Abstract
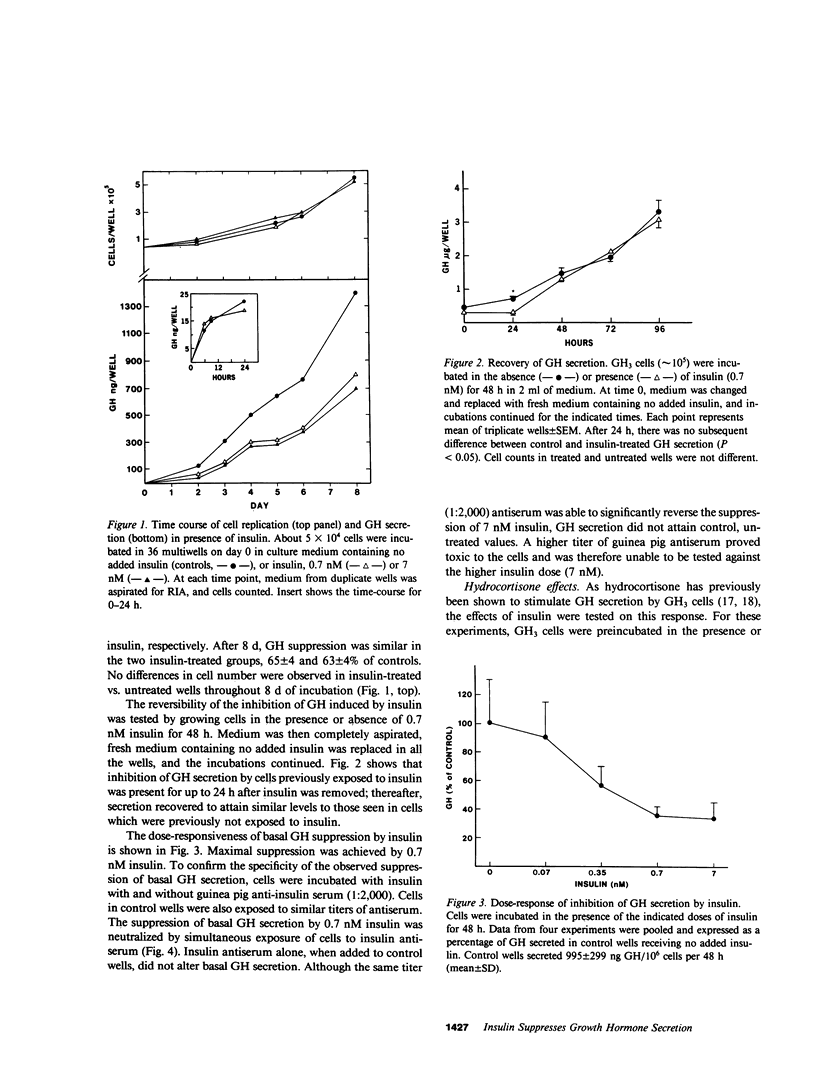
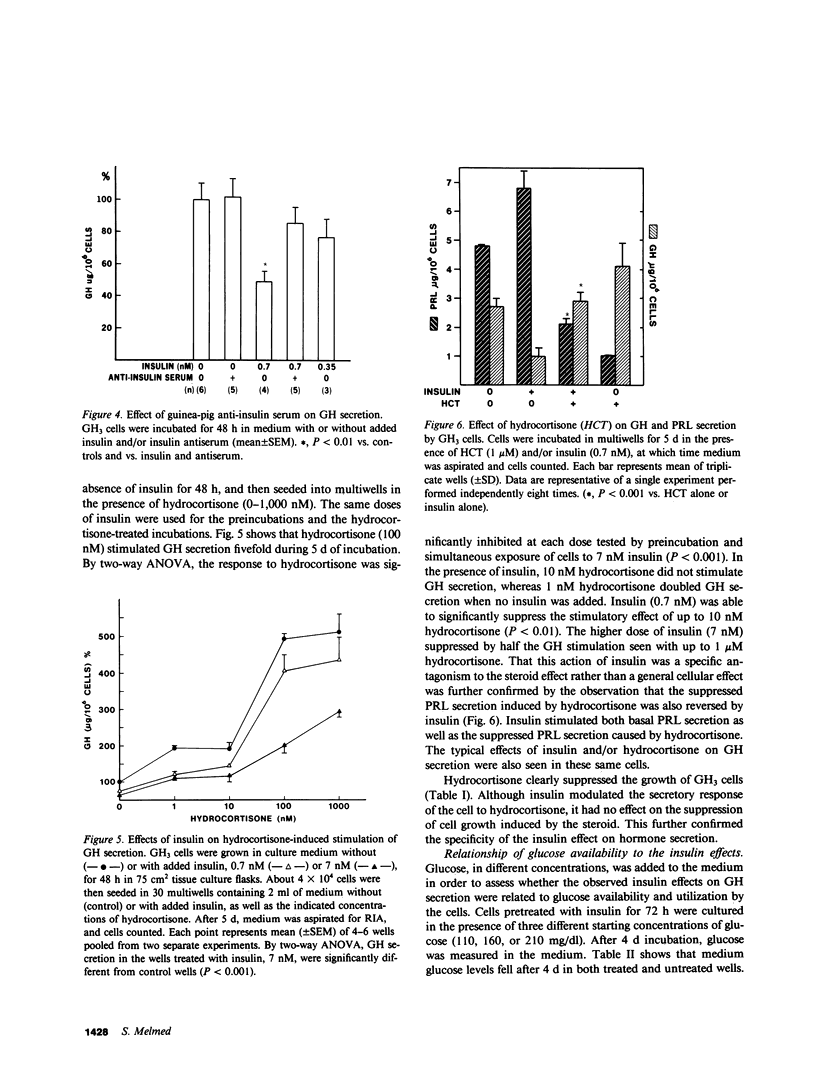
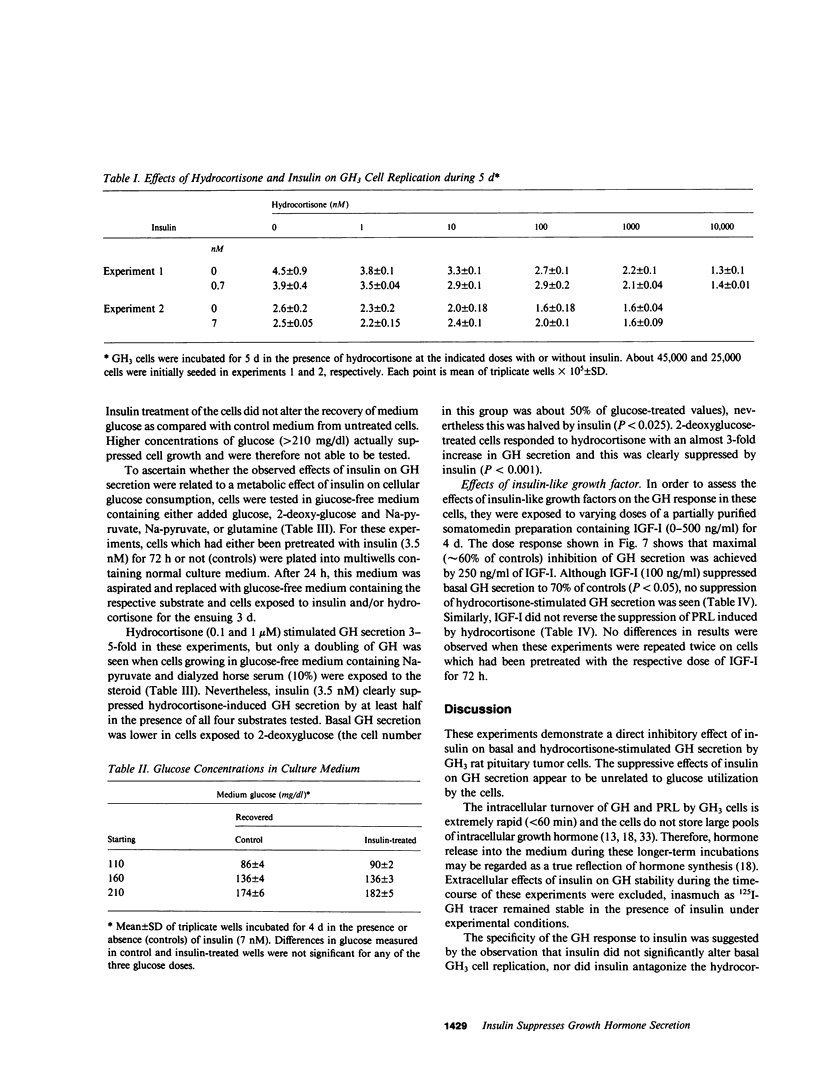
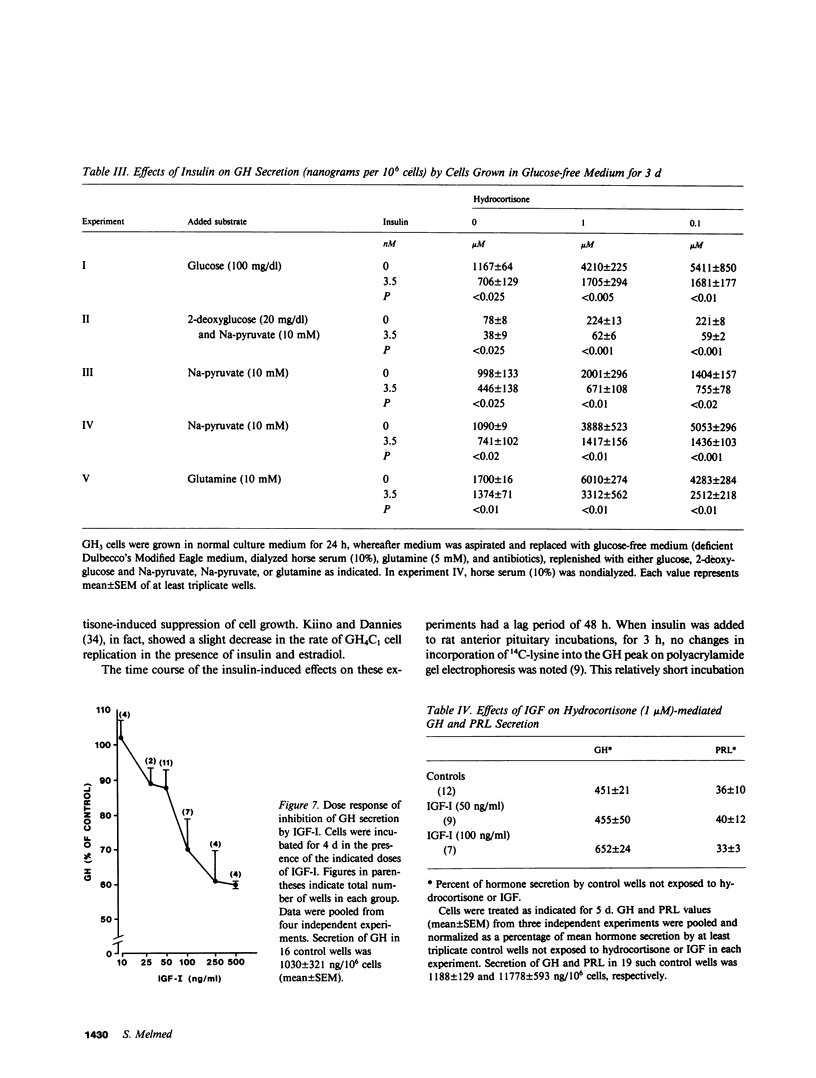
The effects of insulin on basal and hydrocortisone-induced growth hormone (GH) secretion were studied in rat pituitary tumor cells (GH3). Cells were grown in monolayer culture and exposed to exogenously added insulin for up to 8 d. Basal GH secretion was inhibited by insulin (0.7 nM) after a 48-h lag period by approximately 50% (P less than 0.01, vs. untreated control cells). The suppression of GH secretion was reversible, as removal of added insulin resulted in return of GH secretion to normal levels after 24 h. Maximal suppression of basal GH secretion was achieved by 0.7 nM insulin, and these effects were prevented by simultaneous exposure of the cells to guinea pig anti-insulin serum (1:2,000). No effects of insulin on cell replication were evident, and glucose concentration in the medium did not differ in control or insulin-treated wells. Insulin (7 nM) significantly suppressed the fivefold hydrocortisone-induced GH stimulation during 5 d of incubation with up to 1,000 nM of the steroid (P less than 0.001). These inhibitory effects were similarly observed in glucose- and pyruvate-free medium, and in the presence of 2-deoxyglucose. Insulin also reversed the suppression of prolactin (PRL) secretion induced by hydrocortisone (1 uM), and actually stimulated basal PRL secretion by over 50%. Insulin did not alter the inhibitory effect of hydrocortisone on GH3 cell proliferation. Although higher doses (13 nM) of insulin-like growth factor (IGF-I) also suppressed basal GH secretion, IGF-I did not alter the GH and PRL secretory changes induced by hydrocortisone. The results show that insulin exerts a direct, specific inhibitory effect on basal and hydrocortisone-induced GH secretion by GH3 cells unrelated to glucose utilization by the cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft F. C., Levine L., Tashjian A. H., Jr Control of growth hormone production by a clonal strain of rat pituitary cells. Stimulation by hydrocortisone. J Cell Biol. 1969 Dec;43(3):432–441. doi: 10.1083/jcb.43.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D. Glucocorticoid hormone action. Pharmacol Ther B. 1976;2(3):605–669. doi: 10.1016/0306-039x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Berelowitz M., Szabo M., Frohman L. A., Firestone S., Chu L., Hintz R. L. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981 Jun 12;212(4500):1279–1281. doi: 10.1126/science.6262917. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Guillemin R., Ling N., van Wyk J., Humbel R. Inhibition par les somatomédines de la sécrétion de l'hormone de croissance stimulée par le facteur hypothalamique somatocrinine (GRF) ou le peptide de synthèse hpGRF. C R Seances Acad Sci III. 1982 Nov 29;295(11):651–654. [PubMed] [Google Scholar]
- Czech M. P. Cellular dynamics of insulin action. Fed Proc. 1982 Sep;41(11):2717–2718. [PubMed] [Google Scholar]
- Czech M. P. Structural and functional homologies in the receptors for insulin and the insulin-like growth factors. Cell. 1982 Nov;31(1):8–10. doi: 10.1016/0092-8674(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Melmed S. Hepatocyte insulin binding and action in rats with somatomammotrophic tumours. Diabetologia. 1983 Jul;25(1):60–65. doi: 10.1007/BF00251899. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Venkatesan N. Persistence of a curvilinear Scatchard plot for insulin binding despite correcting for degradation. Metabolism. 1982 Dec;31(12):1206–1209. doi: 10.1016/0026-0495(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Birnberg N. C., Rosenfeld M. G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODNER C. J., FREINKEL N. Studies of anterior pituitary tissue in vitro: effects of insulin and experimental diabetes mellitus upon carbohydrate metabolism. J Clin Invest. 1961 Feb;40:261–272. doi: 10.1172/JCI104252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodner C. J., Krouse H. A. Studies of the effect of insulin on synthesis of electrophoretically separated proteins in anterior pituitary in vitro. Endocrinology. 1972 Jun;90(6):1639–1642. doi: 10.1210/endo-90-6-1639. [DOI] [PubMed] [Google Scholar]
- HAZELWOOD R. L., HAZELWOOD B. S. INFLUENCE OF ALLOXAN DIABETES ON GROWTH HORMONE CONTENT OF THE RAT HYPOPHYSIS. Am J Physiol. 1964 May;206:1137–1144. doi: 10.1152/ajplegacy.1964.206.5.1137. [DOI] [PubMed] [Google Scholar]
- Havrankova J., Roth J., Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978 Apr 27;272(5656):827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Hintz R. L., Liu F. A radioimmunoassay for insulin-like growth factor II specific for the C-peptide region. J Clin Endocrinol Metab. 1982 Feb;54(2):442–446. doi: 10.1210/jcem-54-2-442. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Kasuga M., Van Obberghen E., Nissley S. P., Rechler M. M. Demonstration of two subtypes of insulin-like growth factor receptors by affinity cross-linking. J Biol Chem. 1981 Jun 10;256(11):5305–5308. [PubMed] [Google Scholar]
- Kiino D. R., Burger D. E., Dannies P. S. Prolactin storage in a clonal strain of rat pituitary tumor cells is cell-cycle dependent. J Cell Biol. 1982 May;93(2):459–462. doi: 10.1083/jcb.93.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiino D. R., Dannies P. S. Insulin and 17 beta-estradiol increase the intracellular prolactin content of GH4C1 cells. Endocrinology. 1981 Oct;109(4):1264–1269. doi: 10.1210/endo-109-4-1264. [DOI] [PubMed] [Google Scholar]
- Kohler P. O., Bridson W. E., Rayford P. L. Cortisol stimulation of growth hormone production by monkey adenohypophysis in tissue culture. Biochem Biophys Res Commun. 1968 Dec 9;33(5):834–840. doi: 10.1016/0006-291x(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kohler P. O., Frohman L. A., Bridson W. E., Vanha-Perttula T., Hammond J. M. Cortisol induction of growth hormone synthesis in a clonal line of rat pituitary tumor cells in culture. Science. 1969 Oct 31;166(3905):633–634. doi: 10.1126/science.166.3905.633. [DOI] [PubMed] [Google Scholar]
- LUKENS F. D. INSULIN AND PROTEIN METABOLISM. Diabetes. 1964 Sep-Oct;13:451–461. doi: 10.2337/diab.13.5.451. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S., Nelson M., Kaplowitz N., Yamada T., Hershman J. M. Glutathione-dependent thyroxine 5'-monodeiodination modulates growth hormone production by cultured nonthyrotropic rat pituitary cells. Endocrinology. 1981 Mar;108(3):970–976. doi: 10.1210/endo-108-3-970. [DOI] [PubMed] [Google Scholar]
- Monier S., Le Cam A., Le Marchand-Brustel Y. Insulin and insulin-like growth factor I. Effects on protein synthesis in isolated muscles from lean and goldthioglucose-obese mice. Diabetes. 1983 May;32(5):392–397. doi: 10.2337/diab.32.5.392. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSON S. A. Secretion of human growth hormone: physiologic and experimental modification. Metabolism. 1963 Jul;12:577–579. [PubMed] [Google Scholar]
- Rosenfeld R. G., Dollar L. A. Characterization of the somatomedin-C/insulin-like growth factor I (SM-C/IGF-I) receptor on cultured human fibroblast monolayers: regulation of receptor concentrations by SM-C/IGF-I and insulin. J Clin Endocrinol Metab. 1982 Sep;55(3):434–440. doi: 10.1210/jcem-55-3-434. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Hintz R. L. Characterization of a specific receptor for somatomedin C (SM-C) on cultured human lymphocytes: evidence that SM-C modulates homologous receptor concentration. Endocrinology. 1980 Dec;107(6):1841–1848. doi: 10.1210/endo-107-6-1841. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Hintz R. L., Dollar L. A. Insulin-induced loss of insulin-like growth factor-I receptors on IM-9 lymphocytes. Diabetes. 1982 May;31(5 Pt 1):375–381. doi: 10.2337/diab.31.5.375. [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Samuels H. H., Yaffe B. M. Thyroid and glucocorticoid hormones synergistically control growth hormone mRNA in cultured GH1 cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):45–49. doi: 10.1073/pnas.75.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. T., Giroud C. J., Robert M., Avery M. E. Insulin antagonism of cortisol action on lechithin synthesis by cultured fetal lung cells. J Pediatr. 1975 Dec;87(6 Pt 1):953–955. doi: 10.1016/s0022-3476(75)80916-4. [DOI] [PubMed] [Google Scholar]
- Stachura M. E. Sequestration of an early-release pool of growth hormone and prolactin in GH3 rat pituitary tumor. Endocrinology. 1982 Dec;111(6):1769–1777. doi: 10.1210/endo-111-6-1769. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Guyda H. J., Posner B. I. Insulin-like growth factors: a role in growth hormone negative feedback and body weight regulation via brain. Science. 1983 Apr 1;220(4592):77–79. doi: 10.1126/science.6338593. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Bancroft F. C., Levine L. Production of both prolactin and growth hormone by clonal strains of rat pituitary tumor cells. Differential effects of hydrocortisone and tissue extracts. J Cell Biol. 1970 Oct;47(1):61–70. doi: 10.1083/jcb.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Van Wyk J. J., Underwood L. E., Hintz R. L., Clemmons D. R., Voina S. J., Weaver R. P. The somatomedins: a family of insulinlike hormones under growth hormone control. Recent Prog Horm Res. 1974;30(0):259–318. [PubMed] [Google Scholar]
- Vanha-Perttula T., Kohler P. O., Frohman L. A. Effect of insulin on growth hormone (GH) induction by cortisol in rat pituitary tumor cells. Life Sci II. 1970 Jul 22;9(14):805–813. doi: 10.1016/0024-3205(70)90085-8. [DOI] [PubMed] [Google Scholar]
- WOOL I. G., KRAHL M. E. An effect of insulin on peptide synthesis independent of glucose or amino-acid transport. Nature. 1959 May 16;183(4672):1399–1400. doi: 10.1038/1831399a0. [DOI] [PubMed] [Google Scholar]
- Wegnez M., Schachter B. S., Baxter J. D., Martial J. A. Hormonal regulation of growth hormone mRNA. DNA. 1982;1(2):145–153. doi: 10.1089/dna.1.1982.1.145. [DOI] [PubMed] [Google Scholar]
- Wehrenberg W. B., Baird A., Ling N. Potent interaction between glucocorticoids and growth hormone-releasing factor in vivo. Science. 1983 Aug 5;221(4610):556–558. doi: 10.1126/science.6408735. [DOI] [PubMed] [Google Scholar]


