Abstract
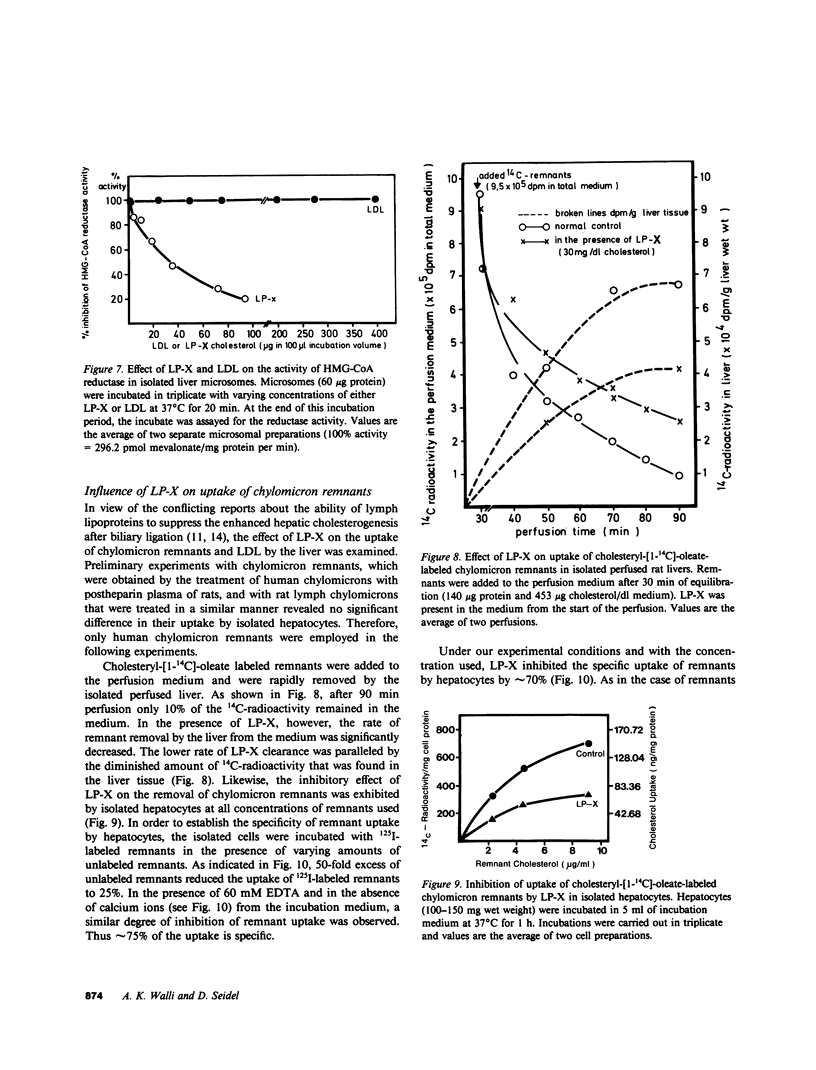
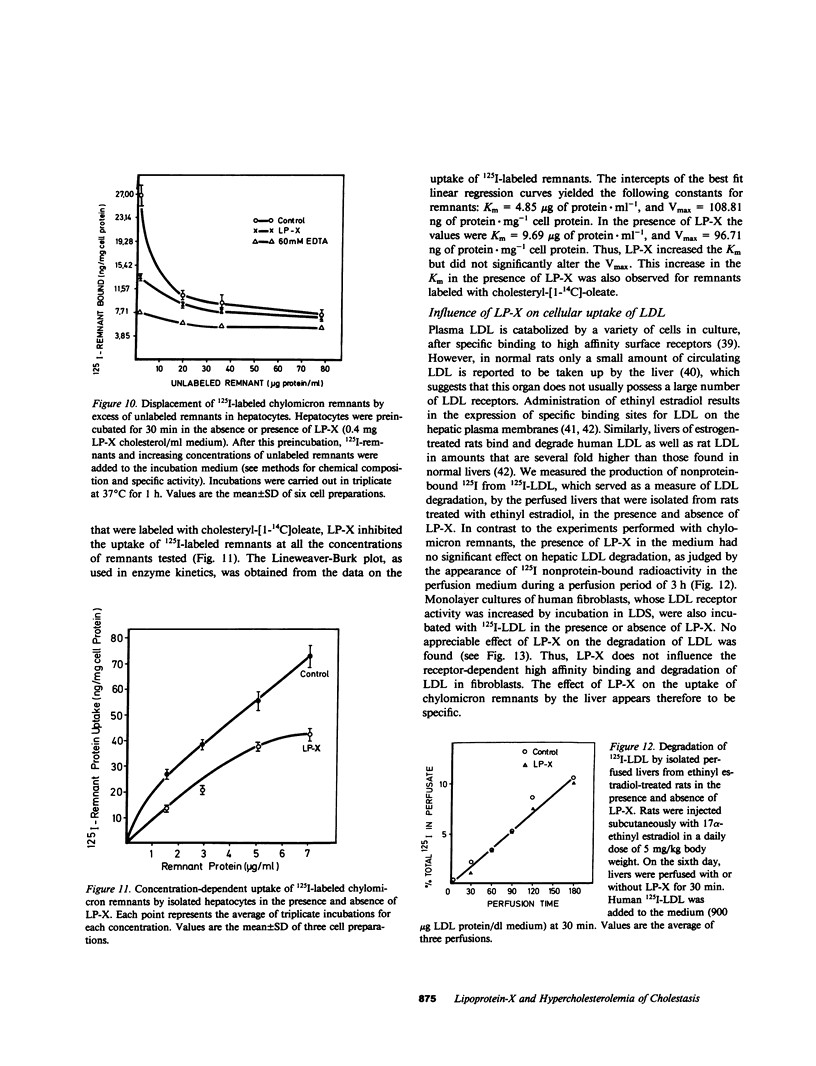
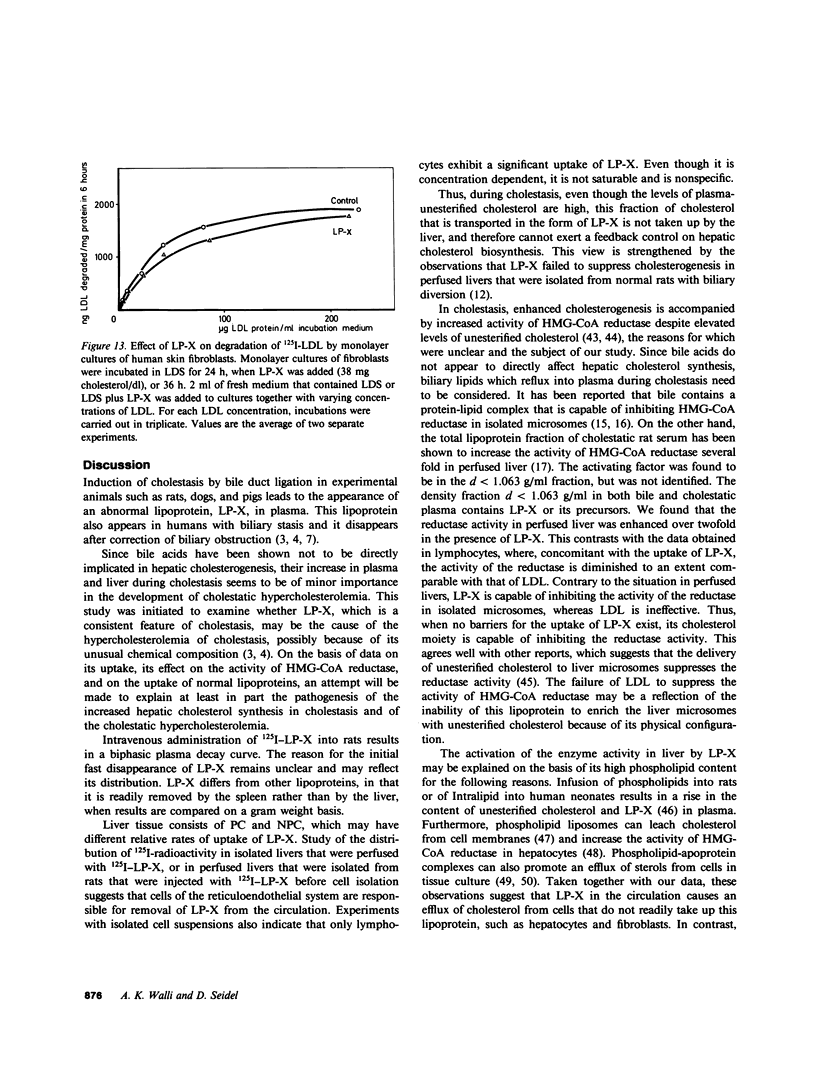
Cholestasis is accompanied by the appearance of lipoprotein-X (LP-X) in plasma. This lipoprotein has a high content of unesterified cholesterol and phospholipids and appears to be ineffective in suppressing the enhanced hepatic cholesterogenesis of cholestasis. Its role as a possible causative factor for cholestatic hypercholesterolemia was investigated. When 125I-LP-X was injected into rats, it disappeared rapidly from the circulation. Calculated on the basis of gram wet weight, spleen took up more LP-X than liver. Prior ligation of the bile duct reduced the uptake in spleen. Experiments with isolated perfused rat liver showed that nonparenchymal cells (NPC) took up over eightfold more 125I-LP-X than hepatic parenchymal cells (PC). Incubation of PC, NPC, human lymphocyte suspensions, or fibroblast cultures with LP-X showed that NPC bound more LP-X than PC or fibroblasts. Lymphocytes took up 20-fold more LP-X than PC and the activity of 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) reductase was depressed by LP-X. Lymphocytes isolated from cholestatic patients showed low activity of this enzyme. The activity was increased by LP-X in isolated perfused livers, but suppressed in isolated microsomes. LP-X competitively inhibited the uptake of chylomicron remnants in isolated perfused livers and hepatocytes. In contrast, degradation of LDL by perfused livers, which were isolated from ethinyl estradiol-treated rats or human fibroblast cultures, remained unchanged in the presence of LP-X. The results indicate that cholesterol transported by LP-X is mainly taken up by the cells of the reticuloendothelial system. It increases the activity of hepatic HMG-CoA reductase and suppresses remnant uptake, thus emphasizing a major role of LP-X in cholestatic hypercholesterolemia.
Full text
PDF


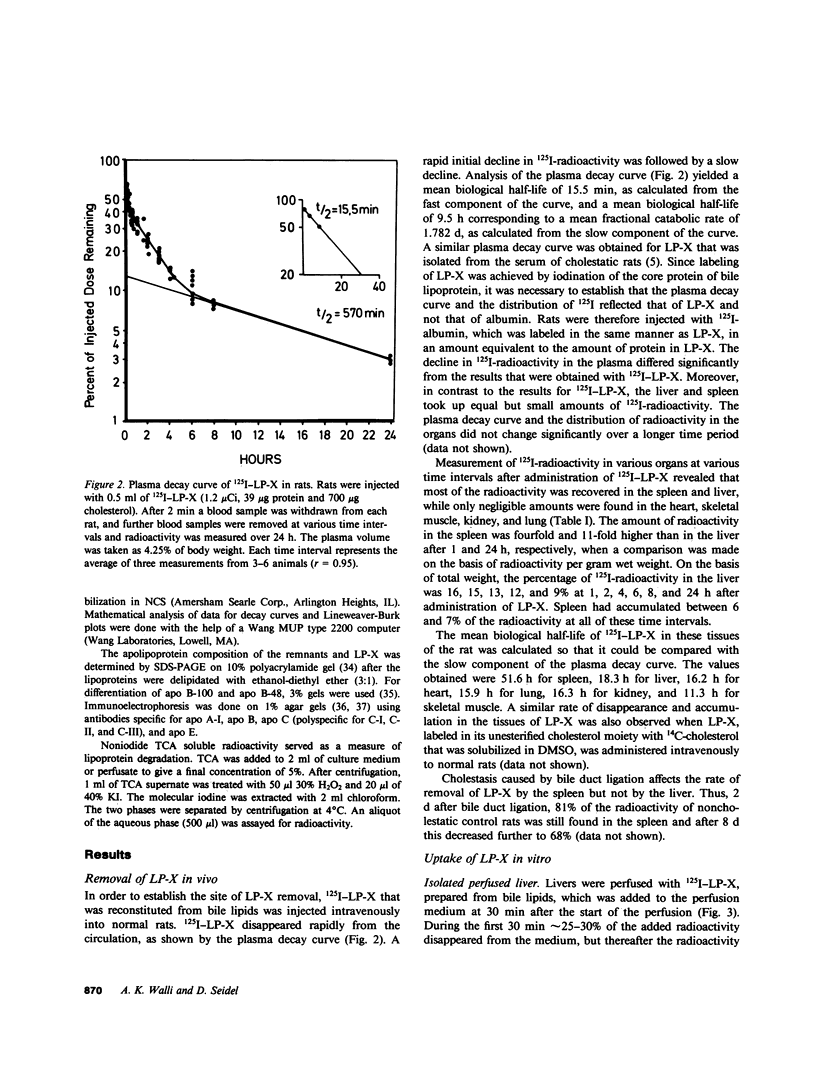
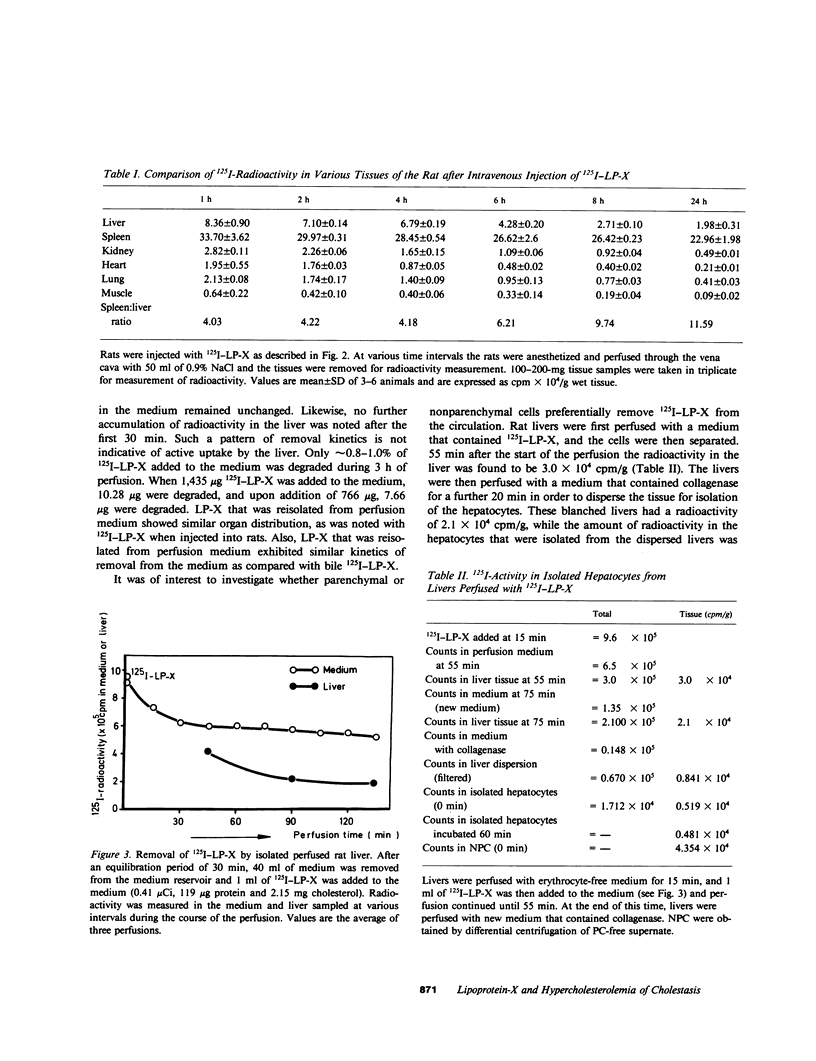
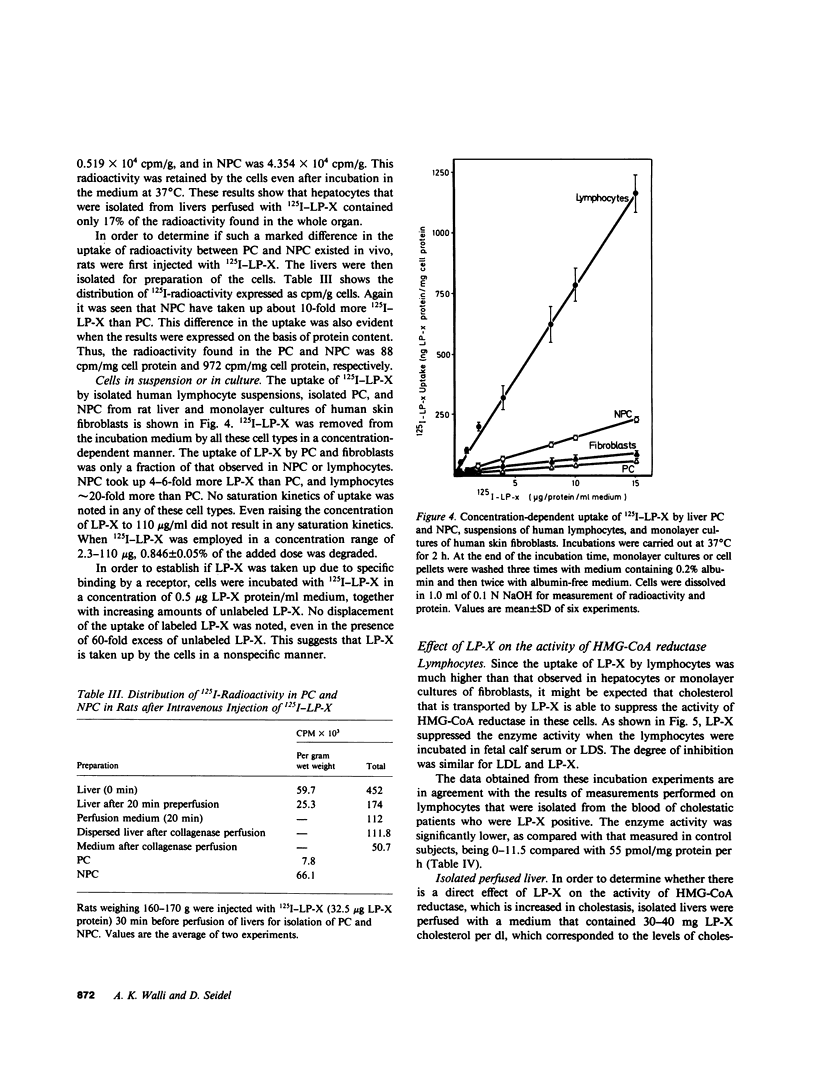
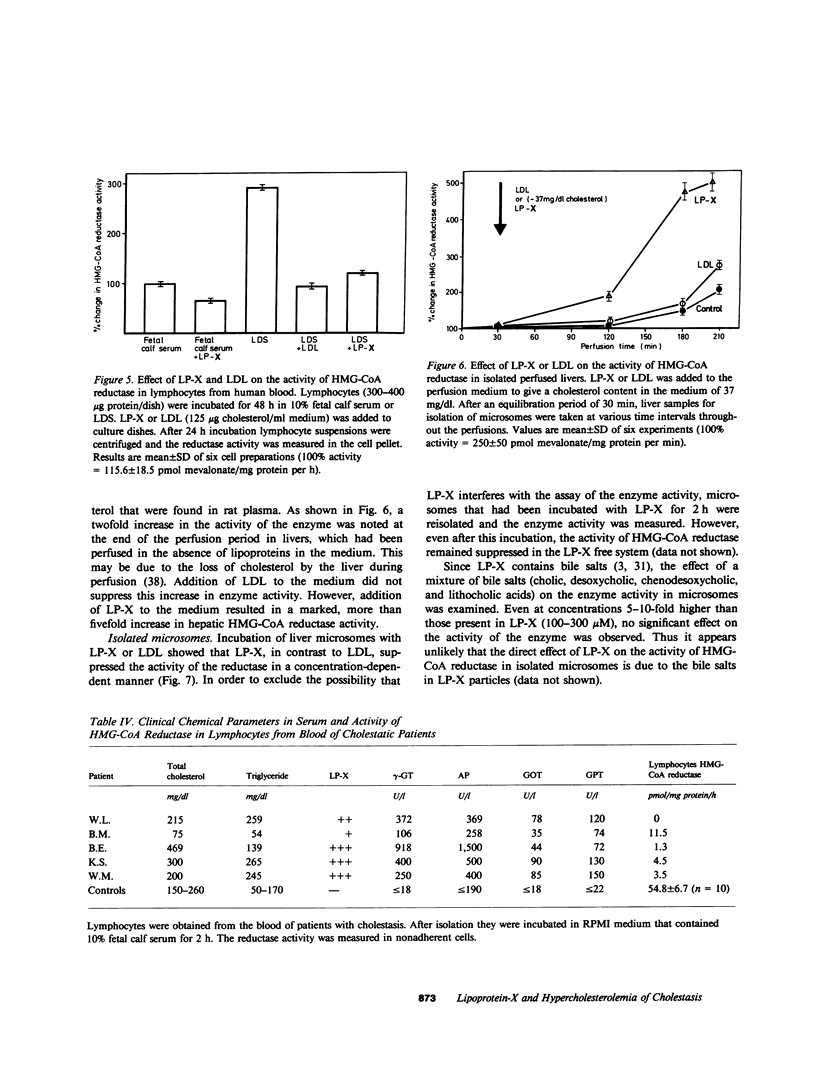



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelin B., Raviola C. A., Innerarity T. L., Mahley R. W. Regulation of hepatic lipoprotein receptors in the dog. Rapid regulation of apolipoprotein B,E receptors, but not of apolipoprotein E receptors, by intestinal lipoproteins and bile acids. J Clin Invest. 1983 Apr;71(4):816–831. doi: 10.1172/JCI110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio G., Müller P., Wieland H., Niedmann P. D., Seidel D. Influence of bile acids and free fatty acids on physicochemical properties of LP-X. Res Exp Med (Berl) 1978 Jun 12;172(3):211–221. doi: 10.1007/BF01855831. [DOI] [PubMed] [Google Scholar]
- Barak A. J., Sorrell M. F., Tuma D. J. Effect of serum lipoproteins of bile obstructed rats on 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in perfused rat liver. Lipids. 1979 Nov;14(11):883–887. doi: 10.1007/BF02533500. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975 Dec;56(6):1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Cooper A. D., Erickson S. K., Nutik R., Shrewsbury M. A. Characterization of chylomicron remnant binding to rat liver membranes. J Lipid Res. 1982 Jan;23(1):42–52. [PubMed] [Google Scholar]
- Cooper A. D., Ockner R. K. Studies of hepatic cholesterol synthesis in experimental acute biliary obstruction. Gastroenterology. 1974 Apr;66(4):586–595. [PubMed] [Google Scholar]
- Cooper A. D. The regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the isolated perfused rat liver. J Clin Invest. 1976 Jun;57(6):1461–1470. doi: 10.1172/JCI108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Arner E. C., Wiley J. S., Shattil S. J. Modification of red cell membrane structure by cholesterol-rich lipid dispersions. A model for the primary spur cell defect. J Clin Invest. 1975 Jan;55(1):115–126. doi: 10.1172/JCI107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. 3. N Engl J Med. 1970 May 28;282(22):1241–1249. doi: 10.1056/NEJM197005282822206. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Effect of plasma lipoproteins and lecithin-cholesterol dispersions on the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase of isolated rat hepatocytes. Biochim Biophys Acta. 1975 Oct 21;409(1):39–50. doi: 10.1016/0005-2760(75)90078-8. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., LOUD A. V., HINKELMAN B. T., SCHNEIDER H. S., FRANTZ I. D., Jr The effect of ligation of the common bile duct on cholesterol synthesis in the rat. J Exp Med. 1954 Jan 1;99(1):43–53. doi: 10.1084/jem.99.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker T. E., Hamilton R. L., Havel R. J. Secretion of lipoprotein-X by perfused livers of rats with cholestasis. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3459–3463. doi: 10.1073/pnas.75.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florén C. H., Albers J. J., Kudchodkar B. J., Bierman E. L. Receptor-dependent uptake of human chylomicron remnants by cultured skin fibroblasts. J Biol Chem. 1981 Jan 10;256(1):425–433. [PubMed] [Google Scholar]
- Goldfarb S., Pitot H. C. Improved assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1971 Jul;12(4):512–515. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Greim H., Trülzsch D., Roboz J., Dressler K., Czygan P., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972 Nov;63(5):837–845. [PubMed] [Google Scholar]
- Griffin E., Breckenridge W. C., Kuksis A., Bryan M. H., Angel A. Appearance and characterization of lipoprotein X during continuous intralipid infusions in the neonate. J Clin Invest. 1979 Dec;64(6):1703–1712. doi: 10.1172/JCI109633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattermann R., Creutzfeldt W. The effect of experimental cholestasis on the negative feedback regulation of cholesterol synthesis in rat liver. Scand J Gastroenterol. 1970;5(5):337–342. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakshmanan M. R., Muesing R. A., LaRosa J. C. Regulation of cholesterol biosynthesis and 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by chylomicron remnants in isolated hepatocytes and perfused liver. J Biol Chem. 1981 Mar 25;256(6):3037–3043. [PubMed] [Google Scholar]
- Larsson B., Nilsson A. Inhibition of hepatic chylomicron remnant uptake in the cholestatic rat. Scand J Gastroenterol. 1980;15(8):959–967. doi: 10.3109/00365528009181798. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liersch M., Baggio G., Heuck C. C., Seidel Effect of lipoprotein-X on hepatic cholesterol synthesis. Atherosclerosis. 1977 Apr;26(4):505–514. doi: 10.1016/0021-9150(77)90118-6. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzato E., Fellin R., Baggio G., Walch S., Neubeck W., Seidel D. Formation of lipoprotein-X. Its relationship to bile compounds. J Clin Invest. 1976 May;57(5):1248–1260. doi: 10.1172/JCI108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara D. J., Rodwell V. W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. In vitro inhibition by a protein present in bile. Arch Biochem Biophys. 1975 Jun;168(2):378–385. doi: 10.1016/0003-9861(75)90266-0. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Reeves B. E., Balasubramaniam S. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase and of acyl-CoA--cholesterol acyltransferase by the transfer of non-esterified cholesterol to rat liver microsomal vesicles. Biochem J. 1981 Jan 15;194(1):265–271. doi: 10.1042/bj1940265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Felin R., Lambrecht J., Agostini B., Wieland H., Rost W., Seidel D. Hypertriglyceridaemia secondary to liver disease. Eur J Clin Invest. 1974 Dec 5;4(6):419–428. doi: 10.1111/j.1365-2362.1974.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Narayanan S. Lipoprotein-X. CRC Crit Rev Clin Lab Sci. 1979 Aug;11(1):31–51. doi: 10.3109/10408367909105853. [DOI] [PubMed] [Google Scholar]
- Nestruck A. C., Niedmann P. D., Wieland H., Seidel D. Chromatofocusing of human high density lipoproteins and isolation of lipoproteins A and A-I. Biochim Biophys Acta. 1983 Aug 29;753(1):65–73. doi: 10.1016/0005-2760(83)90099-1. [DOI] [PubMed] [Google Scholar]
- Ogilvie J. W., Kaplan B. H. The inhibition of sterol biosynthesis in rat liver homogenates by bile. J Biol Chem. 1966 Oct 25;241(20):4722–4730. [PubMed] [Google Scholar]
- Patsch J. R., Aune K. C., Gotto A. M., Jr, Morrisett J. D. Isolation, chemical characterization, and biophysical properties of three different abnormal lipoproteins: LP-X1, LP-X2, and LP-X3. J Biol Chem. 1977 Mar 25;252(6):2113–2120. [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Seidel D., Alaupovic P., Furman R. H. A lipoprotein characterizing obstructive jaundice. I. Method for quantitative separation and identification of lipoproteins in jaundiced subjects. J Clin Invest. 1969 Jul;48(7):1211–1223. doi: 10.1172/JCI106085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel D., Büff H. U., Fauser U., Bleyl U. On the metabolism of lipoprotein-X (LP-X). Clin Chim Acta. 1976 Jan 16;66(2):195–207. doi: 10.1016/0009-8981(76)90057-7. [DOI] [PubMed] [Google Scholar]
- Sherrill B. C., Dietschy J. M. Characterization of the sinusoidal transport process responsible for uptake of chylomicrons by the liver. J Biol Chem. 1978 Mar 25;253(6):1859–1867. [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Catabolism of the apoprotein of low density lipoproteins by the isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):628–634. [PubMed] [Google Scholar]
- Stein O., Stein Y. The removal of cholesterol from Landschütz ascites cells by high-density apolipoprotein. Biochim Biophys Acta. 1973 Nov 29;326(2):232–244. doi: 10.1016/0005-2760(73)90249-x. [DOI] [PubMed] [Google Scholar]
- Stein O., Vanderhoek J., Stein Y. Cholesterol content and sterol synthesis in human skin fibroblasts and rat aortic smooth muscle cells exposed to lipoprotein-depleted serum and high density apolipoprotein/phospholipid mixtures. Biochim Biophys Acta. 1976 May 27;431(2):347–358. doi: 10.1016/0005-2760(76)90155-7. [DOI] [PubMed] [Google Scholar]
- Walli A. K., Seidel D. Effects of clofibric acid and bezafibrate administration on activities of alkaline phosphatase and other enzymes in livers of rats. Res Exp Med (Berl) 1981;179(2):153–161. doi: 10.1007/BF01851983. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Failure of bile acids to control hepatic cholesterogenesis: evidence for endogenous cholesterol feedback. J Clin Invest. 1969 Dec;48(12):2398–2408. doi: 10.1172/JCI106206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Presence of an intact cholesterol feedback mechanism in the liver in biliary stasis. Gastroenterology. 1971 Jul;61(1):77–84. [PubMed] [Google Scholar]
- Wieland H., Seidel D. Eine neue und vereinfachte Methode zum Nachweis des LP-X, eines cholestasespezifischen Lipoproteins. Dtsch Med Wochenschr. 1973 Aug;98(31):1474–1475. doi: 10.1055/s-0028-1107058. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., van Tol A. In vivo uptake of human and rat low density and high density lipoprotein by parenchymal and nonparenchymal cells from rat liver. Biochim Biophys Acta. 1978 Aug 25;530(2):299–304. doi: 10.1016/0005-2760(78)90015-2. [DOI] [PubMed] [Google Scholar]



