Abstract
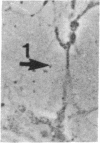
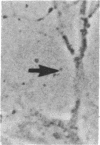
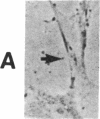
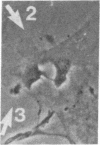
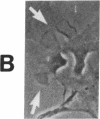
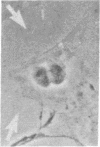
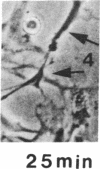
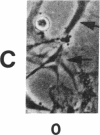
The interaction of inflammatory cells and glomerular prostaglandins (PG) may be important during glomerulonephritis. We therefore examined the influence of platelet-activating factor (PAF), (a mediator of inflammation released from leukocytes) and of phagocytosis of zymosan on arachidonic acid metabolism and on cell contractility in rat glomerular mesangial cells in culture. PAF increased PGE2 synthesis (determined by radioimmunoassay) within minutes (threshold: 10(-10)M; maximal effect: 10(-7)M). Serum-treated zymosan also stimulated PGE2, but with a slower onset. In cells prelabeled with [14C]arachidonic acid both PAF and serum-treated zymosan released 14C from phospholipids and increased free [14C]arachidonate. The ratio of 14C-release to PGE2 was, however, different with PAF and serum-treated zymosan, indicating different phospholipid pools. Under phase-contrast microscopy, PAF caused contraction of mesangial cells with a dose-response and time-course parallel to that for PGE2 synthesis. Serum-treated zymosan caused no contraction. The PAF-induced contraction was enhanced by PG synthesis inhibition and was attenuated by addition of PGE2, indicating a feedback mechanism. The mesangial contraction by PAF may be important in favoring deposition of immune complexes, while the PGE2 synthesis stimulated by PAF and by phagocytosis of zymosan may counteract the deleterious effects of PAF during induction of glomerulonephritis.
Full text
PDF

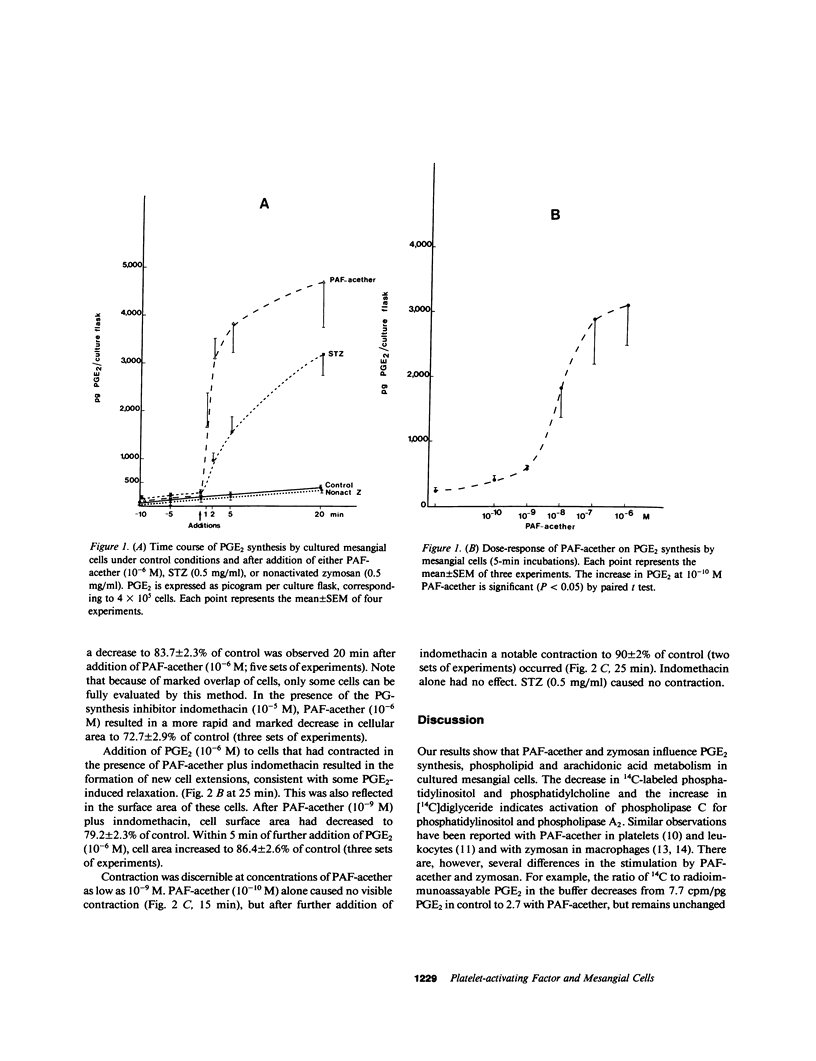
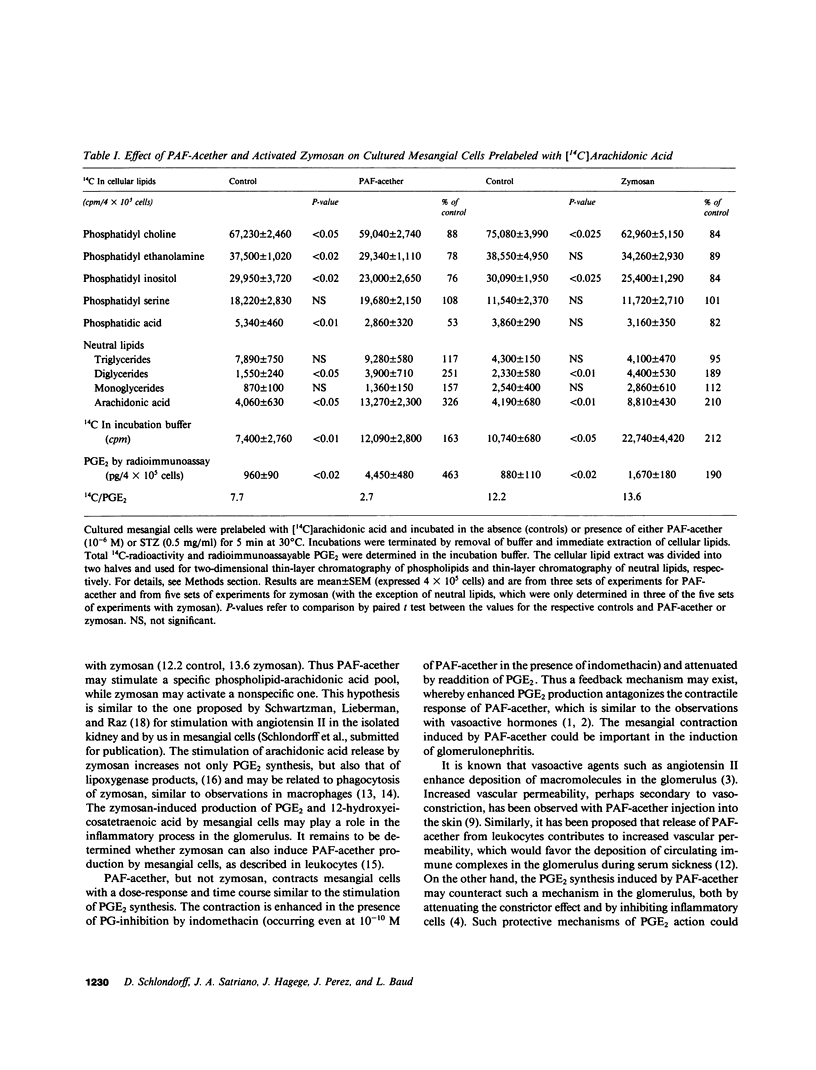

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso F., Gil M. G., Sánchez-Crespo M., Mato J. M. Activation of 1-alkyl-2-lysoglycero-3-phosphocholine. Acetyl-CoA transferase during phagocytosis in human polymorphonuclear leukocytes. J Biol Chem. 1982 Apr 10;257(7):3376–3378. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Walsh C. E., Thomas M. J., Wykle R. L., DeChatelet L. R., Waite B. M. Platelet activating factor. Stimulation of the lipoxygenase pathway in polymorphonuclear leukocytes by 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine. J Biol Chem. 1982 May 25;257(10):5402–5407. [PubMed] [Google Scholar]
- Findlay S. R., Lichtenstein L. M., Hanahan D. J., Pinckard R. N. Contraction of guinea pig ileal smooth muscle by acetyl glyceryl ether phosphorylcholine. Am J Physiol. 1981 Sep;241(3):C130–C133. doi: 10.1152/ajpcell.1981.241.3.C130. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Desai U., Gonzalez-Crussi F., Lamb R., Chu A. Two phospholipase pools for prostaglandin synthesis in macrophages. Nature. 1981 Apr 23;290(5808):710–713. doi: 10.1038/290710a0. [DOI] [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Satouchi K., Hanahan D. J., Pinckard R. N. Vasoactive properties of acetyl glyceryl ether phosphorylcholine and analogues. Lab Invest. 1982 Apr;46(4):422–427. [PubMed] [Google Scholar]
- Kelley V. E., Winkelstein A. Effect of prostaglandin E1 treatment on murine acute immune complex glomerulonephritis. Clin Immunol Immunopathol. 1980 Jul;16(3):316–323. doi: 10.1016/0090-1229(80)90137-3. [DOI] [PubMed] [Google Scholar]
- Kröner E. E., Peskar B. A., Fischer H., Ferber E. Control of arachidonic acid accumulation in bone marrow-derived macrophages by acyltransferases. J Biol Chem. 1981 Apr 25;256(8):3690–3697. [PubMed] [Google Scholar]
- Lapetina E. G. Platelet-activating factor stimulates the phosphatidylinositol cycle. Appearance of phosphatidic acid is associated with the release of serotonin in horse platelets. J Biol Chem. 1982 Jul 10;257(13):7314–7317. [PubMed] [Google Scholar]
- Levenson D. J., Simmons C. E., Jr, Brenner B. M. Arachidonic acid metabolism, prostaglandins and the kidney. Am J Med. 1982 Feb;72(2):354–374. doi: 10.1016/0002-9343(82)90826-9. [DOI] [PubMed] [Google Scholar]
- Scharschmidt L. A., Dunn M. J. Prostaglandin synthesis by rat glomerular mesangial cells in culture. Effects of angiotensin II and arginine vasopressin. J Clin Invest. 1983 Jun;71(6):1756–1764. doi: 10.1172/JCI110931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M., Liberman E., Raz A. Bradykinin and angiotensin II activation of arachidonic acid deacylation and prostaglandin E2 formation in rabbit kidney. Hormone-sensitive versus hormone-insensitive lipid pools of arachidonic acid. J Biol Chem. 1981 Mar 10;256(5):2329–2333. [PubMed] [Google Scholar]
- Sraer J., Foidart J., Chansel D., Mahieu P., Kouznetzova B., Ardaillou R. Prostaglandin synthesis by mesangial and epithelial glomerular cultured cells. FEBS Lett. 1979 Aug 15;104(2):420–424. doi: 10.1016/0014-5793(79)80866-2. [DOI] [PubMed] [Google Scholar]
- Stein H. D., Feddergreen W., Kashgarian M., Sterzel R. B. Role of angiotensin II-induced renal functional changes in mesangial deposition of exogenous ferritin in rats. Lab Invest. 1983 Sep;49(3):270–280. [PubMed] [Google Scholar]