Abstract
Autonomic, pain, and neuropsychologic comorbidities appear in heart failure (HF), likely resulting from brain changes, indicated as loss of structural integrity and functional deficits. Among affected brain sites, the anterior insulae are prominent in serving major regulatory roles in many of the disrupted functions commonly seen in HF. Metabolite levels, including N-acetylaspartate (NAA), creatine (Cr), choline (Cho), and myo-inositol (MI), could indicate the nature of anterior insula tissue injury in HF. The study aim was to assess anterior insular metabolites to determine processes mediating autonomic, pain, and neuropsychologic disruptions in HF. We performed magnetic resonance spectroscopy in bilateral anterior insulae in 11 HF and 53 controls, using a 3.0-Tesla magnetic resonance imaging scanner. Peaks for NAA at 2.02ppm, Cr at 3.02ppm, Cho at 3.2ppm, and MI at 3.56ppm were assigned, peak areas calculated, and metabolites expressed as ratios, including NAA/Cr, Cho/Cr, and MI/Cr. HF patients showed significantly increased Cho/Cr ratios, indicative of glial proliferation or injury, on the left anterior insula, and reduced NAA/Cr levels, suggesting neuronal loss/dysfunction, on the right anterior insula over controls. No differences in MI/Cr ratios appeared between groups. Right anterior insular neuronal loss and left glial alterations may contribute to distorted autonomic, pain, and neuropsychologic functions found in HF.
Keywords: Magnetic resonance spectroscopy, N-acetylaspartate, Choline, Myoinositol, Depression, Pain
1. Introduction
Heart failure (HF) patients show a substantial range of autonomic [1], pain, and neuropsychologic deficits [2, 3], including altered sympathetic and parasympathetic regulation, high pain prevalence, diminished cognitive, and increased mood and anxiety symptoms [2-6]. Of those deficits, autonomic aberrations are perhaps the most concerning for the condition, since the failure to control chronic and dynamic changes in blood pressure and potentiation of cardiac arrhythmia compromise survival. Multiple brain areas in HF show structural and functional deficits, with autonomic regulatory areas especially affected, including the insular cortices. These brain changes have been shown by voxel based morphometry [7], T2-relaxometry [8], diffusion tensor imaging [9], and functional magnetic resonance techniques [1, 10]. The injuries and aberrant functional responses likely contribute to the classic symptoms of HF [2, 3]. However, neither structural nor functional non-invasive procedures are able to differentiate between neuronal vs non-neuronal injury in brain sites, which is essential for identification of potential therapeutic strategies for HF.
The anterior insular cortices, which are topographically organized, are key structures for regulating autonomic, pain, mood, and anxiety functions [11-14]. Anterior sub-regions show different functional responses to autonomic challenges, and principally serve autonomic [12], as well as pain, mood, attention, and anxiety regulation roles. The mid- and caudal regions serve additional neuropsychologic and sensory integrative functions. The anterior insula receives fibers from, and projects to, the hypothalamus, an essential autonomic regulatory structure, with projections to the brainstem, ventral tegmental area, amygdala, and other limbic and cortical sites. Since both structural and functional deficits appear in the anterior insula in HF, we expect that metabolite changes in anterior insular sites will impact HF symptoms. The left and right anterior insulae must be considered separately, since physiological roles for the two sides differ; the right anterior insula principally serves sympathetic roles, while the left primarily serves parasympathetic action (although both sides interact) [12]. Determining metabolite levels of both anterior insulae in HF would provide insights into the particular types of tissue damage, and potential interventions for tissue protection in the condition.
Proton magnetic resonance spectroscopy (PMRS) procedures can non-invasively assess brain metabolites, partition major neuronal and non-neuronal changes, and may indicate regional tissue integrity [15]. Brain metabolites include N-acetylaspartate (NAA), a marker of neuronal integrity/functionality, with reduced levels largely considered as neuronal loss or dysfunction, creatine (Cr), an indicator of energy metabolism, choline (Cho), a measure of non-neuronal cell (glia) membrane turnover or density, and myo-inositol (MI), a marker of glial cell status [15]. Thus, changes in metabolite levels can differentiate neuronal vs non-neuronal insular pathology (glial vs neuronal changes), and would provide valuable insights into mechanisms of site-specific injury in HF. Metabolic alterations, detected by the PMRS procedures, appear in several neurologic disorders [15], and in chronic hypoxic/ischemic processes [16], which commonly operate in HF. The PMRS technique sensitivity allows examination of brain metabolites, and may detect subtle changes in anterior insular tissue integrity.
The aim of this study was to evaluate metabolite patterns in bilateral anterior insula in HF and control subjects using PMRS procedures. We hypothesized that HF patients would differ in metabolite levels that would indicate the relative extent of neuronal and glial injury in the condition. That information would provide useful insights into mechanisms underlying anterior insular injury, and the processes contributing to functional concerns in HF.
2. Material and Methods
2.1. Subjects
We studied 11 hemodynamically-optimized HF and 53 control subjects. The demographic data and other variables of HF and control subjects are summarized in Table 1. The diagnosis of HF was based on national diagnostic criteria, and all subjects were classified New York Heart Association Functional Class II. All HF subjects were recruited from the Ahmanson-University of California at Los Angeles (UCLA) Cardiomyopathy Center and the Los Angeles area. Heart failure subjects underwent similar treatment and care to achieve specific hemodynamic goals, including management with angiotensin receptor blockers or angiotension-converting enzyme inhibitors, beta blockers, and diuretics. Body weight and medication doses of HF subjects were stabilized for at-least six months prior to magnetic resonance imaging (MRI) and PMRS studies, and all studies were performed within one year of HF diagnosis to minimize variability from the disease onset. All control subjects were recruited through advertisements at the UCLA Medical Center and Clinics, UCLA Campus, and Los Angeles area, and were in good health, without any clinical history of cardiovascular, stroke, respiratory, or neurologic disorder. HF subjects with NYHA III and IV were excluded from this study, since HF subjects with such classifications cannot lay supine in the MRI machine for sustained periods. Control and HF subjects were excluded if they were claustrophobic, unable to lay supine, carrying non-removable metal such as braces, embolic coils, pacemakers/implantable cardioverter-defibrillators, stents, or with body weight more than 125 kg to avoid difficulties in comfortably fitting subjects inside the scanner and claustrophobic issues from the limited bore diameter of the scanner. All subjects gave written and informed consent prior to the study, and the study protocol was approved by the Institutional Review Board at UCLA.
Table 1.
Demographic data and other variables of HF and control subjects.
| Variables | HF [n = 11] |
Controls [n = 53] |
p values |
|---|---|---|---|
| Age (years) | 51.60±8.38 | 46.81±8.13 | 0.08 |
| Gender (Male: Female) | 7:4 | 32:21 | 0.84 |
| Handedness | 10 right; 1 left | 40 right; 9 left; 4 ambidextrous |
0.48 |
| BMI (kg/m2) | 27.47±5.39 | 24.71±3.80 | 0.045 |
| LVEF (%) | 0.28±0.08 | - | - |
| PSQI | 6.55±3.70 | 4.15±2.60 | 0.013 |
| ESS | 8.82±3.92 | 5.74±3.29 | 0.008 |
| BDI-II | 10.09±7.67 | 4.04±4.37 | 0.027* |
| BAI | 7.82±6.69 | 4.25±5.12 | 0.05 |
BAI = Back anxiety inventory; BDI-II = Back depression inventory; BMI = Body-mass-index; ESS = Epworth sleepiness scale; LVEF = Left ventricular ejection fraction; PSQI = Pittsburgh sleep quality index;
= Equal variances not assumed.
2.2. Mood and Anxiety Symptoms
We used the Beck Depression Inventory (BDI)-II and Beck Anxiety Inventory (BAI) to evaluate mood and anxiety symptoms in HF and control subjects, respectively. Both are self-report questionnaires, which are used commonly in assessment of medical conditions, and were administered once either immediately prior to or after the MRI and PMRS studies.
2.3. Sleep Quality and Daytime Sleepiness
All HF and control subjects were assessed for daytime sleepiness and sleep quality using the Epworth Sleepiness Scale (ESS) and the Pittsburgh Sleep Quality Index (PSQI), respectively. These self-administered questionnaires were also introduced either immediately before or after MRI and PMRS studies.
2.4. Magnetic Resonance Imaging and Spectroscopy
We used a 3.0-Tesla MRI scanner (Magnetom Trio, Siemens, Germany) for brain studies; subjects lay supine during MRI and PMRS. To minimize head movement, we used foam pads on either side of the head. We performed simultaneous proton density (PD) and T2-weighted imaging [repetition time (TR) = 10,000 ms; echo-time (TE1, 2) = 17, 134 ms; flip angle (FA) = 130°; matrix size = 256×256; field of view (FOV) = 230×230 mm2; slice-thickness = 4.0 mm], covering the whole-brain in the axial plane using a dual-echo turbo spin-echo pulse sequence. High-resolution T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence (TR = 2200 ms; TE = 2.2 ms; inversion time = 900 ms; FA = 9°; matrix size = 256×256; FOV = 230×230 mm2; slice-thickness = 1.0 mm). Both high resolution T1-weighted and T2-weighted images were used for voxel localization of PMRS, and proton MR spectra were acquired from bilateral anterior insular cortices. The single voxel PMRS was performed using point resolved spectroscopy pulse sequence (TR = 3000 ms, TE = 30 ms, spectral points = 2048, bandwidth = 1500 Hz, averages = 144, voxel size = 10×10×10 mm3). After global shimming, manual voxel shimming was performed, and a full-width-at-half-maximum of 13-18 Hz was achieved in all subjects. We used a frequency-selective pulse sequence, known as chemical shift selective suppression, for water suppression prior to MRS data acquisition.
2.5. Spectral Data Processing
We studied only major metabolites, which included N-acetylaspartate (NAA), creatine (Cr), choline (Cho), and myo-inositol (MI). Signal quantification of those metabolites was performed using the standard Siemens software available in the scanner. Peaks of NAA at 2.02 ppm, Cr at 3.02 ppm, Cho at 3.2 ppm, and MI at 3.56 ppm were assigned, and automatic curve-fitting procedures were used to obtain signal integrals. After estimation of NAA, Cr, Cho, and MI metabolite amplitudes, metabolite ratios, including NAA/Cr, Cho/Cr, and MI/Cr were calculated. All spectra were assessed for artifacts, line-width, spectral fitting, and only spectra with adequate quality and signal-to-noise ratio of >3 were included in the analysis. We excluded 4 spectra from the left anterior insula of control subjects and 8 from the right anterior insula of control subjects due to non-acceptable data quality, leaving number of HF and controls subjects presented in Table 2.
Table 2.
Brain metabolite ratios of HF and control subjects.
| Insular side | Metabolite ratio | HF (Mean ± SD) |
Controls (Mean ± SD) |
p values |
|---|---|---|---|---|
| Left (HF, n = 11; Controls, n = 49) |
NAA/Cr | 1.49±0.32 | 1.58±0.22 | 0.17 |
| Cho/Cr | 1.19±0.21 | 1.03±0.16 | 0.02 | |
| MI/Cr | 0.94±0.39 | 0.93±0.30 | 0.80 | |
| Right (HF, n = 11; Controls, n = 45) |
NAA/Cr | 1.62±0.32 | 1.96±0.69 | 0.04 |
| Cho/Cr | 1.00±0.22 | 0.91±0.35 | 0.17 | |
| MI/Cr | 0.81±0.18 | 0.82±0.31 | 0.86 |
Cho = Choline; Cr = Creatine; NAA = N-acetylaspartate; SD = Standard deviation.
2.6. Statistical Analysis
Data were analyzed using the IBM Statistical Package for the Social Sciences (IBM SPSS, v20) software. Numerical demographic data and other variables were compared with independent samples t-tests, and categorical variables were examined with χ2 tests. Both left and right anterior insular metabolite ratios of HF and control subjects were compared with Mann-Whitney U-tests. A non-parametric test was chosen, since the metabolite ratios were not normally distributed (significant values on the Shapiro-Wilks test of normality). A p value ≤ 0.05 was considered statistically significant.
3. Results
Demographic data and other variables of all subjects in both groups are summarized in Table 1. No significant differences in age, handedness, or gender appeared between HF and control subjects. However, body-mass-index, PSQI, ESS, BDI-II, and BAI differed significantly between the two groups (Table 1).
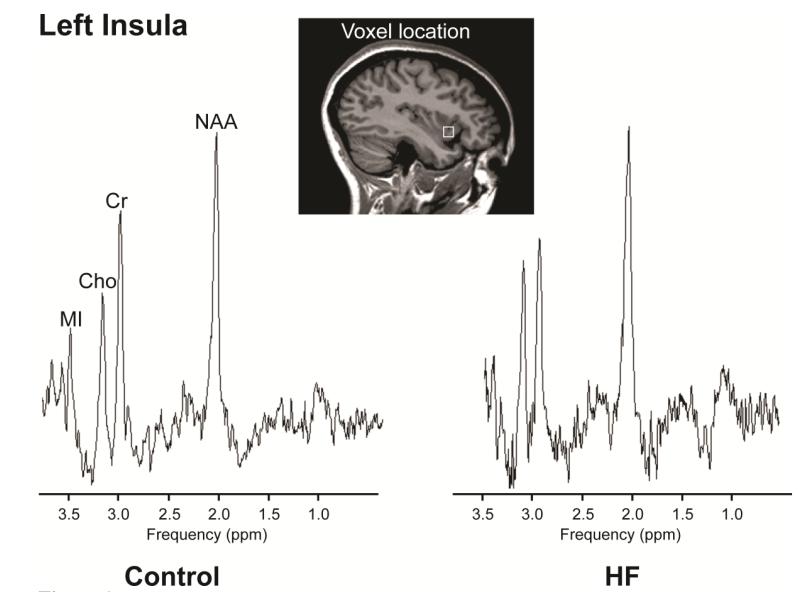
Brain metabolite ratios of HF and control subjects are summarized in Table 2, and representative left insular spectra from a HF and a control subject are displayed in Figure 1. HF patients showed bilateral insular metabolic abnormalities compared to control subjects. The left anterior insular cortex of HF showed increased Cho/Cr ratio (p=0.02), and no significant changes in NAA/Cr (p=0.17) and MI/Cr ratios (p=0.80), compared to control subjects (Table 2). The right insular cortex in HF showed reduced NAA/Cr (p=0.04), and no significant changes in Cho/Cr (p=0.17) and MI/Cr (p=0.86) ratios compared to controls (Table 2).
Figure 1.
PMRS spectra, acquired from the left insula in a HF (age, 42.8 years; female) and a comparable age- and gender-matched control (age, 40.3 years; female) subject, showing NAA, Cr, Cho, and MI metabolites. Java-based MRS software (jMRUI, V 3.0) was used to process and display these spectra.
4. Discussion
4.1. Overview
The changes in metabolite levels suggest that HF patients have preferential damage to neurons on the right anterior insula, as indicated by reduced NAA/Cr ratios, and injury to non-neuronal cells on the left side, as suggested by increased Cho/Cr ratios. The findings support earlier evidence of a substantial tissue volume loss and altered water diffusion [7, 9], indicating injury in the anterior insula bilaterally, and point to more severe damage on the right side, with significant neuronal loss. Functional deficits also appeared in earlier studies to autonomic and cold pressor challenges [1, 10], with more profound functional changes appearing on the right side over left-side patterns [1, 10]. The most profound physiological outcomes that may develop from the current findings relate to sympathetic control, which is primarily mediated by the right side; the neuronal loss likely mediates a substantial portion of the altered, sustained and dynamic sympathetic patterns found to pressor challenges in HF. The left-side metabolic changes presumably modify the parasympathetic patterns in the challenges, and damage to both left and right insulae may affect mood, and anxiety regulation [12, 17].
4.2. Changes of NAA and Cho Metabolites
Ratios of NAA, Cho, and MI were calculated with respect to the Cr metabolite. The right anterior insular cortex showed reduced NAA/Cr ratios, i.e., reduced NAA levels, in HF, relative to control subjects, and no significant changes in Cho/Cr or MI/Cr ratios. The NAA metabolite, a major amino acid in the mammalian central nervous system is primarily localized in neuronal cells, axons, and dendrites [18]. Reduced NAA signals appear in various neurological diseases and conditions, and indicate neuronal/axonal loss or dysfunction [15]. The reduced NAA signals in the acute stage of tissue changes after hypoxia or ischemia may represent neuronal/axonal dysfunction [16], while reduced levels in the chronic condition may indicate neuronal/axonal loss which appears to be the case here, since in other HF patients, substantial tissue loss occurs [7]. However, voxel locations for MRS data acquisition were predominately in gray matter, NAA signal reduction should be interpreted here as neuronal, over axonal loss.
The left anterior insular cortex showed increased Cho/Cr ratios in HF over control subjects, and no significant differences in NAA/Cr or MI/Cr ratios, suggesting increased levels of Cho metabolite. Changes in Cho concentration can originate from phosphorylated choline and alterations in membrane metabolism and glia cell reactions, which may increase Cho levels. Myelin loss or degradation also increases the availability of Cho-containing compounds, and thus, increased Cho signal [15].
Both left and right anterior insulae showed earlier tissue changes based on T2-relaxometry [8], cerebral blood flow changes (unpublished data), and changes in axons near the anterior insula, based on axial diffusion changes [9]. The metabolic changes found here replicate structural changes observed in earlier studies [7-9].
4.3. Insular Cortices: Autonomic Roles
Insular cortices serve major autonomic roles [12, 17], and integrate autonomic actions, together with other limbic areas. Stimulation of the insulae alters pulse rate and blood pressure, and modifies respiration [19]. The anterior insular sites examined project to hypothalamic areas, which receive baroreceptor input and project to multiple brainstem areas that serve sympathetic and parasympathetic roles, and thus, modify cardiovascular control [20, 21]. Hypothalamic stimulation independently modifies heart rate, cardiac rhythm, and blood pressure [22], and the hypothalamus, like the insular cortices, also shows structural injury in HF, but the altered neural and non-neuronal cellular changes from the metabolite indications found here will compromise insular influences on the hypothalamus, and consequently, hypothalamic output. The anterior insular cortices also project to the cingulate and ventral medial prefrontal cortex, major contributors to autonomic regulation [21].
4.4. Insular Cortices: Neuropsychologic, Cognitive, and Pain Regulation
The anterior insular cortices participate in mood and anxiety regulation and dyspnea perception [13, 14, 23]. Multiple brain structures, including the anterior insula, cingulate, hippocampus, ventral medial prefrontal cortex, and cerebellar areas are injured in subjects with depression only [24], and are also injured in HF subjects. Increased levels of affective symptoms, as well as elevated signs of dyspnea, which are regulated by the insular cortices and other brain structures (cingulate and cerebellar areas), are common in HF [1, 8, 9], and abnormal anterior insular metabolites may contribute to such deficits.
HF subjects frequently report both acute and chronic pain [25]. The anterior insula receives afferents from the thalamus, and encodes aspects of pleasantness of challenges, including the stimulus location and sensory properties [11]; it can, for example, differentially respond to cutaneous vs visceral pain. Both thalamic and insular sites showed structural injury in the same HF subjects’ cohort [9]. The anterior insular metabolic deficits found here may contribute to pain characteristics experienced by HF subjects.
Cognitive deficits, including attention inadequacies, difficulty with complex reasoning, and confusion are also common in HF patients [2-4, 6]. The anterior insula receives from, and projects to multiple limbic and cortical regions, sites that serve cognitive roles. Thus, the anterior insular metabolic abnormalities observed here may also play a role in these deficient functions.
4.5. Pathological Mechanisms
The precise pathological mechanisms underlying alterations in anterior insular metabolites in HF are unknown, but several possibilities emerge. HF patients show compromised cerebral perfusion, secondary to low cardiac output, with localized changes in blood flow and axons [9]. Hypoxic/ischemic processes resulting from sleep-disorder breathing, which is commonly reported in HF [26], may also contribute to injury. HF subjects show both obstructive and central (Cheyne-Stokes) sleep apnea. Apneic events may lead to hypoxic exposure in the brain, and thus, alter brain metabolites; intermittent hypoxic events trigger oxidative and inflammatory injurious mechanisms leading to neural injury [26]. Several limbic sites, including the insular cortices, show injury in obstructive sleep apnea in humans [27], and animal studies simulating intermittent hypoxia also show tissue damage in multiple limbic and cerebellar sites [28].
Both acute and chronic hypoxic/ischemic conditions show reduced levels of NAA [16] and increased levels of Cho [29]. Animal studies simulating irreversible cerebral ischemia showed reduced NAA levels prior to changes appearing on routine MR imaging [16]. All HF patients included in this study were diagnosed at least one year prior to this study, and the metabolic changes likely resulted from chronic hypoxic/ischemic conditions, as opposed to acute process.
4.6. Clinical Significance
Several injurious processes, including reduced regional cerebral perfusion, as evaluated by arterial spin labeling (unpublished data), probably resulting from low cardiac output and sleep-disordered breathing, operate in HF, and may lead to altered levels of brain metabolites. Abnormal insular metabolites may affect neurons mediating insular functions, resulting in autonomic, mood, pain, and cognitive abnormalities. The findings of increased Cho and decreased NAA levels indicate that additional support for protection of glial cells may be required over neuronal protection alone in the condition. Interventions that improve cerebral perfusion, secondary to enhanced cardiac output, and reduction in sleep-disordered breathing in HF may partially recover functional deficits.
4.7. Limitations
Several limitations should be discussed, including the BMI differences between groups, the small sample size of HF subjects, disease onset variation, and inclusion of HF subjects with NYHA functional Class II. HF subjects significantly differed in BMI from control subjects, and that difference may contribute to metabolic differences. However, the regression analysis failed to show any significant effect of BMI on metabolite levels in HF and controls, indicating that the metabolic findings do not influence BMI variations between groups. The limited number of HF subjects in this study requires replicating findings with larger numbers; the study was insufficiently powered to resolve all significant correlations between metabolites and sleep and affective variables. The disease onset duration of HF may have introduced some variability in our findings. However, studies were performed within one year of HF diagnosis to minimize variability from disease onset, and all HF subjects underwent the same treatment plan and care. We included only those HF subjects of NYHA Functional Class II. Subjects with NYHA III and IV were excluded due to logistic reasons, since HF subjects with those classes cannot lay supine in the MRI machine for a long period. Thus, findings should not be generalized to all HF Functional Classes.
5. Conclusions
Heart failure subjects show bilateral abnormal anterior insular metabolites, including NAA and Cho levels, compared to control subjects. Those findings indicate that both neurons and glia are affected, providing a basis for protective interventions in both cellular aspects. The metabolic changes likely contribute to the altered insular functions, including the autonomic, pain, mood, and cognitive deficits frequently found in HF. The pathological mechanisms underlying the metabolic deficits are unclear, but likely include reduced brain perfusion resulting from low cardiac output, and hypoxia/ischemia from sleep disordered breathing; both those conditions are common in HF.
Highlights.
Insular metabolites in HF were examined with PMRS procedures.
Decreased NAA appeared on right side and increased Cho on left side.
Lower NAA show neuronal injury and higher Cho indicate glial activation.
Abnormal metabolites may contribute to autonomic, pain and cognitive deficits.
Metabolic changes may results from brain hypoxia and/or ischemia.
Acknowledgements
We thank Ms. Rebecca Harper, Mr. Edwin Valladares, and Drs. Rebecca Cross and Stacy Serber for assistance with data collection. This research work was supported by National Institutes of Health R01 NR 013625.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors have no conflict of interest to declare.
References
- [1].Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, et al. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail. 2005;11:437–46. doi: 10.1016/j.cardfail.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [2].Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs. 2010;25:189–98. doi: 10.1097/JCN.0b013e3181ca36fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Evangelista LS, Sackett E, Dracup K. Pain and heart failure: unrecognized and untreated. Eur J Cardiovasc Nurs. 2009;8:169–73. doi: 10.1016/j.ejcnurse.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr., et al. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest. 1990;85:1362–71. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Neckel H, Quagliotto E, Casali KR, Montano N, Dal Lago P, Rasia-Filho AA. Glutamate and GABA in the medial amygdala induce selective central sympathetic/parasympathetic cardiovascular responses. Can J Physiol Pharmacol. 2012;90:525–36. doi: 10.1139/y2012-024. [DOI] [PubMed] [Google Scholar]
- [6].Schiffer AA, Pedersen SS, Broers H, Widdershoven JW, Denollet J. Type-D personality but not depression predicts severity of anxiety in heart failure patients at 1-year follow-up. J Affect Disord. 2008;106:73–81. doi: 10.1016/j.jad.2007.05.021. [DOI] [PubMed] [Google Scholar]
- [7].Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–84. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- [8].Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214–23. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307:106–13. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, et al. Aberrant central nervous system responses to the Valsalva maneuver in heart failure. Congest Heart Fail. 2007;13:29–35. doi: 10.1111/j.1527-5299.2007.05856.x. [DOI] [PubMed] [Google Scholar]
- [11].Henderson LA, Peck CC, Petersen ET, Rae CD, Youssef AM, Reeves JM, et al. Chronic pain: lost inhibition? J Neurosci. 2013;33:7574–82. doi: 10.1523/JNEUROSCI.0174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131–40. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- [13].Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- [14].Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum Brain Mapp. 2011;32:1836–46. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999;56:919–26. doi: 10.1001/archneur.56.8.919. [DOI] [PubMed] [Google Scholar]
- [16].Penrice J, Lorek A, Cady EB, Amess PN, Wylezinska M, Cooper CE, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1997;41:795–802. doi: 10.1203/00006450-199706000-00001. [DOI] [PubMed] [Google Scholar]
- [17].Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philo Trans R Soc Lond B, Biol Sci. 2009;364:1933–42. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- [20].Kimmerly DS, O’Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol. 2005;569:331–45. doi: 10.1113/jphysiol.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. NeuroImage. 2007;35:698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- [22].Oppenheimer SM, Saleh T, Cechetto DF. Lateral hypothalamic area neurotransmission and neuromodulation of the specific cardiac effects of insular cortex stimulation. Brain Res. 1992;581:133–42. doi: 10.1016/0006-8993(92)90352-a. [DOI] [PubMed] [Google Scholar]
- [23].Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–20. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- [24].Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- [26].Schmalgemeier H, Bitter T, Fischbach T, Horstkotte D, Oldenburg O. C-Reactive Protein Is Elevated in Heart Failure Patients with Central Sleep Apnea and Cheyne-Stokes Respiration. Respiration. 2013 Aug 28; doi: 10.1159/000351115. doi: 10.1159/000351115. Epub. [DOI] [PubMed] [Google Scholar]
- [27].Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–8. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- [29].Glunde K, Shah T, Winnard PT, Jr., Raman V, Takagi T, Vesuna F, et al. Hypoxia regulates choline kinase expression through hypoxia-inducible factor-1 alpha signaling in a human prostate cancer model. Cancer Res. 2008;68:172–80. doi: 10.1158/0008-5472.CAN-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]