Abstract
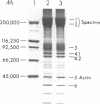
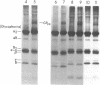
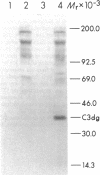
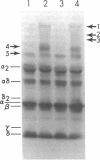
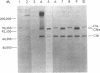
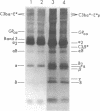
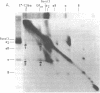
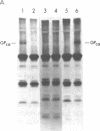
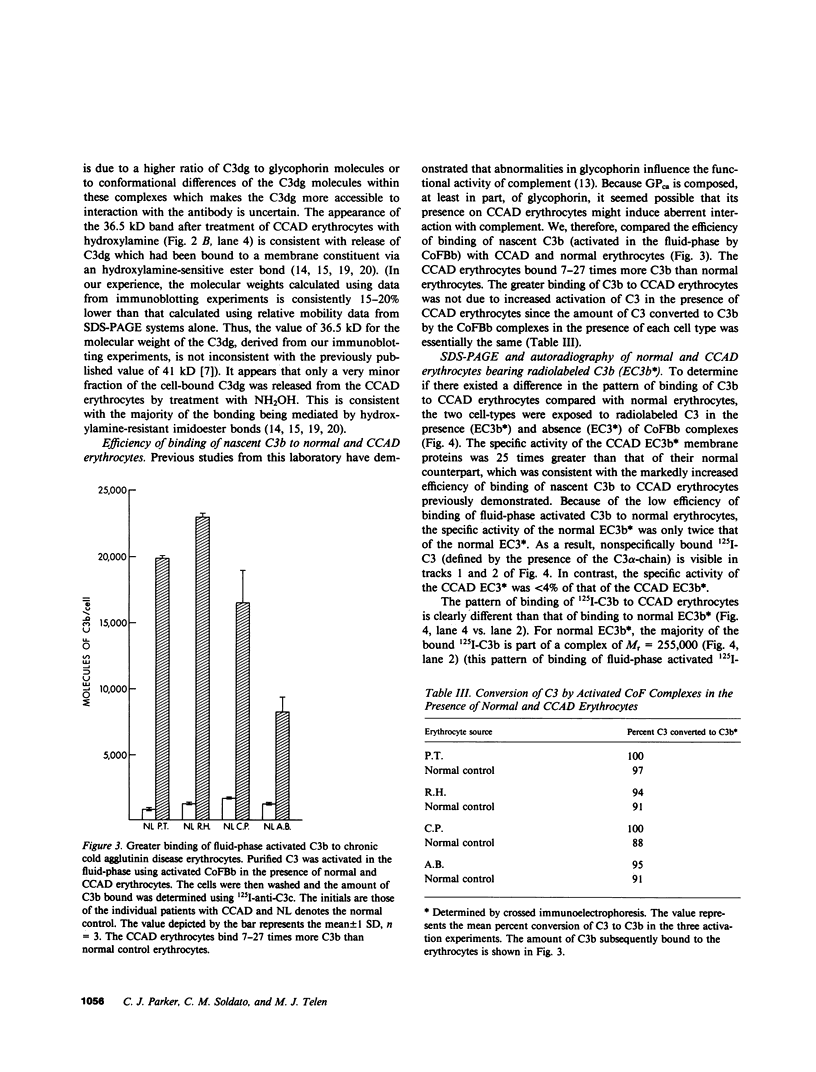
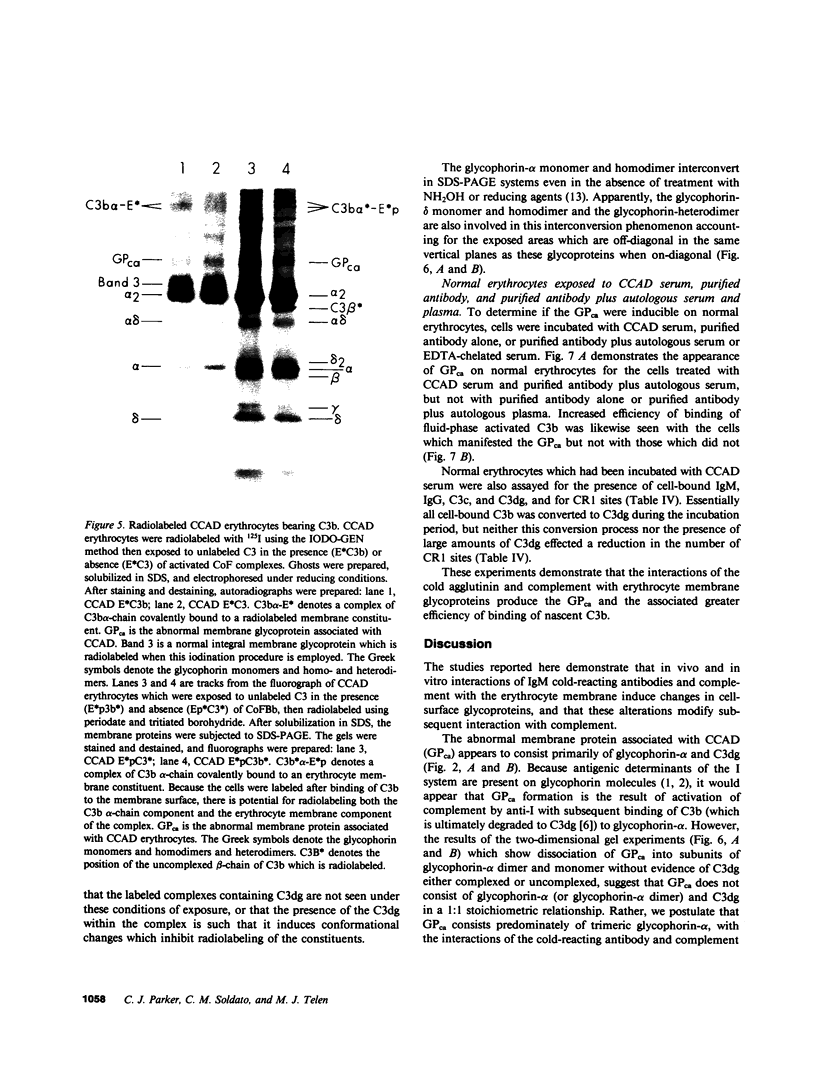
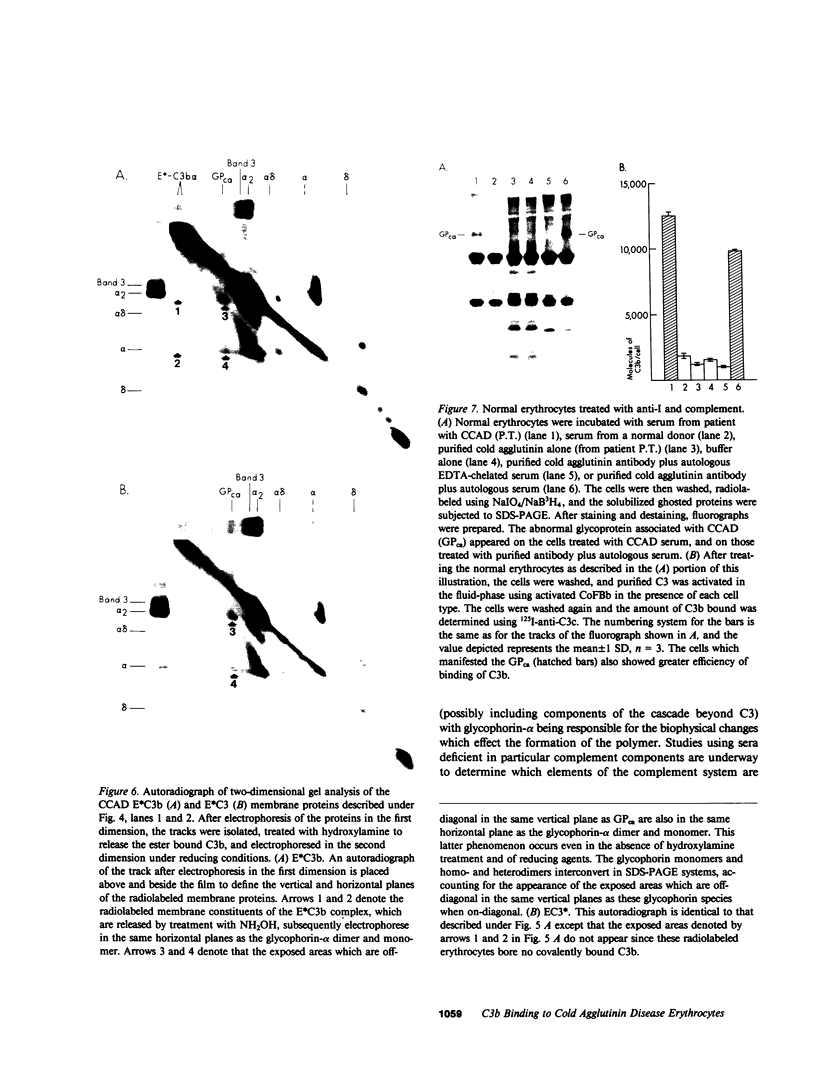
The pathogenesis of chronic cold agglutinin disease (CCAD) has been enigmatic. To determine if abnormal erythrocyte membrane constituents might provide the stimulus for antibody production, we compared the electrophoretic pattern of radiolabeled membrane glycoproteins of four patients with CCAD to that of normal control erythrocytes. For the CCAD erythrocytes, fluorographs revealed the appearance of an abnormal band whose molecular weight was estimated at 126,000 D. Using two-dimensional gel analysis and immunoblotting techniques, it was determined that the 126,000 D glycoprotein consisted predominately of polymeric glycophorin-alpha. Previous investigations had suggested that abnormalities in glycophorin-alpha influence the functional activity of the complement system. When purified complement (C)3 was activated in the fluid-phase by cobra venom factor complexes, CCAD erythrocytes bound nascent C3b 7-27 times more efficiently than normal erythrocytes. Normal erythrocytes could be induced to manifest the appearance of the 126,000 D band, and the increased efficiency of binding of nascent C3b by incubation with CCAD serum or with the purified cold agglutinin antibody plus autologous serum, but not with the purified antibody alone or the purified antibody plus EDTA-chelated autologous serum. These studies demonstrate that the interactions of IgM cold-reacting antibody and complement with glycophorin induce changes in the biophysical properties of the erythrocyte membrane which modify subsequent interactions with complement.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., Frank M. M. Studies on the in vivo effects of antibody. Interaction of IgM antibody and complement in the immune clearance and destruction of erythrocytes in man. J Clin Invest. 1974 Aug;54(2):339–348. doi: 10.1172/JCI107769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel P. J., Groeneboer O., Grosveld G., Pondman K. W. The binding of activated C3 to polysaccharides and immunoglobulins. J Immunol. 1978 Dec;121(6):2566–2572. [PubMed] [Google Scholar]
- Evans R. S., Turner E., Bingham M. Chronic hemolytic anemia due to cold agglutinins: the mechanism of resistance of red cells to C' hemolysis by cold agglutinins. J Clin Invest. 1967 Sep;46(9):1461–1474. doi: 10.1172/JCI105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. S., Turner E., Bingham M., Woods R. Chronic hemolytic anemia due to cold agglutinins. II. The role of C' in red cell destruction. J Clin Invest. 1968 Apr;47(4):691–701. doi: 10.1172/JCI105764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. J., Atkinson J. P., Frank M. M. The role of complement in the clearance of cold agglutinin-sensitized erythrocytes in man. J Clin Invest. 1976 Oct;58(4):942–949. doi: 10.1172/JCI108547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Oldroyd R. G., Milstein C., Wright B. W. Three rat monoclonal antibodies to human C3. Immunology. 1980 Nov;41(3):503–515. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Pangburn M. K., Oldroyd R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J Exp Med. 1982 Jul 1;156(1):205–216. doi: 10.1084/jem.156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Voak D., Oldroyd R. G., Downie D. M., Bevan P. C. Use of monoclonal anti-C3 antibodies to characterise the fragments of C3 that are found on erythrocytes. Vox Sang. 1983;45(5):367–372. doi: 10.1111/j.1423-0410.1983.tb01928.x. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Holcombe F. H., Levine R. P. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980 Aug;125(2):634–639. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Law S. K., Minich T. M., Levine R. P. Binding reaction between the third human complement protein and small molecules. Biochemistry. 1981 Dec 22;20(26):7457–7463. doi: 10.1021/bi00529a020. [DOI] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Comparison of binding characteristics of factors B and H to C3b on normal and paroxysmal nocturnal hemoglobinuria erythrocytes. J Immunol. 1983 Nov;131(5):2484–2489. [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Increased enzymatic activity of the alternative pathway convertase when bound to the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1982 Feb;69(2):337–346. doi: 10.1172/JCI110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Soldato C. M., Rosse W. F. Abnormality of glycophorin-alpha on paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1984 Apr;73(4):1130–1143. doi: 10.1172/JCI111299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W., Shumak K. H. Biologic activity of cold-reacting autoantibodies (first of two parts). N Engl J Med. 1977 Sep 8;297(10):538–542. doi: 10.1056/NEJM197709082971005. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Logue G. L., Adams J., Crookston J. H. Mechanisms of immune lysis of the red cells in hereditary erythroblastic multinuclearity with a positive acidified serum test and paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1974 Jan;53(1):31–43. doi: 10.1172/JCI107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972 Mar;51(3):575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. II. Molecular nature of IgG and IgM complement-fixing sites and effects of their interaction with serum. J Clin Invest. 1972 Mar;51(3):583–589. doi: 10.1172/JCI106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]