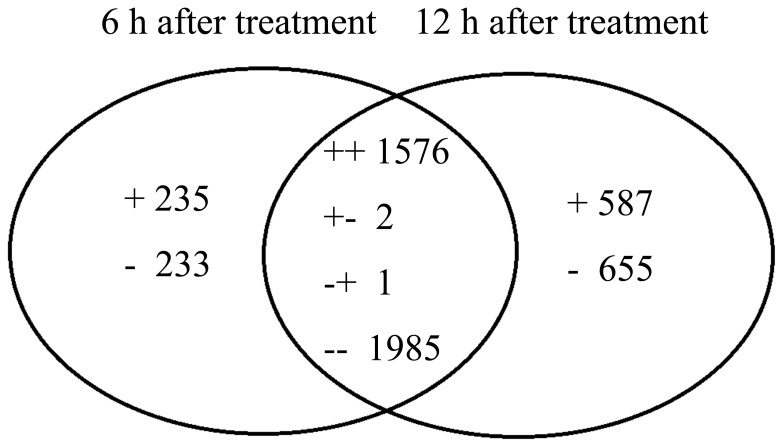Abstract
Background
Only a small amount of solar ultraviolet C (UV-C) radiation reaches the Earth's surface. This is because of the filtering effects of the stratospheric ozone layer. Artificial UV-C irradiation is used on leaves and fruits to stimulate different biological processes in plants. Grapes are a major fruit crop and are grown in many parts of the world. Research has shown that UV-C irradiation induces the biosynthesis of phenols in grape leaves. However, few studies have analyzed the overall changes in gene expression in grape leaves exposed to UV-C.
Methodology/Principal Findings
In the present study, transcriptional responses were investigated in grape (Vitis vinifera L.) leaves before and after exposure to UV-C irradiation (6 W·m−2 for 10 min) using an Affymetrix Vitis vinifera (Grape) Genome Array (15,700 transcripts). A total of 5274 differentially expressed probe sets were defined, including 3564 (67.58%) probe sets that appeared at both 6 and 12 h after exposure to UV-C irradiation but not before exposure. A total of 468 (8.87%) probe sets and 1242 (23.55%) probe sets were specifically expressed at these times. The probe sets were associated with a large number of important traits and biological pathways, including cell rescue (i.e., antioxidant enzymes), protein fate (i.e., HSPs), primary and secondary metabolism, and transcription factors. Interestingly, some of the genes involved in secondary metabolism, such as stilbene synthase, responded intensely to irradiation. Some of the MYB and WRKY family transcription factors, such as VvMYBPA1, VvMYB14, VvMYB4, WRKY57-like, and WRKY 65, were also strongly up-regulated (about 100 to 200 fold).
Conclusions
UV-C irridiation has an important role in some biology processes, especially cell rescue, protein fate, secondary metabolism, and regulation of transcription.These results opened up ways of exploring the molecular mechanisms underlying the effects of UV-C irradiation on grape leaves and have great implications for further studies.
Introduction
In nature, solar radiation comprises electromagnetic radiation of different wavelengths and broadly classified as ultraviolet radiation (UV≈200–400 nm), photosynthetically active radiation (PAR≈400–700 nm), and far red radiation (FR≈700–780 nm). Approximately 7–9% of all solar radiation that reaches the Earth's surface is in the UV range. UV radiation is broadly classified based on wavelength as UV-A radiation (320–400 nm), which cannot be absorbed by the stratospheric ozone layer and is fully transmitted to the Earth's surface. UV-B radiation (280–320 nm), which is filtered through the ozone layer and, therefore makes up only a small amount of the radiation that reaches the Earth's surface; and UV-C radiation (200–280 nm), which is the most hazardous range of UV light, but it is physiologically insignificant because these wavelengths are almost completely absorbed by the atmosphere [1]–[3]. It is therefore important to study the effects of UV radiation on plants in detail. The current knowledge regarding the ecophysiological impact of UV radiation on plants has come largely from field experiments involving natural and artifical UV radiation [4]–[5]. UV-B and UV-C may penetrate plant tissues, damage proteins and membranes, and block replication and transcription of DNA, but UV-A has not been found to have any deleterious effect [5]–[7].
Although more studies have focused on UV-B and UV-A than on UV-C, some recent studies have reported that artificial UV-C has many regulatory effects on plant morphology, physiology, and biochemistry [8]–[13]. UV-C irradiation has been shown to increase the accumulation of flavonoids, triterpene, and resveratrol compounds in lettuce, Quillaja brasiliensis, and peanut leaves [14]–[15]. It also led to a decrease in pea fresh weight and in the concentration of leaf pigments and free proline in pea plants. This was accompanied by an increase in malondialdehyde [16]. UV-C radiation decreased soluble carbohydrates, reducing sugar, chlorophyll, and proline concentrations and increasing the concentrations of UV-absorbing pigments, soluble proteins, and glucosinolate in canola leaves (Brassica napus L.) [17]. It increased jasmonate and polyamine concentrations in leaves of apple seedlings and scoparone content in citrus leaves [10], [18]. The production of these compounds is associated with other inducible defenses, such as cell wall modification, defense enzymes, and antioxidant activity. Pre-storage treatment of table grapes, tomatoes, mangoes, and citrus fruit with low doses of UV-C can reduce postharvest decay [19]–[21]. UV-C was found to promote the expression of an array of genes [8]–[11], [22].
Because they are one of the world's most important commercial crops, grapevines are cultivated worldwide. They are used as raw materials of many consumer products such as juices, liquors, and wines [23]. UV-C exposure has been shown to efficiently induce the biosynthesis of resvertrol and its derivates in grapevine organs, including leaves and berries [24]–[27]. It is here speculated that a large of change in gene expression and metabolism should appear in grape leaves exposed to UV-C irradiation, based on results reported in these plants. However, none of these studies have analyzed the overall changes in gene expression or metabolism induced by UV-C in grape leaves and berries. DNA microarrays permit an overall view of gene expression involved in response to a particular stimulus in a rapid, efficient, and cost-effective manner [28]–[30]. Here, we focus on changes in gene expression of grape leaves in response to UV-C irradiation with Affymetrix Vitis vinifera (Grape) Genome Array, in order to understand the molecular basis of the response of grapevines to UV-C irradiation.
Materials and Methods
Plant materials and treatments
Vines of V. vinifera ‘Hongbaladuo’ were used in these experiments. They were grown in the vineyard at the Institute of Botany, Chinese Academy of Sciences, Beijing. According to the method described by Wang et al., healthy, mature (30-day old) leaves of similar size were detached from the shoot at 08:00–09:00 a.m. [27]. Leaf petioles were immediately inserted into the water in a bucket, and were then rapidly transferred from water buckets to triangular flasks containing ddH2O. All leaves were incubated in the dark at 25°C for half an hour. Then the leaf abaxial surfaces were exposed for 10 min to 6 W·m−2 UV-C irradiation provided by a UV-C lamp (Model ZW30S26W, Beijing Lighting Research Institute, China). The leaves were kept in the flasks until sampling. Samples were collected at 0, 6, and 12 h after the initiation of treatments. Each treatment was performed three times, and each replication consisted of six leaves. After sampling, the leaves were ground into powder in liquid nitrogen and stored at −80°C until analysis.
RNA extraction, amplification, labeling, and hybridization
Total RNA was extracted from grape leaves using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.) according to the manufacturer's instructions and digested with DNase I at 37°C for 15 min to remove any contaminating DNA. The RNA was cleaned with an RNeasy Kit (Qiagen, Hilden, Germany). RNA quantity and quality were determined using spectrophotometry and 1% formaldehyde denaturing gel electrophoresis. Samples with bright bands of ribosomal 28S to 18S RNA in a ratio >1.5∶1 were used for microarray analysis [28]–[30]. An Affymetrix Gene-Chip V. vinifera (Grape) Genome Array, which contains 15,700 probe sets covering 14,000 V. vinifera transcripts and 1,700 transcripts from other Vitis species, was used for microarray analysis. Hybridization, data capture, and analysis were performed by CapitalBio Corporation (Beijing, China), a service provider authorized by Affymetrix Inc. (Santa Clara, CA, U.S.). Briefly, 200 ng of total RNA was used for cDNA synthesis. This produced biotin-tagged cRNA with a MessageAmp™ Premier RNA Amplification Kit (Ambion). A 10 µg fragmented cRNA with control was hybridized to each GeneChip array at 45°C for 16 h (Affymetrix Gene Chip Hybridization Oven 640) according to the manufacturer's instructions. After hybridization, the GeneChip arrays were washed and stained with streptavidin phycoerythrinonan (SAPE) with an Affymetrix Fluidics Station 450 followed by scanning with an Affymetrix GeneChip Scanner 3000 7G. Microarray data processing produced microarray image files (CEL).
Statistical analysis
The signal intensities of each feature were background adjusted and normalized via the quantile normalization performed by Robust Multichip Analysis (RMA) [31]. For defining differentially expressed (DE) probe sets, two group comparisons were performed for all probe sets between 0 and 6 h and between 0 and 12 h were performed via significance analysis of microarrays (SAM 2.10) [32]. Two filtering criteria were used: (1) P-value <0.05; (2) Fold change 12 h/0 h (6 h/0 h) ≧2 or ≤0.5. The lower confidence bound (LCB) of the 95% confidence interval of the fold changes was used [33]. The reliability of the comparison criteria was assessed by checking the false discovery rate (FDR) when permuting samples 1000 times. Probe sets that satisfied the criteria given above were chosen for further analysis. The DE prob sets were clustered with Gene Cluster 3.0. After log2 transformation, hierarchical clustering was performed on genes and arrays. Gene annotation and determination of functional categories were performed using data at PLEXdb (http://www.plexdb.org/) and based upon the findings reported by Deluc et al. [34].
Validation of microarray data with real-time quantitative PCR (qRT-PCR)
Total RNA extraction was the same as that used for microarray analysis, as described above. The total RNA was treated with DNase I (Promega) to avoid DNA contamination. One microgram of RNA was reverse transcribed using the Superscript II reverse transcriptase (Invitrogen) with an oligo(dT)15 primer according to the manufacturer's instructions (Tiangen Biotech, Beijing, China). qRT-PCR experiments were conducted using Real Master Mix (SYBR Green) (Tiangen Biotech, Beijing, China). Reactions were carried out on a Step One Plus Real-Time PCR system (Life Technologies Corporation). The following standard thermal profile was used for all PCR experiments: 94°C for 5 min; 40 cycles of 95°C for 15 s, and 60°C for 60 s. Fluorescence signals were captured at the end of each cycle, and the melting curve analysis was performed from 68°C to 95°C to confirm the specificity of the PCR reaction. Probe set-specific primers were designed using Primer 5 software (Additional file S1). The amplification curves were analyzed with Biogazelle qbase+ software and the amplification efficiency of the primers was 90–110%. The data from the microarrays showed the actin gene (XM_002282480.2, 1606368_s_at) to be expressed stably in response to UV-C, the actin gene was used as the internal control to normalize all the qRT-PCR data. Analyses of qRT-PCR data was performed using the classic (1+E)−ΔΔCT method (CT is the threshold cycles of one gene, E is the amplification efficiency). ΔCT is equal to the difference in threshold cycles for target (X) and reference (R) (CT,X–CT,R), while the ΔΔCT is equal to the difference of ΔCT for control (C, 0 h) and treatment (T, 6 or 12 h) (ΔCT,T–ΔCT,C). The amplification system (e.g., primer and template concentrations) was optimized, and the efficiency was close to 1. The amount of target, normalized to an endogenous reference and relative to a calibrator, was determined as follows: Amount of target = 2−ΔΔCT.
Gene ontology (GO) enrichment analysis of DE probe sets
The GO enrichement analysis of DE probe sets was performed with an online AgriGo tool (http://bioinfo.cau.edu.cn/agriGO) [35]. Specifically, the ‘Parametric Analysis of Gene Set Enrichment (PAGE)’ tool was used for analysis, and ‘vitis vinifera’ was entered as the species. A FDR multi-test adjustment was performed and the P-value <0.01. The other relative parameters were set as default.
Results
Expression and validation of differentially expressed probe sets (DE probe sets)
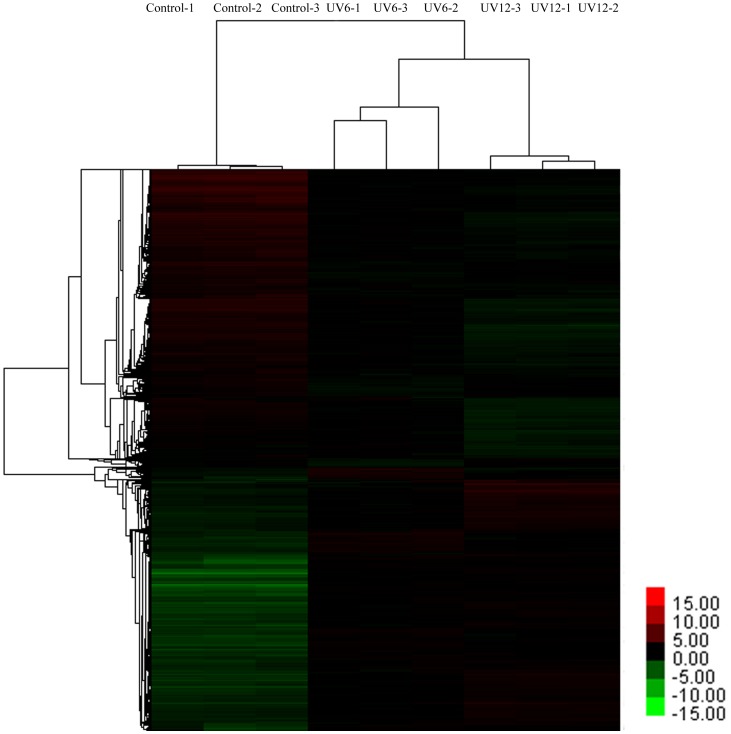
An Affymetrix V. vinifera Genome Array with 15,700 probe sets was used evaluate the transcriptomic changes in grape leaves in response to UV-C. The array data were averaged for three biological replicates and filtered as described in the Materials and methods section. We investigated the transcriptomic change in grape leaves at 6 and 12 h after the initiation of UV-C treatment, and compared it with control (i.e., before UV-C treatment, at 0 h after the initiation of UV-C treatment). A hierarchical clustering was prepared to represent the transcripts of all the DE probe sets at 3 replicates to compare the UV-C responsive transcriptomes (Figure 1). These results indicated that UV-C led to an intense change in the transcriptome. However, only slight differences were observed between 6 and 12 h after exposure to UV-C irradiation. According to the filtering criteria, a total of 5274 (about 33.59% of total probe sets) were defined to be DE probe sets at 6 or 12 h after UV-C treatment. These included 3564 probe sets that appeared at both 6 and 12 h after UV-C treatment, which represented 67.59% of the total DE probe sets. Among DE probe sets, 468 probe sets (8.88%) and 1242 probe sets (23.55%) were differentially expressed specifically at 6 and 12 h respectively (Figure 2). Among the total 5274 DE probe sets, 1576 showed an up-regulated trend and 1985 showed a down-regulated trend at both 6 and 12 h post-exposure to UV-C irradiation, and 3 showed the opposite trends at both points in time. Furthermore, 235 probe sets were uniquely up-regulated at 6 h after UV-C treatment and 233 were down-regulated. There were 587 probe sets that were uniquely up-regulated 12 h after UV-C treatment, and 655 probe sets were down-regulated.
Figure 1. Average linkage hierarchical clustering analysis of the log2 signal values of the 5274 DE probe sets after UV-C.
Control-1, control-2, and control-3 are three replications before UV-C treatment; UV6-1, UV6-2, and UV6-3 are three replications 6 h after UV-C treatment; UV12-1, UV12-2, and UV12-3 are three replications 12 h after UV-C treatment.
Figure 2. Venn diagram of differentially expressed transcripts (both identified and unknown) that were up- and down-regulated 6 and 12 h after UV-C treatment.
The symbols “+” and “−” indicate up- and down-regulated transcripts. A total of 5274 transcripts were significantly (P<0.05) affected by UV-C treatment. There were 235 unique up-regulated transcripts 6 h after UV-C treatment; 587 unique up-regulated transcripts 12 h after UV-C treatment; 233 unique down-regulated transcripts 6 h after UV-C treatment; 655 unique down-regulated transcripts 12 h after UV-C treatment; 1576 transcripts were up-regulated both 6 and 12 h after UV-C treatment; 1985 transcripts were down-regulated both 6 and 12 h after UV-C treatment; 2 transcripts were up-regulated 6 h after UV-C treatment but down-regulated 12 h after UV-C treatment; 1 was down-regulated 6 h after UV-C treatment but up-regulated 12 h after UV-C treatment.
In order to further confirm the results obtained from the microarray analyses, qRT-PCR assays were conducted on 25 probe sets sequences using specific primers (Additional file S1). The qRT-PCR profiles were analyzed using three biological replicates. Linear regression analyses displayed highly significant correlations (r2 = 0.951 for 6 h, and r2 = 0.933 for 12 h) between qRT-PCR and microarray results for the 25 evaluated probe sets (Additional file S2), confirming the validity of the microarray results.
Functional analysis of probe sets responsive to UV-C irradiation
The results of AgriGO enrichment analysis showed 5274 DE probe sets to be enriched in 8 biological processes (Table 1). In general, the up-regulated DE-probe sets were found to be involved in amino acid and derivative metabolic and secondary metabolic processes and response to stimulus. The down-regulated DE-probe sets were enriched in photosynthetic processes. To further determine the pattern of regulation of UV-C response-related probe sets, the probe sets were annotated in PLEXdb (http://www.plexdb.org) and Munich Information Center for Protein (MIPS) sequences, and classified based on MIPS. A total of 2990 probe sets (about 56.70% of total DE probe sets) were functionally annotated and analyzed further. An additional 1002 probe sets (about 19% of total DE probe sets) matched genes with unknown functions or had unclear classifications (unclassified), and 1266 (about 24% of total DE probe sets) probe sets did not have any BLAST hits in public, non-redundant databases. Expression patterns and functional categories of the 2990 annotated probe sets are shown in Additional files S3–S6 and Table 2, respectively. Common probe sets were compared and the probe sets that were specifically expressed at 6 or 12 h after exposure to UV-C irradiation were analyzed based on functional classification.
Table 1. Functional enrichment analysis of DE probe sets.
| GO Term | Onto | Number | Description | Z-score | |
| 6 h | 12 h | ||||
| GO:0006519 | P | 320 | Cellular amino acid and derivative metabolic process | 6.8 | 7.4 |
| GO:0006950 | P | 290 | Response to stress | 6.6 | 6.3 |
| GO:0009607 | P | 73 | Response to biotic stimulus | 5 | 4.7 |
| GO:0019748 | P | 126 | Secondary metabolic process | 4.7 | 4.3 |
| GO:0050896 | P | 472 | Response to stimulus | 4.7 | 4.8 |
| GO:0051704 | P | 64 | Multi-organism process | 4.3 | 4.3 |
| GO:0009056 | P | 212 | Catabolic process | 4.3 | 4.9 |
| GO:0015979 | P | 81 | Photosynthesis | −4.4 | −7.5 |
Z-score is the statistical value in PAGE calculation. P represents biological processes. The positive.
values of Z-score indicate the corresponding biology process is up-regulated; The negative.
values of Z-score indicate the corresponding biology process is dowm-regulated.
Table 2. Functional categories of probe sets and expression pattern.
| Commonly regulated at 6 and 12 h | Uniquely regulated at 6 h | Uniquely regulated at 12 h | ||||
| up | down | up | down | up | down | |
| Metabolism | 206 | 262 | 19 | 25 | 70 | 90 |
| Energy | 26 | 71 | 1 | 4 | 14 | 47 |
| Storage protein | 3 | 1 | 1 | 0 | 0 | 0 |
| Cell cyecle and DNA processing | 8 | 29 | 0 | 8 | 12 | 4 |
| Transcription | 90 | 125 | 24 | 12 | 21 | 33 |
| Protein synthesis | 20 | 59 | 1 | 2 | 92 | 15 |
| Protein fate | 110 | 109 | 12 | 16 | 46 | 50 |
| Protein with binding function | 32 | 35 | 2 | 2 | 13 | 18 |
| Protein activity regulation | 7 | 0 | 0 | 0 | 1 | 0 |
| Transport regulation | 126 | 122 | 21 | 18 | 41 | 38 |
| Signal transduction | 99 | 76 | 19 | 5 | 28 | 23 |
| Cell rescue | 105 | 70 | 9 | 11 | 6 | 30 |
| Interaction with cellular environment | 4 | 3 | 0 | 0 | 0 | 0 |
| Plant/fungal specific systemic Sensing and response | 42 | 66 | 4 | 4 | 6 | 18 |
| Transposable elements | 3 | 5 | 0 | 1 | 0 | 0 |
| Cell fate | 10 | 18 | 1 | 0 | 0 | 2 |
| Development | 3 | 4 | 1 | 1 | 4 | 3 |
| Biogenesis of cellular component | 17 | 47 | 7 | 4 | 7 | 9 |
| Total | 911 | 1102 | 122 | 113 | 361 | 380 |
Common responsive probe sets 6 and 12 h post-exposure to UV-C irradiation
There were 911 annotated probe sets that were up-regulated at both 6 and 12 h after exposure to UV-C treatment compared with pre-exposure. They were divided into groups according to their putative involvement in different cellular events (Additional file S3). Of these, 206 (22.61%) were involved in metabolism, which contained the most probe sets. Among these probe sets, 41 were assigned to lower categories of secondary metabolism, including 13 probe sets representing stilbene synthase (up-regulated 8–700-fold). There were 126, 110, and 105 probe sets involved in transport regulation, protein fate, and cell rescue, respectively. Of these, 99 were related to signal transduction. These included serine/threonine kinase, leucine-like receptor kinase, chitin elicitor receptor kinase, N-acetyl-1-glutamate kinase, mitogen-activated protein kinase, MAP kinase, and MAPK kinase. Some 20 probe sets were involved in G-protein mediated signal transduction, and 24 probe sets were involved in Ca2+-mediated signal transduction. There were 90 probe sets associated with transcription, such as MYB family transcription factors, WRKY family transcription factors, and zinc finger family proteins. The probe set representing VvMYB14 (NM_001281203.1) was up-regulated by about 206-fold 6 h after exposure to UV-C irradiation, and it was still up-regulated 211-fold at 12 h. WRKY57-like (XM_002275540.1) and ethylene responsive element binding factor (XR_077949.1) were also strongly up-regulated 170- and 117-fold, respectively. Additional file S4 shows 1102 probe sets to be down-regulated at both 6 and 12 h after UV-C irradiation. Of these, 262 were involved in metabolism. Likewise, 48 probe sets were associated with secondary metabolism, including probe sets involved the in biosynthesis of alkaloids, porphyrins, lignins, and flavonoids. There were 125 probe sets associated with transcription, including MYB family genes, WRKY family genes, and others. VvMYBPA1, which regulates tannin biosynthesis, was among these probe sets. It was down-regulated by 2.7 and 3.13-fold 6 and 12 h after exposure to UV-C irradiation, respectively. There were 122 and 109 probe sets involved in transport regulation and protein fate, respectively. Moreover, 76 probe sets were involved in signal transduction, which included several protein kinases, probe sets associated with G-protein, small GTPase, Ca2+, fatty acid derivatives, polyphosphoinositde, and receptor enzyme-mediated signal transduction, and probe sets involved in transmembrane receptor protein tyrosine kinase and serine/threonine kinase signaling pathways. There was only one annotated gene that showed an opposite trend at 6 and 12 h after UV-C treatment. The probe set representing serine carboxy peptidase was up-regulated at 6 h and down-regulated at 12 h after the treatment.
Probe sets specifically up- and down-regulated 6 h post-exposure to UV-C irradiation
As shown in Additional file S5, there were a total of 122 up-regulated probe sets identified, which were uniquely responsive to UV-C at 6 h post-exposure to UV-C irradiation. Of these, 24 probe sets were involved in transcription regulation, including two MYB family transcription factors, one WRKY DNA-binding protein, two bHLH DNA-binding proteins, and three zinc finger family proteins. There were 21 probe sets associated with transporters and 19 associated with metabolism. There were 19 signal transduction-related probe sets, including one NAD kinase, two MAP kinases, one shaggy-related kinase, one ras-related protein, three calcium-binding EF-hand family proteins, and one calcium-dependent protein kinase.
There were 113 down-regulated probe sets responding to UV-C uniquely at 6 h in grape leaves (Additional file S5). Of these, 25 probe sets were metabolism-related and involved in amino acid, pyrimidine nucleotide, phosphate, carbohydrate compounds, lipids, fatty acids, isoprenoid metabolism, and biosynthesis of phenylpropanoids. There were 18 probe sets associated with down-regulated transporters, including three sec14p-like phosphatidylinositol transfer family proteins, two sugar transporters, one amino acid transporter, and two H(+)-ATPases. Transcript regulatory probe sets included one GRA family transcription factor, one GAGA-binding transcriptional activator, and one transcriptional co-activator p15 (PC4) family protein.
Probe sets specifically up- or down-regulated 12 h post-exposure to UV-C irradiation
In Additional file S6, a total of 361 up-regulated probe sets were found to respond to UV-C uniquely at 12 h. Of these, 25 probe sets were found to be associated with metabolism. There were 41, 28, and 21 probe sets involved in transport regulation, signal transduction, and transcription, respectively. There were 92 probe sets related to protein synthesis. These included probe sets associated with ribosomal proteins and translation-initiation-related proteins. Similarly, 46 probe sets were found to be related to protein fate. These contribute to protein folding and stabilization, targeting, sorting and translocation, and modification and degradation.
In contrast, 380 probe sets were down-regulated (Additional file S6). Of these, 90 probe sets were associated with metabolism and 65 were involved in protein synthesis and protein fate. There were 47 energy-related probe sets down-regulated at 12 h after treatment, and 38 down-regulated probe sets were found to be transporter-related, including the three phosphate translocator related proteins, three antiporters, one transport ATPase, and several ABC transporters.
Discussion
UV-C irradiation disrupts cellular homeostasis in plants, severely retarding growth and development, sometimes causing death. Plants exposed to UV-C irradiation exhibit a characteristic set of cellular and metabolic responses [8]–[13]. The results of the present work showed that some genes were repressed and others were triggered after exposure to UV-C irradiation. In the leaves of Vitis vinifera, biological functions and regulatory networks of genes were coordinated and mobilized in response to UV-C irradiation. Based on these results, the discussion section focuses on the following factors.
Antioxidant enzymes and defense response
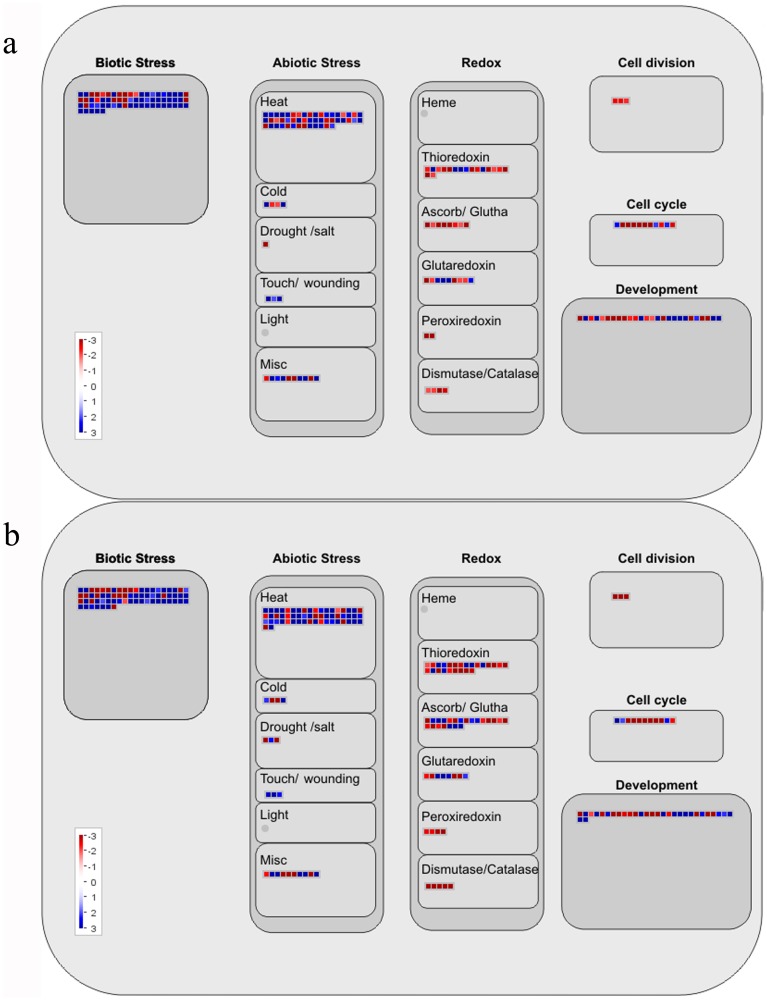
The generation of reactive oxygen species (ROS) is a common feature of plant responses to different environmental stresses [36]. Oxidative stress due to UV-B exposure has previously been shown to increase the activity levels of different antioxidant enzymes, such as superoxide dismutase, ascorbate peroxidase, glutathione-S-transferases, glutathione-reductase, peroxidases. and catalases [37]–[39]. Although UV-C irradiation can cause DNA lesions and other damage to plants in a manner similar to UV-B, the response of plants to UV-C may manifest in different pathways [6], [40]. There is little available information regarding enzymatic antioxidant defense response to UV-C irradiation. In the present study, at 6 and 12 h after exposure to UV-C irradiation, the expression of two peroxidase probe sets and two thylakoid ascorbate peroxidase probe sets increased significantly. The expression of a stromal ascorbate peroxidase probe set was found to increase slightly at 6 and 12 h. The expression of most genes involved in oxygen and radical detoxification, such as superoxide dismutase, peroxidase, dehydroascorbate reductase, glutathione S-transferase, ascorbate peroxidase, glutathione peroxidase, and thioredoxin peroxidase, showed decreased expression (Table 3, Figure 3). This indicated that the present UV-C intensity could be stronger for grape leaves, or that the responses to UV-C may differ from UV-B in grape leaves. This is worth studying further. In addition, other genes contributing to stress defense, such as ß-1,3-glucanase and chitinase probe sets, were greatly up-regulated at 6 (67.98-fold) and 12 h (273.06-fold) after exposure to UV-C irradiation (Table 3, Figure 3).
Table 3. Genes involved in stress defense.
| Probe set ID | Fold change at 6 h | Fold change at 12 h | Annotation |
| 1609321_at | 9.85 | 21.65 | Peroxidase superfamily protein |
| 1615967_at | 3.41 | 8.64 | Peroxidase precursor |
| 1621336_at | 1.83 | 2.70 | Stromal ascorbate peroxidase |
| 1609231_at | 1.73 | 2.48 | Thylakoidal ascorbate peroxidase |
| 1620826_s_at | 1.65 | 2.30 | Thylakoidal ascorbate peroxidase |
| 1616657_at | 0.49 | 0.56 | Superoxide dismutase [Cu-Zn] |
| 1622739_at | 0.48 | 0.48 | Peroxidase superfamily protein |
| 1609478_s_at | 0.84 | 0.46 | Class III peroxidase |
| 1611203_at | 0.64 | 0.45 | Dehydroascorbate reductase 1 |
| 1615206_s_at | 0.51 | 0.45 | Glutathione S-transferase |
| 1611993_at | 0.61 | 0.43 | Ascorbate peroxidase 3 |
| 1608089_at | 0.50 | 0.42 | Glutathione peroxidase |
| 1611871_at | 0.63 | 0.41 | Dehydroascorbate reductase |
| 1620356_x_at | 0.60 | 0.35 | Glutathione S-transferase |
| 1618599_at | 0.37 | 0.33 | Superoxide dismutase |
| 1609324_at | 0.58 | 0.32 | Glutathione S-transferase |
| 1619210_at | 0.47 | 0.29 | Superoxide dismutase [Cu-Zn] |
| 1614776_a_at | 0.51 | 0.28 | Superoxide dismutase [Cu-Zn] |
| 1617515_at | 0.48 | 0.25 | L-ascorbate peroxidase |
| 1614361_at | 0.53 | 0.24 | Peroxidase superfamily protein |
| 1608433_at | 0.29 | 0.11 | Thioredoxin superfamily protein |
| 1614204_at | 0.30 | 0.09 | Thioredoxin superfamily protein |
| 1613132_s_at | 0.15 | 0.07 | Peroxidase superfamily protein |
| 1612707_at | 0.14 | 0.07 | Superoxide dismutase |
| 1618920_at | 0.07 | 0.05 | Peroxidase superfamily protein |
| 1608586_at | 0.08 | 0.04 | Peroxidase superfamily protein |
| 1613461_s_at | 241.76 | 341.8 | Class IV chitinase |
| 1611710_at | 169.87 | 263.66 | Class IV chitinase |
| 1613999_x_at | 55.75 | 78.07 | Chitinase A |
| 1617192_at | 107.00 | 68.16 | Class IV chitinase |
| 1608864_s_at | 43.94 | 55.17 | Acidic endochitinase precursor |
| 1618373_at | 25.89 | 35.31 | Chitinase A |
| 1607557_at | 5.18 | 31.96 | Class IV chitinase |
| 1621319_s_at | 4.24 | 17.00 | Class IV chitinase |
| 1613871_at | 13.92 | 11.82 | Chitinase |
| 1617430_s_at | 4.50 | 7.05 | Basic chitinase |
| 1620505_at | 3.50 | 4.67 | Chitinase class I |
| 1608262_at | 2.15 | 3.66 | Class I extracellular chitinase |
| 1611876_s_at | 3.33 | 3.13 | Acidic endochitinase precursor |
| 1606625_at | 3.76 | 2.61 | Class IV chitinase |
| 1612050_at | 2.08 | 1.00 | Chitinase A |
| 1621583_at | 0.34 | 0.23 | Chitinase-like protein 2 |
| 1619916_s_at | 55.41 | 273.06 | ß -1,3-glucanase 3 |
| 1615595_at | 67.98 | 114.52 | ß -1,3-glucanase |
| 1620063_at | 39.61 | 75.38 | ß -1,3-glucanase 1 |
| 1610722_at | 8.03 | 10.36 | ß -1,3-glucanase 1 |
| 1618425_at | 7.36 | 6.66 | ß -1,3-glucanase 3 |
| 1619828_at | 0.94 | 0.38 | ß -1,3-glucanase 2 |
| 1618409_at | 0.29 | 0.17 | ß -1,3-glucanase-like protein |
Figure 3. MapMan visualization of changes in the celluar responses pathway at (a) 6 h and (b) 12 h after UV-C treatment.
Red box represent up-regulated probsets; Blue boxesrepresent down-regulated probsets.
Secondary metabolism related to phenylpropanoid (PAL) pathway
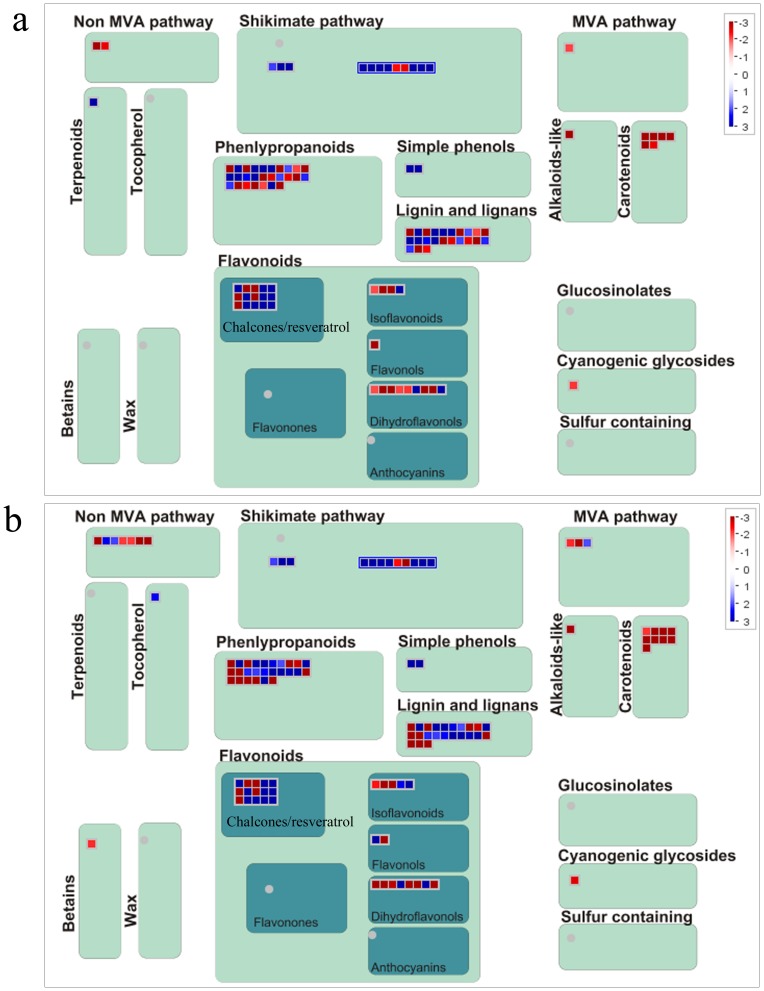
UV-C displayed a significant inductive effect on secondary metabolism in grapevine leaves, especially on the PAL pathway (Table 4, Figure 4). Phenol is an important nonenzymatic compound. It functions by scavenging ROS to protect plants. Flavonoids are antioxidant molecules that act as free radical scavengers and contribute to the protection of plant components (such as chloroplasts and other organelles) from damage caused by UV-B irradiation [41]–[42]. However, whether flavonoids provide a similar response to UV-C irradiation is not clear. The results of the microarray performed here showed that four probe sets representing CHS, encoding the key enzyme for the synthesis of flavonoids, did not increase after UV-C treatment. In contrast, they were down-regulated about 2 to 10-fold (Table 4). Except anthocyanidin 3-O-glucosyltransferase and flavonol synthase were up-regulated 75 and 10-fold respectively, most of enzymes, such as flavanone 3-hydroxylase, dihydroflavonol-4-reductase, leucoanthocyanidin reductase 2, and anthocyanidin reductase, were down-regulated (Table 4, Figure 4). These enzymes were involved in isoflavonoid, dihydroflavonol and flavonol biosythsis.
Table 4. Genes involved in the biosynthesis of resveratrol and flavonoids.
| Probe set ID | Fold change at 6 h | Fold changeat 12 h | Annotation |
| 1609696_x_at | 539.05 | 753.74 | Stilbene synthase 1 |
| 1610850_at | 515.92 | 707.8 | Stilbene synthase 1 |
| 1620964_s_at | 465.19 | 646.25 | Stilbene synthase 1 |
| 1611190_s_at | 360.37 | 489.72 | Stilbene synthase 1 |
| 1610824_s_at | 371.21 | 387.27 | Stilbene synthase 2 |
| 1612804_at | 227.87 | 236.81 | Resveratrol synthase |
| 1622638_x_at | 224.51 | 224.8 | Resveratrol synthase |
| 1609697_at | 306.96 | 200.09 | Stilbene synthase 4 |
| 1608009_s_at | 182.29 | 153.71 | Resveratrol synthase 2 |
| 1616575_at | 7.55 | 27.51 | Resveratrol synthase |
| 1614621_at | 23.26 | 26.37 | Stilbene synthase 1 |
| 1610070_at | 11.91 | 17.84 | Stilbene synthase |
| 1606750_at | 6.84 | 8.28 | Stilbene synthase 3 |
| 1619011_at | 0.49 | 0.48 | Chalcone synthases |
| 1606663_at | 0.23 | 0.17 | Chalcone synthase |
| 1607732_at | 0.11 | 0.09 | Chalcone synthase |
| 1617019_at | 0.09 | 0.09 | Chalcone synthase |
| 1610206_at | 6.47 | 7.9 | Phenylalanine ammonia-lyase 2 |
| 1619642_at | 0.28 | 0.31 | Phenylalanine ammonia-lyase |
| 1613113_at | 0.26 | 0.22 | Phenylalanine ammonia-lyase |
| 1610821_at | 10.71 | 20.52 | Cinnamate-4-hydroxylase |
| 1616191_s_at | 7.72 | 14.31 | Cinnamate-4-hydroxylase |
| 1609307_at | 8.96 | 7.22 | 4-coumarate:CoA ligase 1 |
| 1606753_at | 1.44 | 2.64 | 4-coumarate–CoA ligase-like |
| 1619320_at | 3.19 | 2.03 | 4-coumarate:CoA ligase 3 |
| 1607228_at | 1.19 | 3.25 | Resveratrol/hydroxycinnamic acid O-glucosyltransferase |
| 1608579_at | 1.02 | 2.43 | Resveratrol/hydroxycinnamic acid O-glucosyltransferase |
| 1620342_at | 133.44 | 211.13 | Caffeic acid O-methyltransferase |
| 1607475_s_at | 56.11 | 90.11 | Caffeic acid O-methyltransferase |
| 1621563_x_at | 16.04 | 13.2 | Caffeic acid O-methyltransferase |
| 1612124_at | 16.1 | 13.07 | Caffeic acid O-methyltransferase |
| 1616434_s_at | 6.53 | 6.09 | Caffeic acid O-methyltransferase |
| 1619682_x_at | 3.32 | 2.99 | Caffeic acid O-methyltransferase |
| 1614191_s_at | 0.78 | 2.24 | Caffeic acid O-methyltransferase-like |
| 1619450_s_at | 2.55 | 2.89 | Caffeic acid 3-O-methyltransferase |
| 1615085_at | 0.69 | 2.12 | Caffeic acid 3-O-methyltransferase 1 |
| 1615401_at | 89.65 | 75.24 | Anthocyanidin 3-O-glucosyltransferase |
| 1618155_at | 2.94 | 2.15 | Anthocyanidin 3-O-glucosyltransferase 6 |
| 1621051_at | 2.56 | 1.64 | Anthocyanidin 3-O-glucosyltransferase 2 |
| 1609876_at | 0.33 | 0.43 | Anthocyanidin 5,3-O-glucosyltransferase |
| 1618389_at | 0.47 | 0.38 | Anthocyanin 5-O-glucoside-4″′-O-malonyltransferase |
| 1614045_at | 29.06 | 37.72 | Ferulic acid 5-hydroxylase 1 |
| 1608791_at | 1.78 | 10.38 | Flavonol synthase |
| 1618551_at | 0.07 | 0.05 | Flavonol synthase |
| 1610780_at | 4.14 | 3.71 | Shikimate kinase 1 |
| 1617079_at | 0.42 | 0.42 | Shikimate kinase like 1 |
| 1612989_at | 0.39 | 0.25 | Shikimate kinase like 1 |
| 1614643_at | 1.92 | 3.27 | Caffeoyl-CoA O-methyltransferase |
| 1611897_s_at | 0.48 | 0.36 | Caffeoyl-CoA O-methyltransferase |
| 1607939_at | 0.54 | 0.33 | Caffeoyl-CoA O-methyltransferase |
| 1607607_s_at | 0.46 | 0.55 | Flavanone 3-hydroxylase |
| 1607739_at | 0.13 | 0.19 | Flavanone 3-hydroxylase |
| 1611847_at | 0.09 | 0.09 | Flavonoid 3′,5′-hydroxylase |
| 1607760_at | 0.07 | 0.06 | Flavonoid 3′,5′-hydroxylase |
| 1616437_at | 0.3 | 0.54 | Dihydroflavonol-4-reductase |
| 1615174_s_at | 0.49 | 0.43 | Leucoanthocyanidin reductase 2 |
| 1612134_at | 0.64 | 0.29 | Anthocyanidin reductase |
| 1619986_s_at | 0.13 | 0.17 | UDP-glucose:flavonoid 3-O-glucosyltransferase |
| 1621418_at | 0.22 | 0.08 | UDP-glucose flavonoid 3-O-glucosyltransferase |
| 1615481_at | 0.09 | 0.1 | Cytochrome B5 isoform D |
| 1614485_at | 0.05 | 0.08 | Putative anthranilate N-hydroxycinnamoyl/benzoyltransferase |
| 1618112_at | 0.14 | 0.14 | Putative anthocyanidin-3-glucoside rhamnosyltransferase |
| 1612436_s_at | 13.45 | 13.86 | Isoflavone reductase-like protein 3 |
| 1611389_at | 0.19 | 0.12 | Isoflavone reductase-like protein 6 |
| 1610923_a_at | 1.31 | 2.56 | Isoflavone reductase-like protein 5 |
| 1618991_s_at | 0.23 | 0.22 | Isoflavone reductase-like protein 6 |
| 1617421_at | 8.05 | 10.78 | Isoflavone reductase-like protein 3 |
| 1615912_at | 10.09 | 10.89 | Chalcone-flavanone isomerase family protein |
| 1620424_at | 0.21 | 0.18 | Chalcone-flavanone isomerase family protein |
| 1616977_at | 4.98 | 3.87 | Putative iron/ascorbate-dependent oxidoreductase |
| 1609765_s_at | 0.09 | 0.11 | Leucoanthocyanidin dioxygenase |
| 1614441_at | 75.67 | 174.22 | Leucoanthocyanidin dioxygenase-like |
| 1607805_s_at | 14.12 | 3.84 | Flavonoid 3′-monooxygenase |
Figure 4. MapMan visualization of changes in the secondary metabolism pathway at (a) 6 h and (b) 12 hafter UV-C treatment.
Red boxesrepresent up-regulated probsets; Blue boxesrepresent down-regulated probsets. Probsets for PAL, C4H and 4CL are covered in phenlypropanoids pathway; probsets for CHS, STS and resveratrol O-glucosyltransferase are covered in the chalcones/resveratrol pathway.
Res is a nonflavonoid phenol present in the tissues and organs of several plant families, such as Arachaceae, Vitaceae, and Pinaceae [43]. Previous studies have reported the biological activity of this polyphenol, i.e., estrogenic activity, cardiovascular protective effects, neuroprotective capacity, and cancer chemopreventive activity [44]. The ability of UV-C to induce Res accumulation in grape leaves and berries has attracted special attention [24], [45]–[46]. Cantos et al. irradiated grape berries with UV-C, and the resveratrol concentration was 11 times higher than that of controls. Bonomelli et al. showed that grape leaves treated with UV-C irradiation accumulated Res and that the concentration reached 400 µg/g DW even though no Res was detected in leaves exposed to natural sunlight alone. Some studies have indicated that the accumulation of Res is caused by up-regulation of STS expression [24], [26], [47]. The results of the microarray performed here show 13 STS probe sets to be up-regulated by 8.28- to 753.74-fold at 6 and 12 h after exposure to UV-C irradiation (Table 4, Figure 4). The different levels of expression of STS probe sets here suggest that different STS genes are regulated differently in response to UV-C irradiation. Dai et al. reported that, in grape leaves, individual STS genes respond differentially to powdery mildew infection [48]. In the present study, the expression of one PAL probe set, two C4H probe sets, three 4CL probe sets, and two resveratrol O-glucosyltransferase probe sets was also up-regulated (Table 4, Figure 4). It is here suggested that, in grape leaves, although both flavonoids and Res are produced through the same phenylpropanoid pathway, they respond to UV-C in different ways. Some transcription factors may up-regulate the expression of PAL, C4H, 4CL, STS, and resveratrol O-glucosyltransferase.
Transcription factors (TFs)
The regulation of gene expression plays a fundamental role in plant response to environmental stimuli. Transcription factors (TFs) belonging to the MYB, ERF, bZIP, and WRKY families have been linked to a suite of mechanisms leading to defense and stress responses [49]–[50]. For this reason, the present discussion focused more on differentially expressed probe sets belonging to these gene families (Table 5, Figure 5). A few members of the R2R3-MYB family have been implicated in plant stress response to cold, UV-B, and wounding. These have been preliminarily shown to regulate plant secondary metabolism [51]. R2R3-MYBs have been established as positive and negative regulators of the biosynthetic enzymes required for the production of phenylpropanoids and flavonoids [52]–[53]. For example, AtMYB4 encodes a protein similar to AmMYB308, which represses one of the key targets genes encoding C4H. Some studies have reported that R2R3-MYBs are involved in the regulation of flavonoid biosynthesis. In grape berries, the MYB proteins act together with bHLH protein and probably WDR protein to control anthocyanin and/or proanthocyanidin synthesis [54]–[58]. In the present experiment, 18 probe sets representing MYB transcription factors were expressed very differently in grape leaves after exposure to UV-C irradiation, especially, VvMYB14 and VvMYB4 (XM_002285157.2). These were up-regulated 211.7-fold and 113.5-fold, respectively, in UV-C treated grape leaves (Table 5, Figure 5). It is possible that VvMYB14 and VvMYB4 regulate resveratrol and flavonoid synthesis. In a previous study, VvMYBPA1 was found to specifically regulate proanthocyanidin (PA) synthesis, positively regulating both of the PA branch enzymes leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR). This causes the formation of PA and may regulate the entire general flavonoid pathway, inducing the promoters of the general flavonoid pathway genes CHI (chalconeisomerase), F3′5 ′H (flavonoid 3′,5′-hydroxylase) and LDOX (leucoanthocyanidin dioxygenase) [58]. The down-regulation of VvMYBPA1 suggests a decrease in the biosynthesis of PAs and even total flavonoids. In this study, the gene VvMYBPA1 was down-regulated more than 2.5-fold at both 6 and 12 h after exposure to UV-C irradiation (Table 5, Figure 5). LAR and ANR were down-regulated, but the expression levels of various members of CHS, CHI, F3′5 ′H, and LDOX were either up- or down-regulated (Table 4, Figure 4).
Table 5. Transcription factors responsive to UV-C irradiation.
| Probe set ID | Fold change at 6 h | Fold change at 12 h | Annotation |
| 1620319_s_at | 206.42 | 211.77 | VvMyb14 |
| 1618260_s_at | 73.61 | 133.5 | Vitis vinifera transcription factor Myb4-like |
| 1622064_at | 71.49 | 91.66 | VvMyb14 |
| 1613545_at | 3.53 | 4.91 | MYB transcription factor |
| 1619386_at | 2.57 | 1.71 | MYB transcription factor R3 type |
| 1618514_at | 1.36 | 3.12 | MYB transcription factor |
| 1609021_at | 0.54 | 2.78 | Vitis vinifera transcription factor Myb59-like |
| 1612264_at | 0.32 | 0.44 | MYB transcription factor |
| 1610512_at | 0.31 | 0.43 | MYB transcription factor |
| 1617092_at | 0.28 | 0.26 | MYB transcription factor |
| 1613239_at | 0.26 | 0.22 | MYB transcription factor |
| 1614416_at | 0.19 | 0.14 | MYB transcription factor |
| 1617998_at | 0.18 | 0.22 | Vitis vinifera R2R3 Myb transcription factor |
| 1618884_at | 0.09 | 0.08 | MYB transcription factor |
| 1613486_at | 0.09 | 0.07 | Vitis vinifera transcription factor MYB1R1-like |
| 1611920_at | 0.04 | 0.04 | MYB transcription factor |
| 1616094_at | 0.37 | 0.32 | VvMYBPA1 |
| 1621872_s_at | 0.13 | 0.14 | Vitis vinifera transcription factor MYB1R1-like |
| 1613407_at | 9.94 | 6.29 | Vitis vinifera probable WRKY transcription factor 33-like |
| 1610775_s_at | 170.22 | 156.51 | Vitis vinifera probable WRKY transcription factor 57-like |
| 1607465_at | 52.85 | 39.2 | Vitis vinifera probable WRKY transcription factor 57-like |
| 1622778_at | 43.54 | 79.12 | Vitis vinifera WRKY-type DNA binding protein 1 mRNA |
| 1606659_s_at | 39.34 | 30.29 | Vitis vinifera probable WRKY transcription factor 65-like |
| 1609130_at | 31.36 | 9.12 | Vitis vinifera probable WRKY transcription factor 48-like |
| 1609636_at | 22.79 | 14.78 | Vitis vinifera probable WRKY transcription factor 33-like |
| 1614806_s_at | 17 | 11.83 | Vitis vinifera probable WRKY transcription factor 40-like |
| 1610064_at | 12.84 | 12.68 | Vitis vinifera probable WRKY transcription factor 33-like |
| 1611285_s_at | 11.04 | 4.93 | Vitis vinifera WKRY protein |
| 1622399_at | 10.72 | 4.46 | Vitis vinifera WKRY protein |
| 1616623_at | 8.14 | 5.05 | Vitis vinifera probable WRKY transcription factor 28-like |
| 1611550_at | 6.68 | 7.04 | Vitis vinifera probable WRKY transcription factor 46-like |
| 1612649_s_at | 4.68 | 3.99 | Vitis vinifera probable WRKY transcription factor 7-like |
| 1611650_at | 4.55 | 3.66 | Vitis vinifera probable WRKY transcription factor 7-like |
| 1619424_at | 2.85 | 7.28 | Vitis vinifera probable WRKY transcription factor 11-like |
| 1613318_at | 2.64 | 1.75 | Vitis vinifera WKRY protein |
| 1622333_at | 2.51 | 0.93 | Vitis vinifera probable WRKY transcription factor 23-like |
| 1620175_at | 0.48 | 0.49 | Vitis vinifera WRKY transcription factor 44-like |
| 1619311_at | 117.45 | 74.79 | Ethylene responsive element binding factor 1 |
| 1619585_at | 13.1 | 12.88 | Ethylene-responsive factor-like protein 1 |
| 1621552_at | 7.02 | 12.62 | Ethylene-responsive transcriptional coactivator-like protein |
| 1609683_at | 3.33 | 6.54 | Ethylene-responsive element binding protein |
| 1610300_at | 2.42 | 3.41 | Ethylene responsive element binding protein |
| 1606975_at | 2.08 | 1.19 | Putative ethylene response factor ERF3a |
| 1608511_at | 0.74 | 0.27 | Ethylene responsive element binding factor 5 |
| 1617671_s_at | 3.01 | 1.8 | Ethylene response factor domain protein 9 |
| 1611910_s_at | 0.69 | 0.25 | Similar to putative ethylene response factor |
Figure 5. MapMan visualization ofchanges in the transcription factor pathway at (a) 6 h and (b) 12 h after UV-C treatment.
Red boxesrepresent up-regulated probsets; Blue boxesrepresent down-regulated probsets.
The WRKY proteins share a DNA binding domain, which contains an invariant WRKYGQK sequence. The WRKY TFs super-family is involved in a diverse set of biological functions including pathogen defense, abiotic stress responses, and plant development [59]. In this study, 18 probe sets representing WRKY factors showed increased expression in response to UV-C treatment, especially WRKY57-like (Table 5, Figure 5). This suggests that these factors protect the grapevines from potentially damaging UV-C irradiation.
It has been established that ethylene and ethylene response factor (ERF) proteins play important regulatory roles in plant pathogen resistance and abiotic stress [60]–[61]. In grapevines, ethylene plays an important role in berry development and ripening, including the regulation of gene expression for anthocyanin biosynthesis and accumulation [62]. It was here found that several genes, annotated as ERFs and ethylene responsive proteins, are strongly expressed (the maximum may reach 75-fold) post-exposure to UV-C treatment (Table 5, Figure 5). In this way, ethylene may affect the response of leaves to UV-C irradiation.
Conclusions
In conclusion, the study identified UV-C irradiation-regulated genes using Affymetrix Grape Genome Array and qRT-PCR techniques. The leaf transcriptome of the grapevines was affected by UV-C irradiation. The responsive probe sets were found to belong to a large number of important factors and biological pathways, such as cell rescue (i.e., antioxidant enzymes), protein fate (i.e., HSPs), secondary metabolism (i.e., STS were up-regulated 750-fold), transcription factors, and signal transduction. These results may provide novel insight into the grape leaf response to UV-C and may have considerable implications for further study and application.
Supporting Information
Probe set-specific primers for RT-PCR.
(DOC)
Linear correlation analysis (r2 = 0.951 for 6 h and r2 = 0.933 for 12 h) between qRT-PCR and microarray results for 25 probe sets. X: log2 fold change value from qRT-PCR data; Y: log2 fold change value from microarray data.
(DOCX)
Probe sets commonly up-regulated at 6 and 12 h after exposure to UV-C irradiation.
(DOCX)
Probe sets commonly down-regulated at 6 and 12 h after exposure to UV-C irradiation.
(DOCX)
Probe sets specifically up- and down-regulated at 6 h after exposure to UV-C treatment.
(DOCX)
Probe sets specifically up- and down-regulated at 12 h after exposure to UV-C treatment. This information is available free of charge via the Internet at http://pubs.acs.org.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All microarray expression data produced in our study are publically available at the Gene Expression Omnibus database under the accession number GSE62315.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31171918). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Coohill TP (1989) Ultraviolet action spectra (280 nm to 380 nm) and solar effectiveness spectra for higher plants. Photochemestry and Photobiology 50:451–457. [Google Scholar]
- 2. Frederick JE (1993) Ultraviolet sunlight reaching the Earth's surface. A review of recent research. Photochemestry and Photobiology 57:175–178. [Google Scholar]
- 3. Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiology 133:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madronich S, McKenzie RL, Caldwell M, Bjorn LO (1995) Changes in ultraviolet radiation reaching the Earth's surface. AMBIO. 24:143–152. [DOI] [PubMed] [Google Scholar]
- 5. Nawkar GM, Maibam P, Park JH, Sahi VP, Lee SY, et al. (2013) UV-induced cell death in plants. International Journal of Molecular Sciences 14:1608–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stapleton AE (1992) Ultraviolet radiation and plants: burning questions. Plant Cell 4:1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Britt AB (1999) Molecular genetics of DNA repair in higher plants. Trends in Plant Science 4:20–25. [DOI] [PubMed] [Google Scholar]
- 8. Kovacik J, Klejdus B, Backor M (2010) Physiological responses of scenedesmus quadricauda (Chlorophyceae) to UV-A and UV-C Light. Photochemistry and Photobiology 86:612–616. [DOI] [PubMed] [Google Scholar]
- 9. Huyskens-Keil SK, Hassenberg K, Herppich WB (2011) Impact of postharvest UV-C and ozone treatment on textural properties of white asparagus (Asparagus officinalis L.). Journal of Applied Botany and Food Quality 84:229–234. [Google Scholar]
- 10. Kondo S, Fiebig A, Okawa K, Ohara H, Kowitcharoen L, et al. (2011) Jasmonic acid, polyamine, and antioxidant levels in apple seedlings as affected by Ultraviolet-C irradiation. Plant Growth Regulation 64:83–89. [Google Scholar]
- 11. Darras AI, Demopoulos V, Bali L, Tiniakou C (2012) Photomorphogenic reactions in geranium (Pelargonium × hortotum) plants stimulated by brief exposures of ultraviolet-C irradiation. Plant Growth Regulation 68:343–350. [Google Scholar]
- 12. Poubol J, Lichanporn I, Puthmee T, Kanlayanarat S (2012) Quality and microbiological changes of Asparagus spearpackaged in polyvinylchloride film and treated with ultraviolet-C. Acta Horticulturae 943:235–240. [Google Scholar]
- 13. Rodoni LM, Concellon A, Chaves AR, Vicente AR (2012) Use of UV-C treatments to maintain quality and extend the shelf life of green fresh-cut bell pepper (Capsicum annuum L.). Journal of Food and Sciences 77:632–639. [DOI] [PubMed] [Google Scholar]
- 14. de Costa F, Yendo ACA, Fleck JD, Gosmann G, Fett-Neto AG (2013) Accumulation of a bioactive triterpene saponin fraction of Quillaja brasiliensis leaves is associated with abiotic and biotic stresses. Plant Physiology and Biochemistry 66:56–62. [DOI] [PubMed] [Google Scholar]
- 15. Lee MJ, Son JE, Oh MM (2014) Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A, -B, or -C lamp. Journal of the Science of Food and Agriculture 94:197–204. [DOI] [PubMed] [Google Scholar]
- 16. Todorova D, Katerova Z, Shopova E (2013) Polyamine spermine protects young pea plants against ultraviolet-C radiation. Biotechnol & Biotec Equipment 27:3798–3802. [Google Scholar]
- 17. Tohidi-Moghadam HR, Ghooshchi F, Jamshidpour F (2012) Effect of UV radiation and elevated CO2 on physiological attributes of canola (Brassica napus l.) grown under water deficit stress. Poloar Journal of Environment Study 21:1417–1427. [Google Scholar]
- 18. Kuniga T, Nesumi H (2011) UV-C irradiation affects accumulation of scoparone in citrus. Acta Horticulturae 907:81–85. [Google Scholar]
- 19. Stevens C, Liu J, Khan VA, Lu JY, Kabwe MK et al. (2004) The effects of low-dose ultraviolet light-C treatment on polygalacturonase activity, delay ripening and Rhizopus soft rot development of tomatoes. Crop Protection 23:551–554. [Google Scholar]
- 20. Romanazzi G, Gabler FM, Smilanick JL (2006) Preharvest chitosan and postharvest UV irradiation treatments suppress gray mold of table grapes. Plant Disease 90:445–450. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez-Aguilar GA, Wang CY, Buta JG, Krizek DT (2011) Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe “Tommy Atkins” mangoes. International Journal of Food Sciences and Technology 36:767–773. [Google Scholar]
- 22. Colas S, Afoufa-Bastien D, Jacquens L, Clement C, Bailliieul F, et al. (2012) Expression and in situ localization of two major PR proteins of grapevine berries during development and after UV-C exposition. PLOS ONE 7:e43681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vivier MA, Pretorius IS (2002) Genetically tailored grapevines for the wine industry. Trends in Biotechnology 20:472–478. [DOI] [PubMed] [Google Scholar]
- 24. Bais AJ, Murphy PJ, Dry IB (2000) The molecular regulation of stilbene phytoalexin biosynthesis in Vitis vinifera during grape berry development. Australia Journal of Plant Physiology 27:425–433. [Google Scholar]
- 25. Borie B, Jeandet P, Parize A, Bessis R, Adrian M (2004) Resveratrol and stilbene synthase mRNA production in grapevine leaves treated with biotic and abiotic phytoalexin elicitors. American Journal of Enology and Viticulture 55:60–64. [Google Scholar]
- 26. Petit AN, Baillieul F, Vaillant-Gaveau N, Jacquens L, Conreux A (2009) Low responsiveness of grapevine flowers and berries at fruit set to UV-C irradiation. Journal of Experimental Botany 60:1155–1162. [DOI] [PubMed] [Google Scholar]
- 27. Wang LJ, Ma L, Xi HF, Duan W, Wang JF, et al. (2013) Individual and combined effects of CaCl2 and UV−C on the biosynthesis of resveratrols in grape leaves and berry skins. Journal of Agricultural and Food Chemistry 61:7135–7141. [DOI] [PubMed] [Google Scholar]
- 28. Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proceedings of National Academy of Sciences USA 97:11655–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhiman N, Bonilla R, O'Kane DJ, Poland G (2011) Gene expression microarrays: a 21st century tool for directed vaccine design. Vaccine 20:22–30. [DOI] [PubMed] [Google Scholar]
- 30. Jiang F, Zheng XD, Chen JS (2009) Microarray analysis of gene expression profile induced by the biocontrol yeast Cryptococcus laurentii in cherry tomato fruit. Gene 430:12–16. [DOI] [PubMed] [Google Scholar]
- 31. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264. [DOI] [PubMed] [Google Scholar]
- 32. Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of National Academy of Sciences USA 98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Wong WH (2001b) Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proceedings of National Academy of Sciences USA 98:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deluc LG, Quilici DR, Decendit A, Grimplet J, Wheatley MD, et al. (2009) Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou X, Su Z (2007) Gene Ontology-based annotation and functional enrichment analysis tool for agronomical species. BMC Genomics 8:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mackerness SAH, Jordan BR, Thomas B (1999) Reactive oxygen species in the regulation of photosynthetic genes by ultraviolet-B radiation (UV-B: 280–320 nm) in green and etiolated buds of pea (Pisum sativum L.). Journal of Photochemistry and Photobiogy B 48:180–188. [Google Scholar]
- 37. Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana . Plant Physiology 110:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takeuchi Y, Kubo H, Kasahara H, Sakaki T (1996) Adaptive alterations in the activities of scavengers of active oxygen in cucumber cotyledons irradiated with UV-B. Journal of Plant Physiology 147:589–592. [Google Scholar]
- 39. Shiu CT, Lee TM (2005) Ultraviolet-B-induced oxidative stress and responses of the ascorbate-glutathione cycle in a marine macroalga Ulva fasciata . Journal of Experimental Botany 56:2851–2865. [DOI] [PubMed] [Google Scholar]
- 40. Xie YJ, Xu DK, Cui WT, Shen WB (2012) Mutation of Arabidopsis HY1 cause hypersensitivity by impairing carotenoid and flavonoid biosynthesis and the down-regulation of antioxidant defence. Journal of Experimental Botany 63:3869–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiology 133:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fini A, Guidi L, Ferrini F, Brunetti C, Ferdinando MD (2012) Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? Journal of Plant Physiology 169:929–939. [DOI] [PubMed] [Google Scholar]
- 44. Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC (2002) The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. British Journal of Cancer 86:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cantos E, Espin JC, Tomas-Barberan FA (2001) Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: A new “functional” fruit? Journal of Agricultural and Food Chemistry 49:5052–5058. [DOI] [PubMed] [Google Scholar]
- 46. Bonomelli A, Mercier L, Franchel K, Baillieul F, Benizri E, et al. (2004) Response of grapevine defenses to UV-C exposure. American Journal of Enology and Viticulture. 55:51–59. [Google Scholar]
- 47. Selma MV, Freitas PM, Almela L, Gonzalez-Barrio R, Espin JC (2008) Ultraviolet-C and induced stilbenes control ochratoxigenic Aspergillus in Grapes. Journal of Agricultural and Food Chemistry 56:9990–9996. [DOI] [PubMed] [Google Scholar]
- 48. Dai R, Ge H, Howard S, Qiu WP (2012) Transcriptional expression of stilbene synthase genes are regulated developmentally and differentially in response to powdery mildew in Norton and Cabernet Sauvignon grapevine. Plant Science 197:70–76. [DOI] [PubMed] [Google Scholar]
- 49. Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion of Plant Biology 9:436–442. [DOI] [PubMed] [Google Scholar]
- 50. Singh KB, Foley RC, Onate-Sanchez L (2012) Transcription factors in plant defense and stress responses. Current Opinion of Plant Biology 5:430–436. [DOI] [PubMed] [Google Scholar]
- 51. Endt DV, Kijne JW, Memelink J (2002) Transcription factors controlling plant secondary metabolism: What regulates the regulators? Phytochemistry 61:107–114. [DOI] [PubMed] [Google Scholar]
- 52. Bomal C, Bedon F, Caron S, Mansfield SD, Levasseur C (2008) Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: a comparative in planta analysis. Journal of Experimental Botany 59:3925–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grotewold E (2005) Plant metabolic diversity: a regulatory perspective. Trends in Plant Science 10:57–62. [DOI] [PubMed] [Google Scholar]
- 54. Kobayashi S, Ishimaru M, Hiraoka K, Honda C (2002) Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 215:924–933. [DOI] [PubMed] [Google Scholar]
- 55. Walker AR, Lee E, Bogs J, McDavid DAJ, Thomas MR, Robinson SP (2007) White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant Journal 49:772–785. [DOI] [PubMed] [Google Scholar]
- 56. Deluc L, Barrieu F, Marchive C, Lauvergeat V, Decendit A (2006) Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiology 140:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Deluc L, Bogs J, Walker AR, Ferrier T, Decendit A (2008) The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiology 147:2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiology 143:1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Current Opinion of Plant Biology 10:366–371. [DOI] [PubMed] [Google Scholar]
- 60. Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-Type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O (2005) Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proceedings of National Academy of Sciences USA 102:10736–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chervin C, El-Kereamy A, Roustan JP, Latche A, Lamon J, et al. (2004) Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Science 167:1301–1305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Probe set-specific primers for RT-PCR.
(DOC)
Linear correlation analysis (r2 = 0.951 for 6 h and r2 = 0.933 for 12 h) between qRT-PCR and microarray results for 25 probe sets. X: log2 fold change value from qRT-PCR data; Y: log2 fold change value from microarray data.
(DOCX)
Probe sets commonly up-regulated at 6 and 12 h after exposure to UV-C irradiation.
(DOCX)
Probe sets commonly down-regulated at 6 and 12 h after exposure to UV-C irradiation.
(DOCX)
Probe sets specifically up- and down-regulated at 6 h after exposure to UV-C treatment.
(DOCX)
Probe sets specifically up- and down-regulated at 12 h after exposure to UV-C treatment. This information is available free of charge via the Internet at http://pubs.acs.org.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All microarray expression data produced in our study are publically available at the Gene Expression Omnibus database under the accession number GSE62315.