoma formation and of vascular invasion in grafts of radiation-initiated thyroid clonogens by unirradiated thyroid cells. Carcinogenesis. 1988 Aug;9(8):1329–1335. doi: 10.1093/carcin/9.8.1329. [DOI] [PubMed] [Google Scholar]
- Yao A., Rubin H. Sensitivity of transformation to small differences in population density during serial passage of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7486–7490. doi: 10.1073/pnas.89.16.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]


