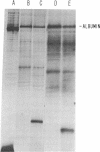
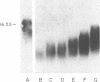
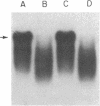
Abstract
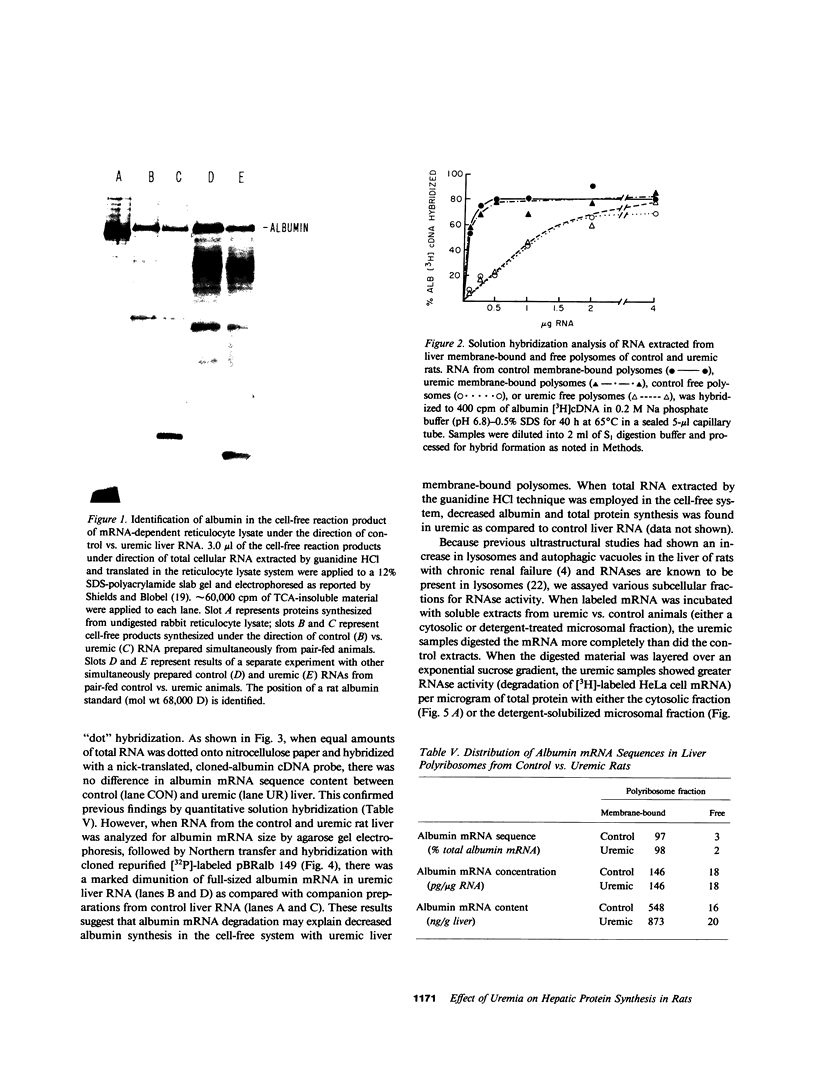
Previously we reported that chronic renal failure in rats leads to preferential disaggregation of liver membrane-bound polysomes associated with a decrease in albumin synthesis. To determine whether reduced albumin synthesis results from reduced cellular levels of albumin messenger RNA (mRNA) or some other molecular mechanism, we have employed mRNA-DNA hybridization in conjunction with cell-free protein synthesis to determine albumin mRNA sequence content and biological activity in subcellular fractions from control and uremic rat liver. Using high specific activity albumin [3H]-complementary DNA prepared from purified-albumin mRNA, we found that total liver polysomes and albumin mRNA sequence content are increased in uremic animals. The extra polysomes are located within the membrane-bound subcellular fraction. These polysomes, however, have reduced ability to synthesize albumin in the cell-free system, and mRNA isolated from membrane-bound polysomes of uremic liver showed reduced albumin synthesis. Evaluation of albumin mRNA size by hybridization analysis revealed a reduced content of intact albumin mRNA molecules per microgram of RNA in the liver of uremic animals. This was associated with increased ribonuclease activity in uremic cytosol. The diminished albumin synthesis by membrane-bound polysomes of uremic rat liver can, therefore, be explained by enhanced degradation of albumin mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Boettiger D., Focht R. J., Holtzer H., Pacifici M. Regulation of the synthesis of extracellular matrix components in chondroblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1982 Sep;30(2):373–384. doi: 10.1016/0092-8674(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M., Biempica L., Goldfischer S., Grossman S., Arias I. M. Effect of chronic renal failure in rats on structure and function of the hepatic endoplasmic reticulum. Exp Mol Pathol. 1977 Dec;27(3):377–391. doi: 10.1016/0014-4800(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brewer E. N., Foster L. B., Sells B. H. A possible role for ribonuclease in the regulation of protein synthesis in normal and hypophysectomized rats. J Biol Chem. 1969 Mar 25;244(6):1389–1392. [PubMed] [Google Scholar]
- Coles G. A., Peters D. K., Jones J. H. Albumin metabolism in chronic renal failure. Clin Sci. 1970 Sep;39(3):423–435. doi: 10.1042/cs0390423. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Enwonwu C. O., Munro H. N. Rate of RNA turnover in rat liver in relation to intake of protein. Arch Biochem Biophys. 1970 Jun;138(2):532–539. doi: 10.1016/0003-9861(70)90378-4. [DOI] [PubMed] [Google Scholar]
- Fleck A., Shepherd J., Munro H. N. Protein synthesis in rat liver: influence of amino acids in diet on microsomes and polysomes. Science. 1965 Oct 29;150(3696):628–629. doi: 10.1126/science.150.3696.628. [DOI] [PubMed] [Google Scholar]
- Gaetani S., Massotti D., Spadoni M. A. Studies of dietary effects on free and membrane-bound polysomes in rat liver. J Nutr. 1969 Nov;99(3):307–314. doi: 10.1093/jn/99.3.307. [DOI] [PubMed] [Google Scholar]
- Grossman S. B., Yap S. H., Shafritz D. A. Influence of chronic renal failure on protein synthesis and albumin metabolism in rat liver. J Clin Invest. 1977 May;59(5):869–878. doi: 10.1172/JCI108709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Kelman L., Saunders S. J., Wicht S., Frith L., Corrigall A., Kirsch R. E., Terblanche J. The effects of amino acids on albumin synthesis by the isolated perfused rat liver. Biochem J. 1972 Oct;129(4):805–809. doi: 10.1042/bj1290805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Mariani G., Bianchi R., Pilo A., Palla R., Toni M. G., Fusani L. Albumin catabolism measurement by a double tracer technique in uraemic patients during a single dialytic treatment. Eur J Clin Invest. 1974 Dec 5;4(6):435–442. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Carey N. H. Rapid inactivation of ovalbumin messenger ribonucleic acid after acute withdrawal of estrogen. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2357–2361. doi: 10.1073/pnas.71.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Schreiber S. S. Alcohol, amino acids, and albumin synthesis. Gastroenterology. 1974 Dec;67(6):1200–1213. [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Schreiber S. S. Effects of carbon tetrachloride on albumin synthesis. J Clin Invest. 1972 Sep;51(9):2310–2314. doi: 10.1172/JCI107041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Drysdale J. W., Isselbacher K. J. Translation of liver messenger ribonucleic acid in a messenger-dependent reticulocyte cell-free system. Properties of the system and identification of ferriitin in the product. J Biol Chem. 1973 May 10;248(9):3220–3227. [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky H., Sarma D. S., Bongiorno M., Verney E. Effect of dietary tryptophan on hepatic polyribosomes and protein synthesis in fasted mice. J Biol Chem. 1968 Mar 25;243(6):1123–1132. [PubMed] [Google Scholar]
- Strair R. K., Yap S. H., Shafritz D. A. Use of molecular hybridization to purify and analyze albumin messenger RNA from rat liver. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4346–4350. doi: 10.1073/pnas.74.10.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap S. H., Strair R. K., Shafritz D. A. Distribution of rat liver albumin mRNA membrane-bound and free in polyribosomes as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5397–5401. doi: 10.1073/pnas.74.12.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap S. H., Strair R. K., Shafritz D. A. Effect of a short term fast on the distribution of cytoplasmic albumin messenger ribonucleic acid in rat liver. Evidence for formation of free albumin messenger ribonucleoprotein particles. J Biol Chem. 1978 Jul 25;253(14):4944–4950. [PubMed] [Google Scholar]
- Zern M. A., Chakraborty P. R., Ruiz-Opazo N., Yap S. H., Shafritz D. A. Development and use of a rat albumin cDNA clone to evaluate the effect of chronic ethanol administration on hepatic protein synthesis. Hepatology. 1983 May-Jun;3(3):317–322. doi: 10.1002/hep.1840030307. [DOI] [PubMed] [Google Scholar]