Abstract
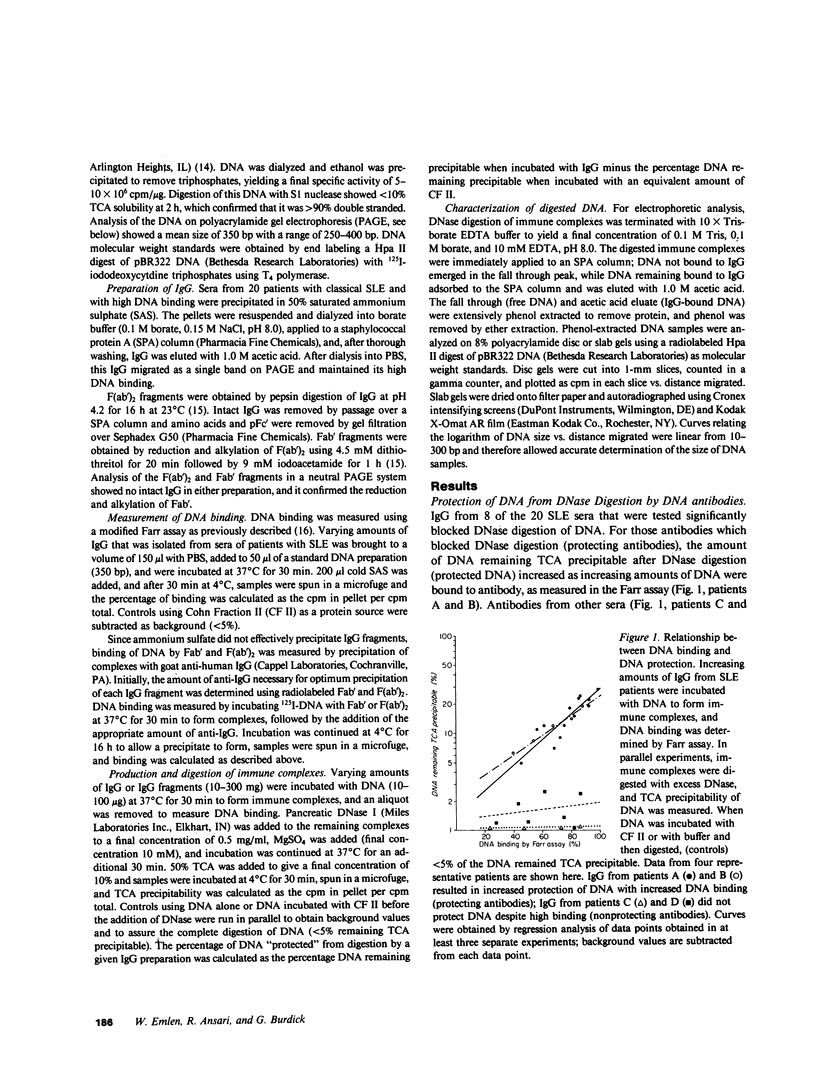
We examined the ability of DNase I to digest DNA that was contained with DNA-anti-DNA immune complexes. IgG isolated from the sera of 20 patients with systemic lupus erythematosus (SLE) and containing antibodies to DNA was incubated with double-stranded DNA to form immune complexes. Excess DNase was added, and digestion of DNA was monitored by the conversion of DNA to TCA soluble products. IgG from 8 of the 20 SLE patients protected DNA from degradation by DNase in direct proportion to the amount of DNA bound to IgG as measured in the Farr binding assay. Using IgG from these sera, we showed that the DNA protected from degradation remained bound to IgG during digestion and was 35-45 base pairs in size. The size of this fragment is the same as that which has been proposed to be the minimal size necessary for monogamous bivalent binding of IgG to DNA. We therefore compared the ability of F(ab')2 and Fab' to protect DNA from DNase digestion and demonstrated that the bivalent F(ab')2 fragments were protective, but that the univalent Fab' fragments were not. These results suggest that some antibodies to DNA that bind to DNA via monogamous bivalent binding can protect a 35-45-base pair DNA fragment from DNase digestion. The implications of this finding are discussed with regard to the in vivo behavior and potential pathogenicity of small DNA-anti-DNA immune complexes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Lakmaker F. Immunology of DNA. V. Analysis of DNA/anti-DNA complexes. J Immunol Methods. 1976;13(3-4):241–252. doi: 10.1016/0022-1759(76)90071-5. [DOI] [PubMed] [Google Scholar]
- Aarden L. A., Lakmaker F., De Groot E. R. Immunology of DNA. IV. Quantitative aspects of the Farr assay. J Immunol Methods. 1976;11(2):153–163. doi: 10.1016/0022-1759(76)90143-5. [DOI] [PubMed] [Google Scholar]
- Adu D., Dobson J., Williams D. G. DNA-anti-DNA circulating complexes in the nephritis of systemic lupus erythematosus. Clin Exp Immunol. 1981 Mar;43(3):605–614. [PMC free article] [PubMed] [Google Scholar]
- Bruneau C., Benveniste J. Circulating DNA:anti-DNA complexes in systemic lupus erythematosus. Detection and characterization by ultracentrifugation. J Clin Invest. 1979 Jul;64(1):191–198. doi: 10.1172/JCI109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia D., Dorsch C., Barnett E. V., Levy L. Metabolism of exogenous single stranded DNA in normal and NZB/W mice. Immunology. 1977 Mar;32(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Chused T. M., Steinberg A. D., Talal N. The clearance and localization of nucleic acids by New Zealand and normal mice. Clin Exp Immunol. 1972 Dec;12(4):465–476. [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Boyer H. W. Solubility and dialysis limits of DNA oligonucleotides. Biochim Biophys Acta. 1972 Mar 14;262(2):116–124. doi: 10.1016/0005-2787(72)90224-9. [DOI] [PubMed] [Google Scholar]
- Emlen W., Burdick G. Purification of DNA antibodies using cibacron blue F3GA affinity chromatography. J Immunol Methods. 1983 Aug 26;62(2):205–215. doi: 10.1016/0022-1759(83)90248-x. [DOI] [PubMed] [Google Scholar]
- Emlen W., Mannik M. Clearance of circulating DNA-anti-DNA immune complexes in mice. J Exp Med. 1982 Apr 1;155(4):1210–1215. doi: 10.1084/jem.155.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Mannik M. Kinetics and mechanisms for removal of circulating single-stranded DNA in mice. J Exp Med. 1978 Mar 1;147(3):684–699. doi: 10.1084/jem.147.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Harbeck R. J., Bardana E. J., Kohler P. F., Carr R. I. DNA:anti-DNA complexes: their detection in systemic lupus erythematosus sera. J Clin Invest. 1973 Apr;52(4):789–795. doi: 10.1172/JCI107242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman I. R. T4 DNA polymerase. Methods Enzymol. 1974;29:46–53. [PubMed] [Google Scholar]
- Liebling M. R., Barnett E. V. Substrate competition between DNase I and anti-DNA antibody. Clin Immunol Immunopathol. 1977 Jul;8(1):80–89. doi: 10.1016/0090-1229(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Liebling M. R., Dorsch C. A., Barnett E. V. Substrate competition in systemic lupus erythematosus: clinical relevance. Clin Immunol Immunopathol. 1977 Sep;8(2):345–352. doi: 10.1016/0090-1229(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Locker J. D., Medof M. E., Bennett R. M., Sukhupunyaraksa S. Characterization of DNA used to assay sera for anti-DNA antibodies; determination of the specificities of anti-DNA antibodies in SLE and non-SLE rheumatic disease states. J Immunol. 1977 Feb;118(2):694–701. [PubMed] [Google Scholar]
- Morimoto C., Sano H., Abe T., Homma M., Steinberg A. D. Correlation between clinical activity of systemic lupus erythematosus and the amounts of DNA in DNA/anti-DNA antibody immune complexes. J Immunol. 1982 Nov;129(5):1960–1965. [PubMed] [Google Scholar]
- Nass K., Frenkel G. D. Adenovirus-specific DNA-binding protein inhibits the hydrolysis of DNA by DNase in vitro. J Virol. 1980 Aug;35(2):314–319. doi: 10.1128/jvi.35.2.314-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalian M., Lafer E., Wong R., Stollar B. D. Reaction of systemic lupus erythematosus antinative DNA antibodies with native DNA fragments from 20 to 1,200 base pairs. J Clin Invest. 1980 Feb;65(2):469–477. doi: 10.1172/JCI109690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Morimoto C. Isolation of DNA from DNA/anti-DNA antibody immune complexes in systemic lupus erythematosus. J Immunol. 1981 Feb;126(2):538–539. [PubMed] [Google Scholar]
- Tan E. M., Schur P. H., Carr R. I., Kunkel H. G. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1966 Nov;45(11):1732–1740. doi: 10.1172/JCI105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. P., Weber D., Broccoli A. V., Winfield J. B. Stability of DNA/anti-DNA complexes. J Immunol. 1979 Jan;122(1):115–120. [PubMed] [Google Scholar]
- Walz A., Pirrotta V. Sequence of the PR promoter of phage lambda. Nature. 1975 Mar 13;254(5496):118–121. doi: 10.1038/254118a0. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Koffler D., Kunkel H. G. Role of DNA-anti-DNA complexes in the immunopathogenesis of tissue injury in systemic lupus erythematosus. Scand J Rheumatol Suppl. 1975;11:59–64. doi: 10.3109/03009747509095630. [DOI] [PubMed] [Google Scholar]





