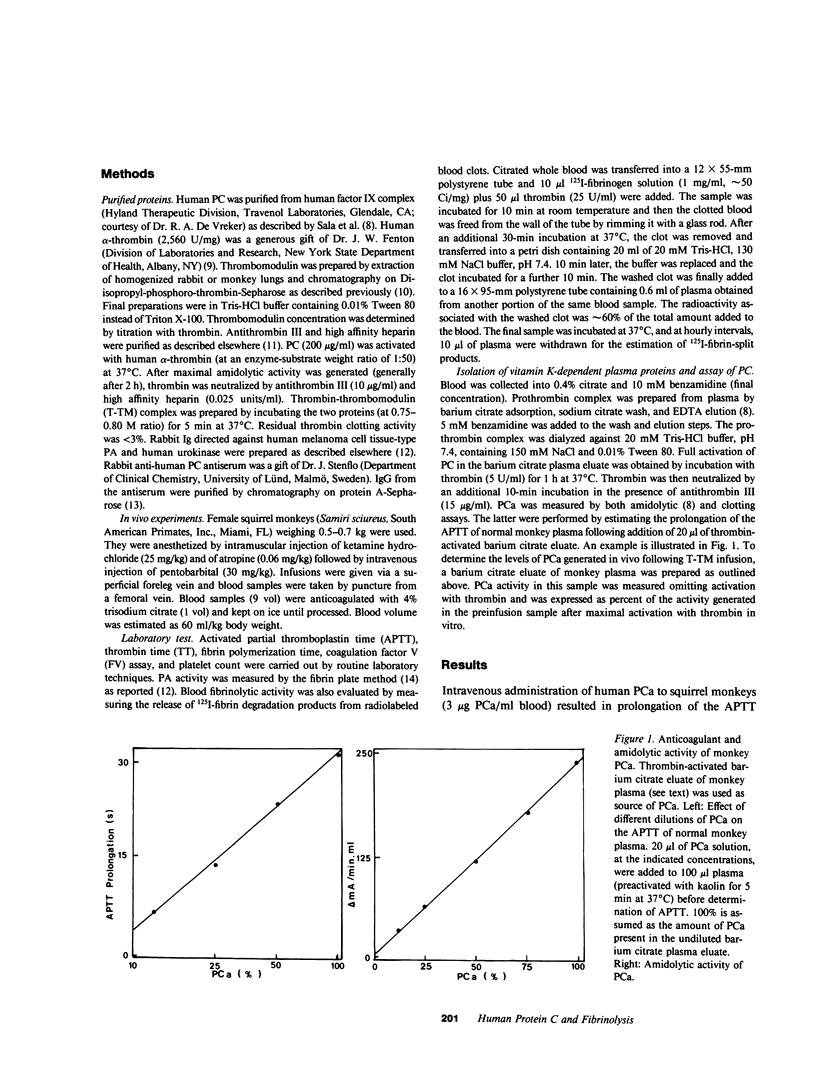
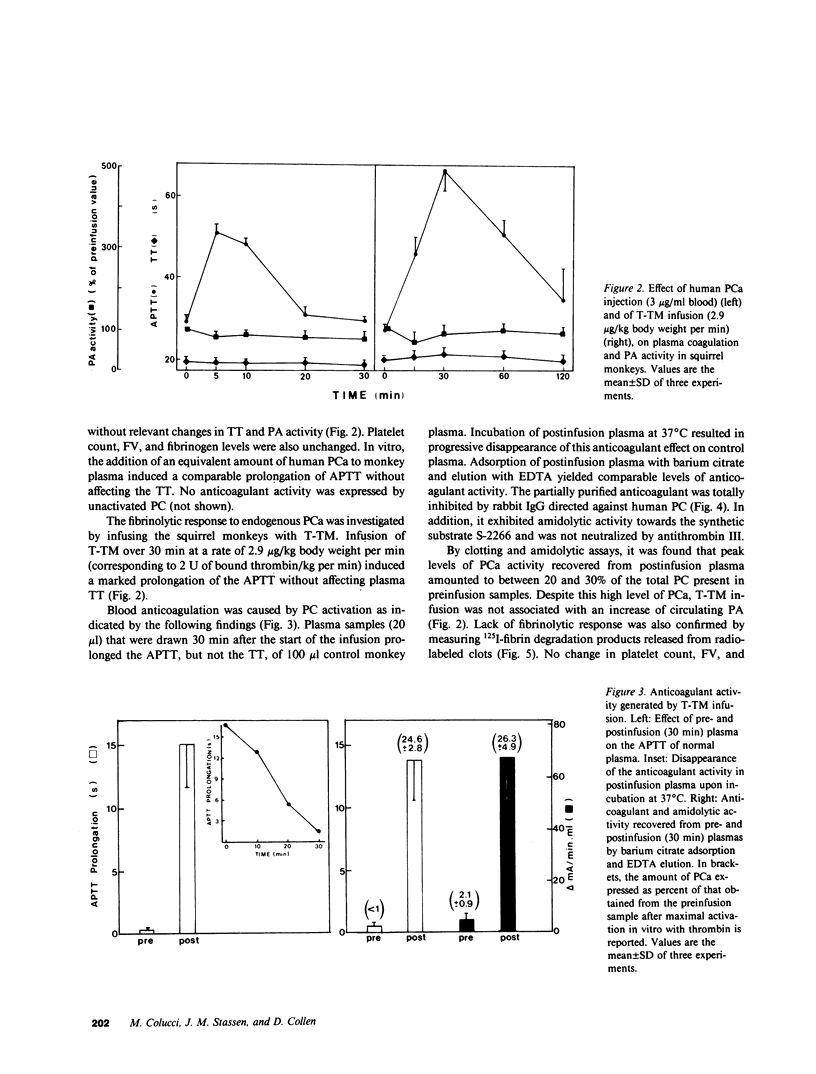
Abstract
Protein C is a circulating proenzyme which, upon activation, exerts a potent anticoagulant activity. Infusion of activated bovine protein C into dogs is accompanied by an increase of circulating tissue plasminogen activator (PA) activity. However, the evidence that human protein C shares a similar profibrinolytic capacity is still lacking. Therefore, we investigated the profibrinolytic properties of human protein C in squirrel monkeys (Samiri sciureus). Injection of activated human protein C resulted in prolongation of the activated partial thromboplastin time but was not associated with increased fibrinolytic activity of blood. Similarly, activation of endogenous protein C (up to 20-30%) by infusion of thrombin-thrombomodulin complex markedly reduced blood coagulability without being accompanied by an increase of circulating PA activity. The in vivo-generated anticoagulant activity was identified as activated protein C by the following observations. It was neutralized by rabbit anti-human protein C-IgG, was slowly inhibited by plasma but not by anti-thrombin III, was adsorbable on barium citrate, and expressed amidolytic activity. Activation of protein C appeared to be selective since other parameters such as thrombin time, platelet count, fibrinogen, and factor V levels were unaffected by thrombin-thrombomodulin infusion. Infusion of human plasma derived from whole blood incubated in vitro with human activated protein C also did not induce a fibrinolytic response, suggesting that no second messengers with PA-releasing activity were being generated in blood. It is concluded that in a primate, neither the administration of activated human protein C nor the activation of endogenous protein C are associated with an increase of fibrinolytic activity. These findings question the role of this enzyme in the regulation of PA release in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- Ceustermans R., Hoylaerts M., De Mol M., Collen D. Preparation, characterization, and turnover properties of heparin-antithrombin III complexes stabilized by covalent bonds. J Biol Chem. 1982 Apr 10;257(7):3401–3408. [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Generation of fibrinolytic activity by infusion of activated protein C into dogs. J Clin Invest. 1981 Nov;68(5):1221–1228. doi: 10.1172/JCI110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comp P. C., Jacocks R. M., Ferrell G. L., Esmon C. T. Activation of protein C in vivo. J Clin Invest. 1982 Jul;70(1):127–134. doi: 10.1172/JCI110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Harris K. W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982 Jul 25;257(14):7944–7947. [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Marciniak E. Inhibitor of human blood coagulation elicited by thrombin. J Lab Clin Med. 1972 Jun;79(6):924–934. [PubMed] [Google Scholar]
- Marlar R. A., Kleiss A. J., Griffin J. H. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982 May;59(5):1067–1072. [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Sala N., Owen W. G., Collen D. A functional assay of protein C in human plasma. Blood. 1984 Mar;63(3):671–675. [PubMed] [Google Scholar]
- Taylor F. B., Jr, Carroll R. C., Gerrard J., Esmon C. T., Radcliffe R. D. Lysis of clots prepared from whole blood and plasma. Fed Proc. 1981 May 15;40(7):2092–2098. [PubMed] [Google Scholar]


