Abstract
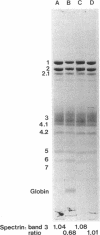
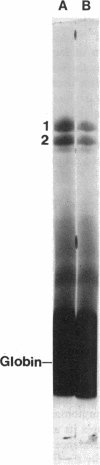
The interaction of spectrin with spectrin-depleted inside-out membrane vesicles was studied in a kindred with an atypical variant of hereditary elliptocytosis inherited in a recessive manner. The probands are characterized by prominent elliptocytosis, decreased erythrocyte thermal stability, an altered limited tryptic peptide pattern of spectrin digested at low ionic strength, and defective spectrin dimer-dimer association. The parents are normal. The spectrin/band 3 ratio determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of isolated membranes of the probands was decreased to approximately 70% of control values, and total erythrocyte spectrin content in one proband was also decreased on SDS-PAGE. When a monospecific antispectrin antibody was used, a faintly labeled fragment of molecular weight approximately 28,000 was detected on immunoblots of whole cell lysates of one proband and a control, but could not account for the decreased erythrocyte spectrin content of the proband on SDS-PAGE. Binding and competitive inhibition studies revealed an alteration in the spectrin-ankyrin interaction due to an abnormality of spectrin in the probands. No defect was found in the mother; the father's spectrin showed decreased binding affinity, although it was not so severe as in the probands. Separation of bound and unbound spectrin dimers from one proband and subsequent conversion to tetramers showed that the self-association defect was detectable only on the bound subpopulation of her spectrin. These findings demonstrate a hitherto undescribed functional abnormality of spectrin in this kindred which could result in decreased stability of the membrane skeleton and contribute to the elliptocytic shape of these erythrocytes.
Full text
PDF



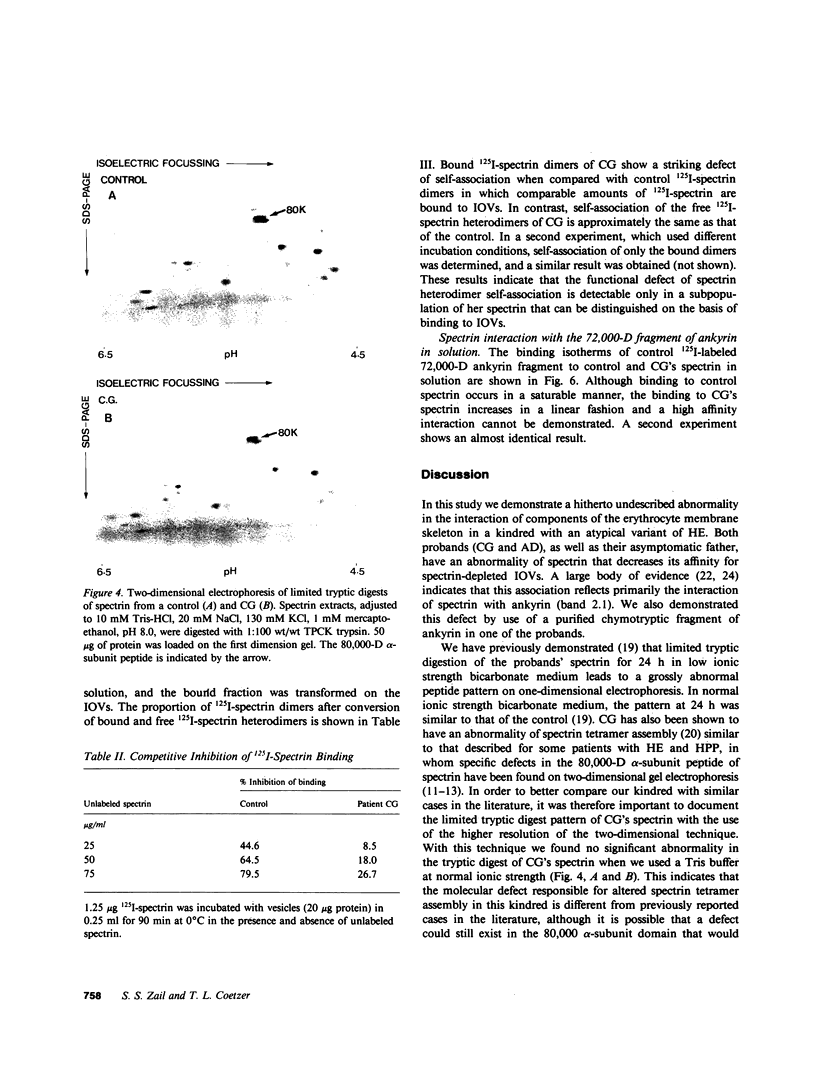
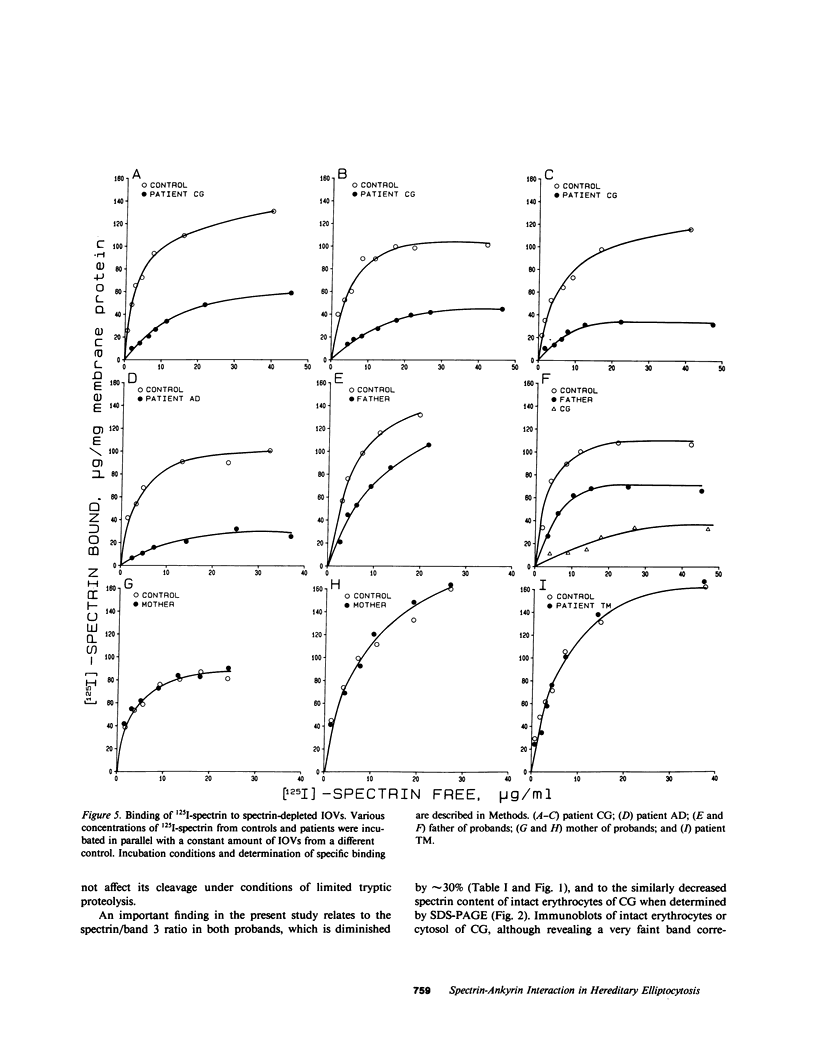
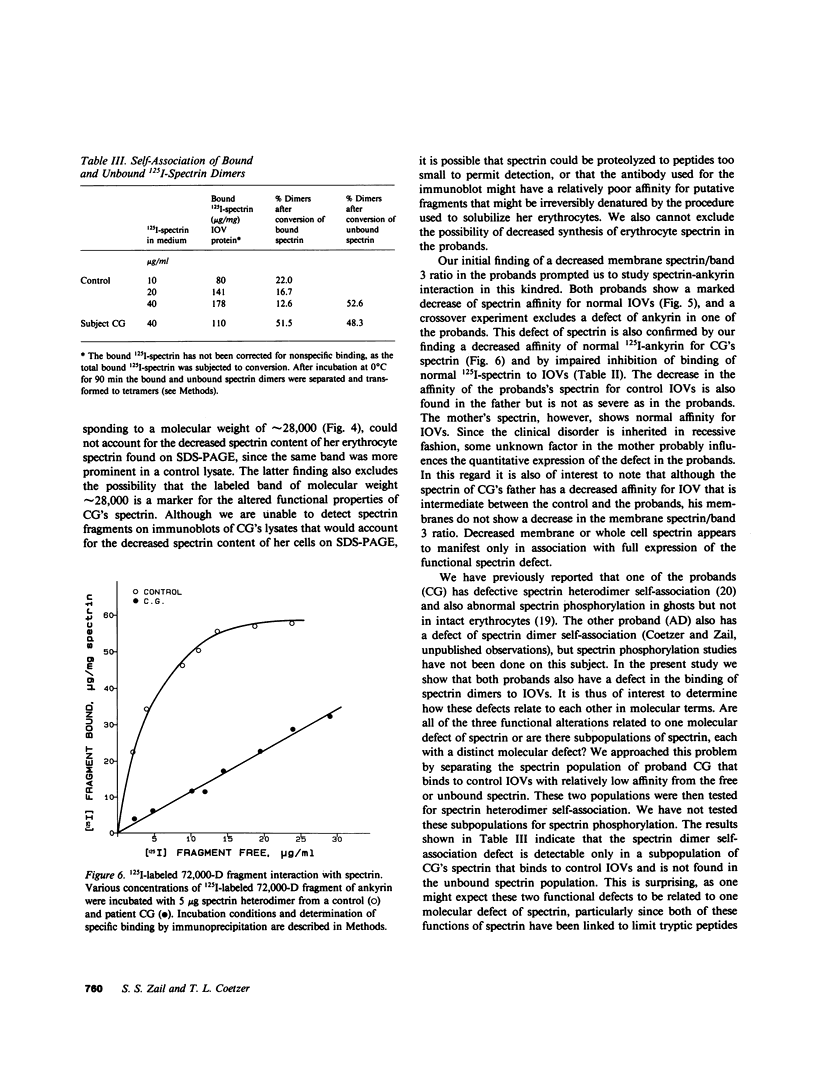


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Chui D. H., Bennett V. A molecular defect in two families with hemolytic poikilocytic anemia: reduction of high affinity membrane binding sites for ankyrin. J Clin Invest. 1981 Dec;68(6):1566–1576. doi: 10.1172/JCI110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Human erythrocyte ankyrin. Purification and properties. J Biol Chem. 1980 Mar 25;255(6):2540–2548. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Spectrin/actin complex isolated from sheep erythrocytes accelerates actin polymerization by simple nucleation. Evidence for oligomeric actin in the erythrocyte cytoskeleton. J Biol Chem. 1980 Feb 25;255(4):1670–1676. [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Tryptic digestion of spectrin in variants of hereditary elliptocytosis. J Clin Invest. 1981 May;67(5):1241–1248. doi: 10.1172/JCI110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzer T., Zail S. Spectrin tetramer-dimer equilibrium in hereditary elliptocytosis. Blood. 1982 May;59(5):900–905. [PubMed] [Google Scholar]
- Cohen C. M. The molecular organization of the red cell membrane skeleton. Semin Hematol. 1983 Jul;20(3):141–158. [PubMed] [Google Scholar]
- Davis J., Bennett V. Microtubule-associated protein 2, a microtubule-associated protein from brain, is immunologically related to the alpha subunit of erythrocyte spectrin. J Biol Chem. 1982 May 25;257(10):5816–5820. [PubMed] [Google Scholar]
- Dhermy D., Lecomte M. C., Garbarz M., Bournier O., Galand C., Gautero H., Feo C., Alloisio N., Delaunay J., Boivin P. Spectrin beta-chain variant associated with hereditary elliptocytosis. J Clin Invest. 1982 Oct;70(4):707–715. doi: 10.1172/JCI110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gomperts E. D., Cayannis F., Metz J., Zail S. S. A red cell membrane protein abnormality in hereditary elliptocytosis. Br J Haematol. 1973 Oct;25(4):415–420. doi: 10.1111/j.1365-2141.1973.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Weidner S. A. Binding of spectrin alpha 2-beta 2 tetramers to human erythrocyte membranes. J Biol Chem. 1980 Sep 10;255(17):8082–8086. [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S., Kulikowski R. R. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W. J., Morrow J. S., Speicher D. W., Zarkowsky H. S., Mohandas N., Mentzer W. C., Shohet S. B., Marchesi V. T. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983 Jun;71(6):1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. Molecular defect of spectrin in hereditary pyropoikilocytosis. Alterations in the trypsin-resistant domain involved in spectrin self-association. J Clin Invest. 1982 Nov;70(5):1019–1030. doi: 10.1172/JCI110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J. T. Defective spectrin dimer-dimer association with hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2072–2076. doi: 10.1073/pnas.79.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- MacDougall L. G., Moodley G., Quirk M. The pyropoikilocytosis-elliptocytosis syndrome in a black South African infant: clinical and hematological features. Am J Pediatr Hematol Oncol. 1982 Fall;4(3):344–349. [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palek J., Lux S. E. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983 Jul;20(3):189–224. [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli M. B., John K. M., Lux S. E. Elliptical erythrocyte membrane skeletons and heat-sensitive spectrin in hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1911–1915. doi: 10.1073/pnas.78.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]