Abstract
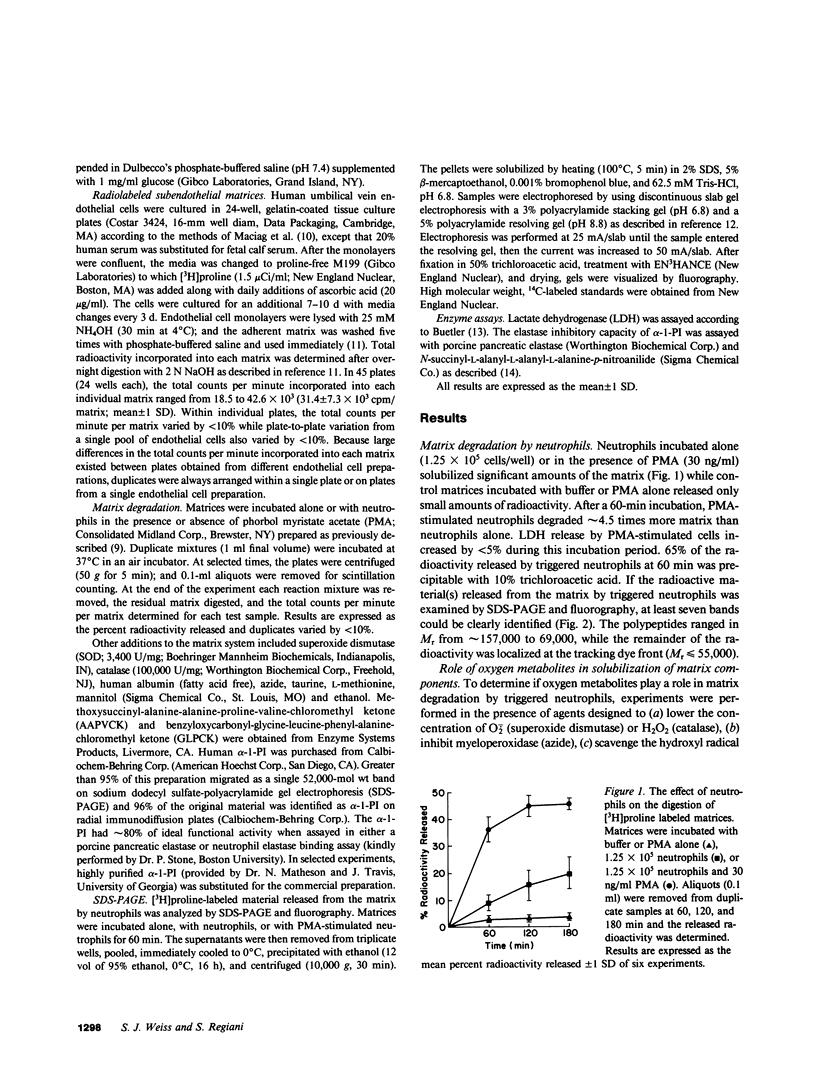
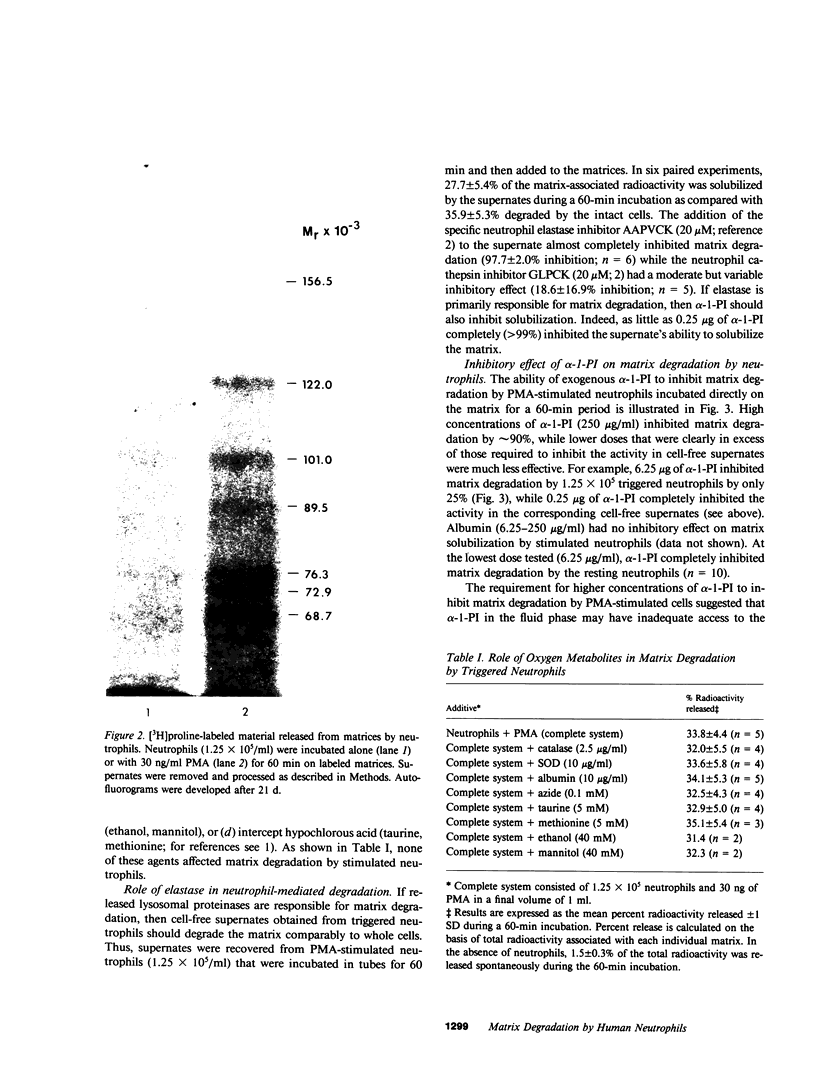
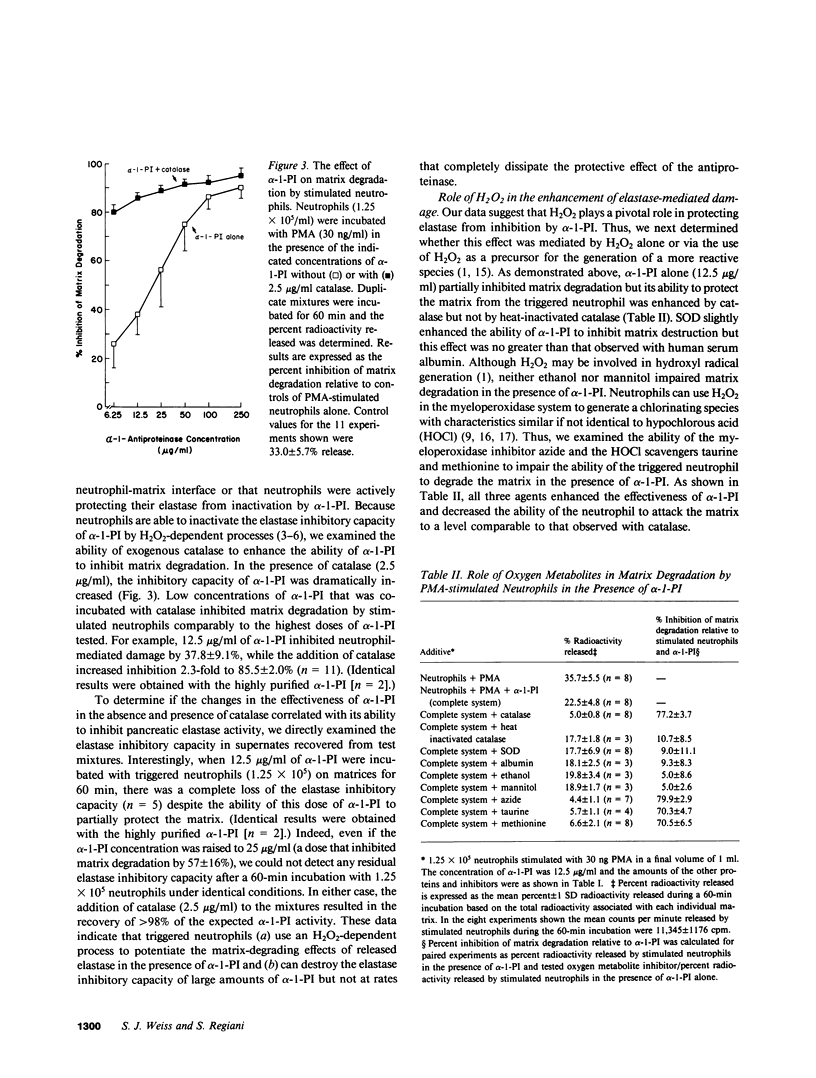
Triggered neutrophils rapidly degraded labeled matrices secreted by cultured, venous endothelial cells via a process dependent on elastase but not oxygen metabolites. In the presence of high concentrations of alpha-1-proteinase inhibitor, the ability of the stimulated neutrophil to solubilize the matrix was impaired. However, at lower concentrations of alpha-1-proteinase inhibitor the neutrophil could enhance the degradative potential of its released elastase by a H2O2-dependent process. Coincident with this increase in matrix damage, the stimulated neutrophil destroyed the elastase inhibitory activity of the alpha-1-proteinase inhibitor via a catalase-inhibitable process. The ability of the triggered neutrophil to solubilize the matrix in the presence of alpha-1-proteinase inhibitor was unaffected by superoxide dismutase or hydroxyl radical scavengers but was markedly impaired by catalase, azide, or hypochlorous acid scavengers. We conclude that neutrophils can cooperatively use an oxidant with characteristics similar, if not identical, to hypochlorous acid and the lysosomal proteinase elastase to negate the protective effects of alpha-1-proteinase inhibitor in order to attack the subendothelial matrix.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Leukocyte elastase. Methods Enzymol. 1981;80(Pt 100):581–588. doi: 10.1016/s0076-6879(81)80046-8. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Miller F., Hoidal J. R., Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B. The effects in vivo and in vitro of oxidative damage to purified alpha1-antitrypsin and to the enzyme-inhibiting activity of plasma. Am Rev Respir Dis. 1979 Jun;119(6):953–960. doi: 10.1164/arrd.1979.119.6.953. [DOI] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Wright D. G., Crystal R. G. Human neutrophil elastase functions as a type III collagen "collagenase". Biochem Biophys Res Commun. 1980 Aug 29;95(4):1815–1822. doi: 10.1016/s0006-291x(80)80110-0. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Stemerman M. B., Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi C. L., Dixit S. N., Kang A. H. Degradation of type IV (basement membrane) collagen by a proteinase isolated from human polymorphonuclear leukocyte granules. J Biol Chem. 1980 Jun 10;255(11):5435–5441. [PubMed] [Google Scholar]
- Mainardi C. L., Hasty D. L., Seyer J. M., Kang A. H. Specific cleavage of human type III collagen by human polymorphonuclear leukocyte elastase. J Biol Chem. 1980 Dec 25;255(24):12006–12010. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Schuyler M., Travis J. Interaction of human alpha-1-proteinase inhibitor with neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):331–336. doi: 10.1021/bi00505a016. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Merritt T. A., Cochrane C. G., Holcomb K., Bohl B., Hallman M., Strayer D., Edwards D. K., 3rd, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983 Aug;72(2):656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983 Aug;72(2):441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Giles P. J., Porcelli L., Reilly C. F., Baugh R., Powers J. Human leucocyte elastase and cathepsin G: structural and functional characteristics. Ciba Found Symp. 1979;(75):51–68. doi: 10.1002/9780470720585.ch4. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- Werb Z., Banda M. J., Jones P. A. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980 Nov 1;152(5):1340–1357. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. S., Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1449–1454. doi: 10.1016/0006-291x(80)90113-8. [DOI] [PubMed] [Google Scholar]
- Zaslow M. C., Clark R. A., Stone P. J., Calore J. D., Snider G. L., Franzblau C. Human neutrophil elastase does not bind to alpha 1-protease inhibitor that has been exposed to activated human neutrophils. Am Rev Respir Dis. 1983 Sep;128(3):434–439. doi: 10.1164/arrd.1983.128.3.434. [DOI] [PubMed] [Google Scholar]