Abstract
Background
The most common adverse effects of oral isotretinoin are cheilitis, skin dryness, dry eyes, and conjunctivitis, whereas evening primrose oil (EPO) is known to improve skin moisture and transepidermal water loss (TEWL) in healthy adults and atopic patients.
Objective
To evaluate the clinical efficacy and safety of EPO in preventing xerotic cheilitis in acne patients being treated with oral isotretinoin.
Methods
Forty Korean volunteers of Fitzpatrick skin types III and IV, having moderate acne, were enrolled and randomized to receive either isotretinoin with or without EPO for 8 weeks. The efficacy of treatment was evaluated on the basis of global acne grading system scores, number of inflammatory and noninflammatory lesions, TEWL, corneometry, physician's global assessment, and patient satisfaction.
Results
The results after 8 weeks of treatment showed that the TEWL of the lip increased significantly during isotretinoin treatment, whereas the TEWL of the hand dorsum showed no significant change. The increase of the TEWL of the lip was more definite in the control group than in the experimental group. The number of acne lesions decreased significantly in both groups, and there were no differences between them.
Conclusion
Our study suggests that the addition of EPO improved xerotic cheilitis in acne patients being treated with oral isotretinoin. However, besides TEWL and corneometry assessments, additional studies are required for a complete understanding of the role of EPO in xerotic cheilitis in acne patients being treated with oral isotretinoin.
Keywords: Acne vulgaris, Evening primrose oil, Isotretinoin, Xerotic cheilitis
INTRODUCTION
Oral isotretinoin (13-cis-retinoic acid) was first approved as a treatment for severe acne by the US Food and Drug Administration in 1982. However, its off-label uses for cases of less severe acne have increased over time1. Isotretinoin exerts its therapeutic effects on acne by reducing sebum excretion and follicular keratinization, as well as reducing colonies of Propionibacterium acnes. Its effectiveness is dependent on the accumulated dose, and any treatment must be continued long enough to reach the standard accumulated dose of 120 to 150 mg/kg2. Although the safety profile of isotretinoin is favorable, this treatment is associated with certain reversible, dose-related adverse effects. The most common adverse effect is mucocutaneous dryness in the lips, eyes, and other epidermal surfaces. Skin dryness together with an increase in transepidermal water loss (TEWL) and erythema during treatment with isotretinoin seem to be the key factors causing patient discomfort, and these adverse effects can compromise patient compliance3,4,5. Therefore, managing these adverse effects and their influence on patient compliance is the key to treatment success. Evening primrose oil (EPO) contain gamma-linolenic acid (GLA, 18:3ω6), a carboxylic acid with an 18-carbon chain and three cis double bonds.6 It is a fatty acid found primarily in vegetable oils; it improves skin hydration in healthy adults and atopic patients7,8, and is indicated for relieving xerosis in patients undergoing therapy with oral isotretinoin. It could be hypothesized that acne patients had better compliance with isotretinoin treatment with EPO because it causes fewer mucocutaneous adverse effects such as xerotic cheilitis. In this study, we investigated the efficacy and tolerability of EPO in preventing xerotic cheilitis in acne patients being treated with oral isotretinoin.
MATERIALS AND METHODS
This was a prospective, randomized, and single-blind study to compare the effectiveness of EPO containing GLA as an adjuvant treatment in patients with moderate acne who are being treated with oral isotretinoin.
Participants
In this study, 40 Korean subjects (23 women and 17 men) of Fitzpatrick skin types III and IV, with moderate acne (mean global acne grading system score, 24.20±2.71), were enrolled. This clinical study was approved by the institutional review board of Chung Ang University Hospital, Seoul, Korea (IRB No. C2011045[493]). Before enrollment, patients were informed of the study procedures, possible risks, and benefits. Signed consent was obtained from each patient. The exclusion criteria included severe acne types, such as acne conglobata or acne fulminans, history of keloid, other systemic diseases, or pregnancy and lactation. The baseline characteristics of the subjects are presented in Table 1.
Table 1.
Summary of demographic data
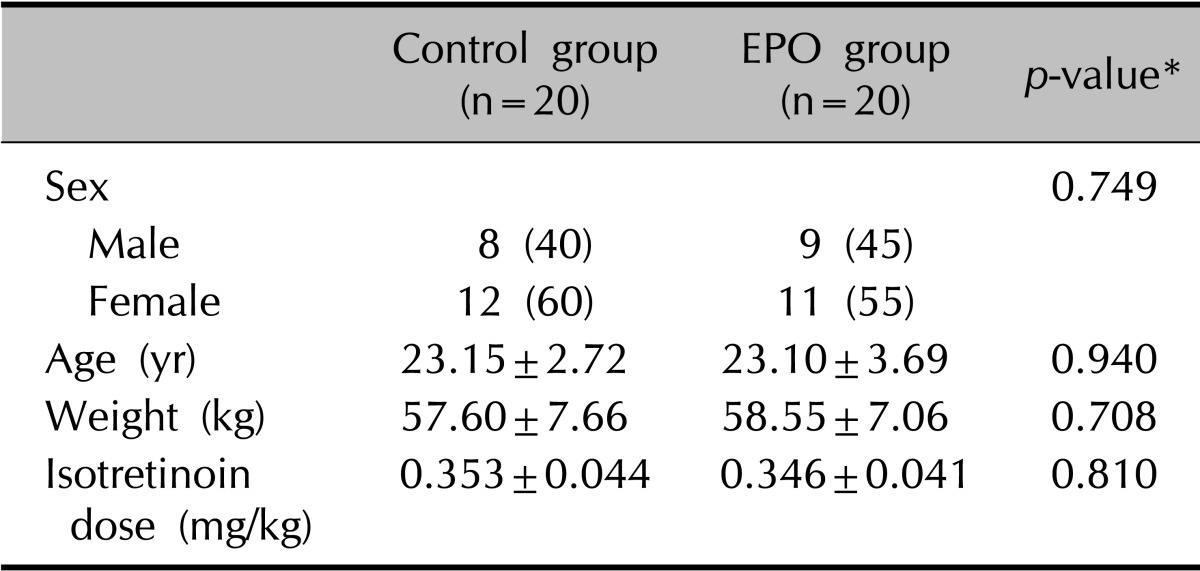
Values are presented as number (%) or mean±standard deviation. EPO: evening primrose oil. *p<0.05, significant.
Interventions
All patients received isotretinoin at daily doses between 0.25 and 0.4 mg/kg for 8 weeks. In the experimental group, the participants simultaneously received six 450 mg EPO capsules thrice daily, providing 240 mg of GLA for 8 weeks. All participants were given a cleansing gel and moisturizer, which they were instructed to use twice a day (morning and night) throughout the study. The use of any other additional cosmetic products on the face was prohibited.
Assessments
Clinical evaluations consisted of assessment of acne severity, counting of facial lesions, and evaluation of the incidence of adverse effects. For the evaluation of the effect on the skin and lip, instrumental evaluations were performed on the skin of the hand dorsum and of the lower lip.
Measurements with Tewameter TM300 (Courage+Khazaka Electronic GmbH, Cologne, Germany) to quantify TEWL and with Corneometer CM 825 (Courage+Khazaka Electronic GmbH) to quantify the moisture content of the stratum corneum were taken at each clinic visit. All measurements on the lip and hand dorsum were taken in sitting position with the subject's forearm lying on the table. Measurements were always performed in the same assessment room. Room temperature (24.0℃~25.5℃) and humidity (18%~24%) were recorded before testing.
Photographic documentation by using identical camera settings, lighting, and positioning was done at each visit. A comparison evaluation of the photographs was done by two independent dermatologists by using the quartile grading scale: 1=poor (0%~25%), 2=moderate (26%~50%), 3=good (51%~75%), 4=excellent (76%~100%). The patient satisfaction score was recorded as follows: unsatisfied, slightly satisfied, satisfied, or very satisfied. Any adverse effect was recorded over the entire study period.
Statistical analysis
Statistical analysis was performed with the SPSS ver. 12.0 for Windows statistical package (SPSS Inc., Chicago, IL, USA). We used the chi-square test, two-sample t-test, or Wilcoxon rank sum test for the analysis of demographic data. The two-sample t-test was used to compare the TEWL and corneometry readings between the groups. Null hypotheses of no difference were rejected if p-values were <0.05.
RESULTS
Forty patients were included in this study, 20 in the experimental group and 20 in the control group, ranging in age from 18 to 31 years (Table 1). The degree of acne was decreased significantly in both groups (p<0.001) after 2 months of treatment. In both groups, the mean number of comedones, pustules, and papules were decreased significantly at 2 months; however, the groups did not differ significantly in terms of the number of comedones and pustules (Table 2).
Table 2.
Mean number of comedones, pustules, and papules in the two groups

Values are presented as mean±standard deviation. EPO: evening primrose oil. *p<0.05, significant.
The clinical evaluation of the other cutaneous symptoms except xerotic cheilitis did not differ significantly between groups, although the experimental group tended to have a lower score than the control group for dryness of the skin (Fig. 1, Table 3, 4). The proportion of patients presenting with xerotic cheilitis after 2 months of therapy was significantly lower in the experimental group. On the lower lip, the experimental group tended to have a lower score than the control group for TEWL, and there was a significant difference between the two groups after 8 weeks of treatment (Fig. 2A, Table 5). Corneometry showed no significant differences between the two groups (Fig. 2B, Table 6).
Fig. 1.

(A) Although the experimental group tended to have a lower score than the control group for dryness of the hand dorsum, transepidermal water loss (TEWL) did not show significant differences between groups. (B) On the skin of the hand dorsum, corneometry did not show significant differences between groups. EPO: evening primrose oil.
Table 3.
Change of TEWL on the hand dorsum during isotretinoin treatment
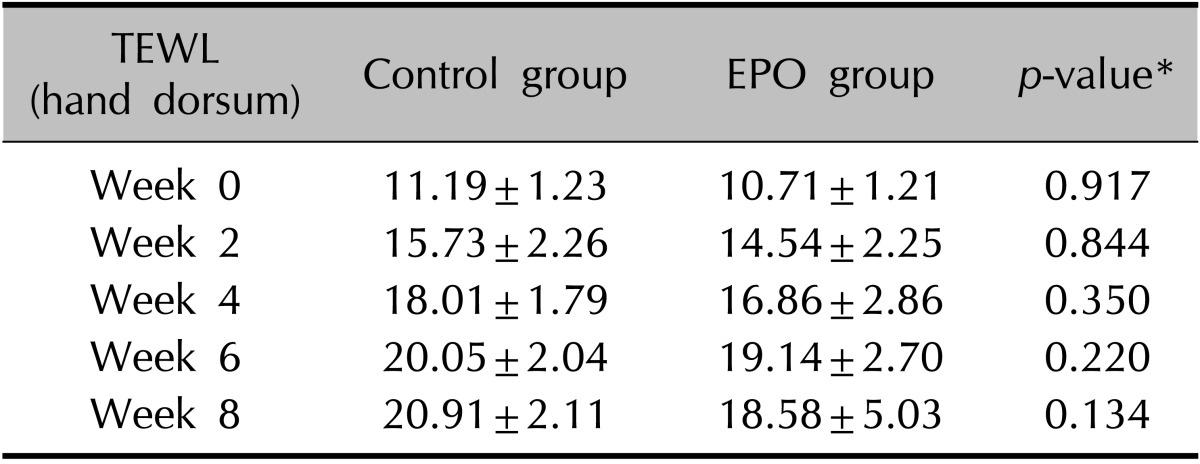
Values are presented as mean±standard deviation. TEWL: transepidermal water loss, EPO: evening primrose oil. *p<0.05, significant.
Table 4.
Change in corneometry readings of the hand dorsum during isotretinoin treatment
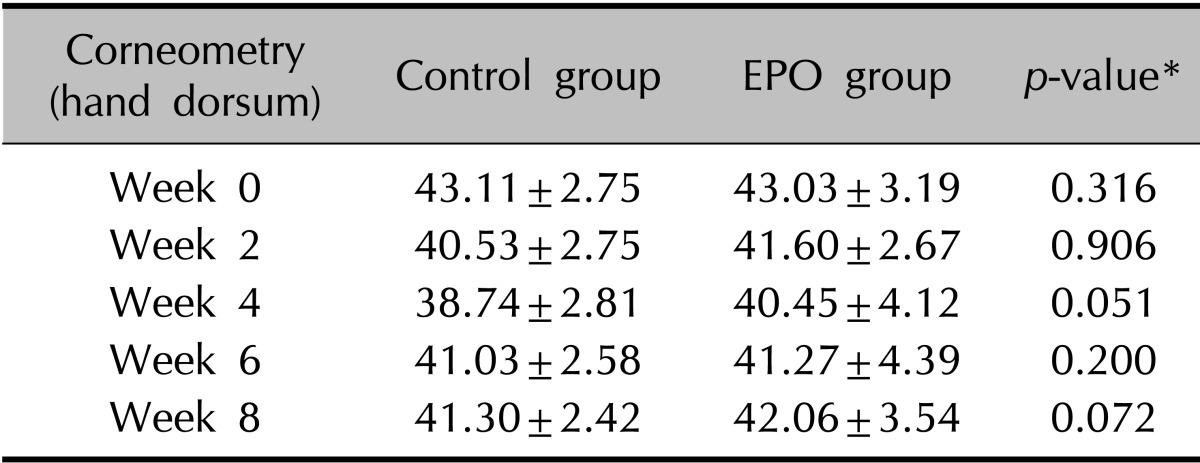
Values are presented as mean±standard deviation. EPO: evening primrose oil. *p<0.05, significant.
Fig. 2.
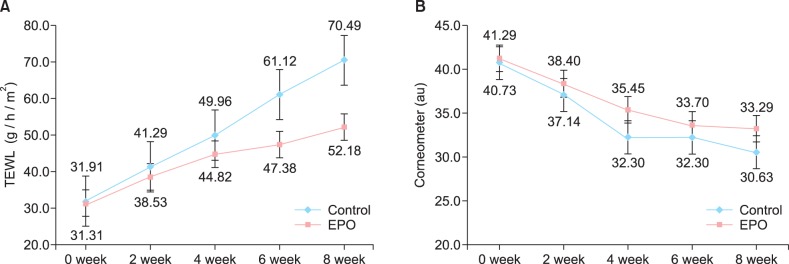
(A) On the lower lip, the experimental group tended to have a lower score than the control group for transepidermal water loss (TEWL), and there was a significant difference between the two groups after 8 weeks of treatment. (B) Corneometry showed no significant differences between the two groups. EPO: evening primrose oil.
Table 5.
Change of TEWL of the lower lip during isotretinoin treatment
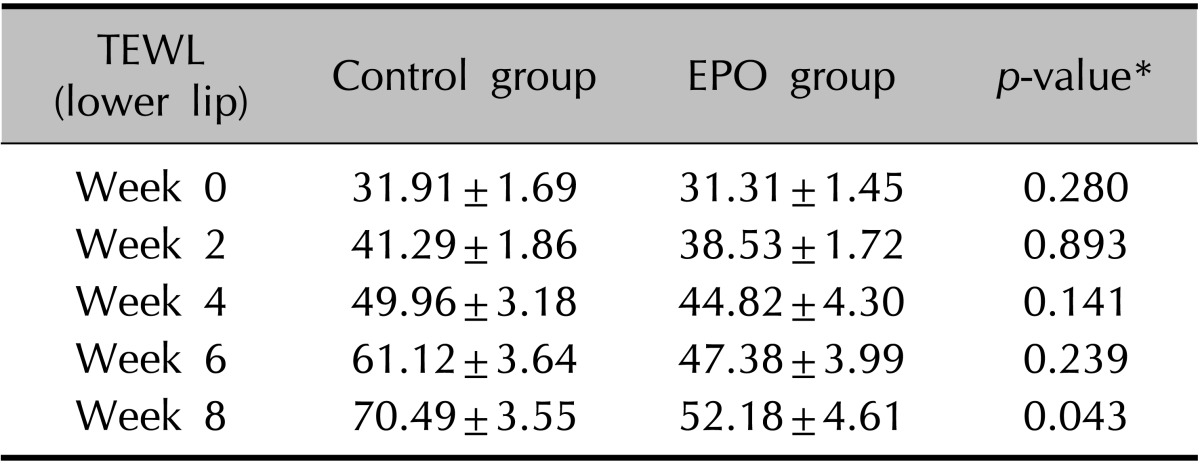
Values are presented as mean±standard deviation. TEWL: transepidermal water loss, EPO: evening primrose oil. *p<0.05, significant.
Table 6.
Change in corneometry readings of the lower lip during isotretinoin treatment
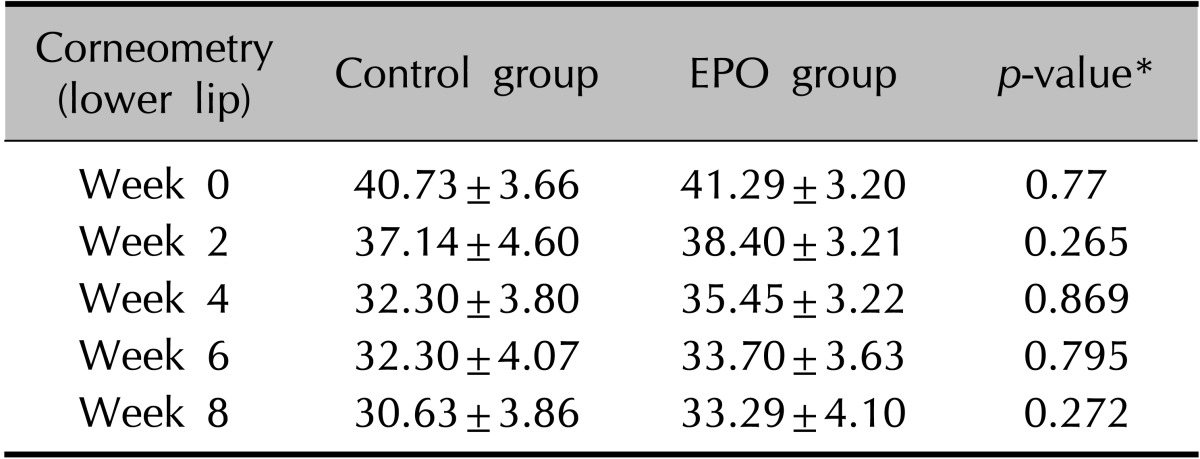
Values are presented as mean±standard deviation. EPO: evening primrose oil. *p<0.05, significant.
On the quartile grading scale, the mean clinical improvement for xerotic cheilitis at 8 weeks after the final treatment was 2.20±0.52 in the control group and 3.15±0.81 in the experimental group (Fig. 3, Table 7). There were significant differences between the control and experimental groups (p<0.05). Concerning patient satisfaction, most of the final ratings were moderate or good in both groups, although the experimental group was significantly satisfied at week 4 (Fig. 4).
Fig. 3.
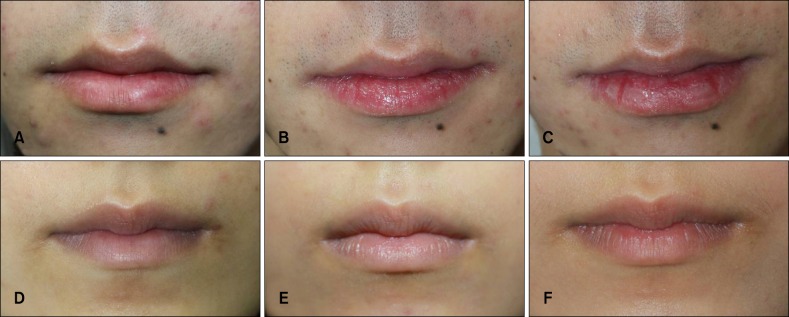
Clinical photographs of the lips during isotretinoin treatment. The control group (A~C) showed prominent xerotic cheilitis during the study period, and the experimental group (D~F) showed no significant cheilitis. From left: before treatment, 2 weeks later, and 8 weeks later.
Table 7.
Grade of clinical improvement of xerotic cheilitis by physicians

Values are presented as mean±standard deviation. EPO: evening primrose oil. *p<0.05, significant.
Fig. 4.
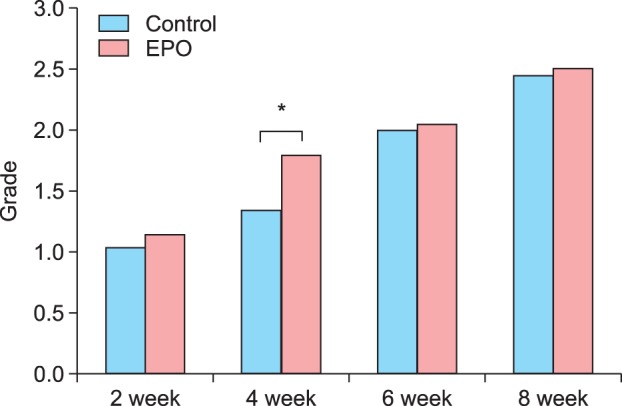
Concerning patient satisfaction levels during the treatment, most of the final ratings of satisfaction were "moderate" or "good" in both groups; *however, the experimental group was "significantly satisfied" at week 4. EPO: evening primrose oil.
DISCUSSION
Isotretinoin is a type of retinoid that has been widely used in the topical and systemic treatment of various dermatoses: psoriasis, keratinization disorders, keratotic genodermatosis, and severe acne. It is also used in the treatment and/or chemoprevention of skin cancer and other neoplasms. Isotretinoin inhibits sebaceous gland function and keratinization; however, its exact mechanism of action is not well understood9. Moreover, this drug has immunomodulator and anti-inflammatory properties10. Oral isotretinoin is the treatment of choice for severe inflammatory acne and is considered an alternative treatment for patients with moderate and long-term mild inflammatory acne with poor response to other treatments11,12.
The recommended dose of oral isotretinoin for acne treatment is 0.5 to 1.0 mg/kg daily for 16 to 32 weeks, reaching 120 to 150 mg/kg2. This regimen is well known to produce good results; however, it causes several dose-dependent adverse effects such as xerotic cheilitis, mild xerosis, and epistaxis13. Because of these adverse effects, some patients have difficulty in complying with the recommended dose. These mucocutaneous adverse effects mainly reflect decreased sebum production, reduced stratum corneum thickness, and altered skin barrier function9. Cheilitis is the most common manifestation and occurs in virtually all patients who receive isotretinoin therapy14. In fact, the absence of cheilitis in an apparent "treatment failure" that should raise the suspicion of noncompliance15. In an effort to overcome this concern, the use of hydrating and emollient products is often recommended to control the most frequent mucocutaneous adverse effects. In this study, we investigated the efficacy of oral EPO instead of a topical emollient for relieving xerotic cheilitis in patients undergoing therapy with oral isotretinoin.
The treatment groups were well matched at baseline concerning demographic data and individual isotretinoin dose; there were no significant differences between the experimental and control groups. The comparison between groups did not show significant differences in terms of the number of comedones and pustules after 8 weeks of treatment. This means that isotretinoin had an about the same curative effect for acne vulgaris in both groups. Oral EPO did not influence the efficacy of oral isotretinoin treatment. GLA inhibits inflammatory responses by regulating nuclear factor-kappaB and activator protein-1 activation, and prostaglandin E1 and 15-hydroxyeicosatrienoic acid converted from GLA have anti-inflammatory properties. For these reasons, the acne lesions were expected to improve somewhat better in the experimental group; however, the results did not show this effect.16,17,18
The results also showed that the group with oral EPO showed lower TEWL and higher corneometry readings than the control group. These suggest that GLA might improve skin properties and protect the skin barrier. This difference can be attributed to the higher concentration of GLA in EPO that is necessary to maintain hydration19. Deficiencies in n-6 essential fatty acids (EFA) correlated with the severity of skin barrier dysfunction and cutaneous inflammation20. GLA efficiently reverses epidermal hyperproliferation owing to its metabolites such as anti-inflammatory eicosanoids that exert antiproliferative properties, and these actions are associated with an increase in ceramide synthesis and improvement of skin barrier function21.
The skin of the lip has many different properties compared with the hand dorsum skin. The lip does not contain hair or sweat glands and sebaceous glands. The lip skin epithelium is characterized by thin and nonkeratinized tissue and a poor water-holding capacity22. We detected a significantly high TEWL on the lip compared with that on the hand dorsum skin in patients treated with oral isotretinoin. The dryness and desquamation of the lip in patients treated with oral isotretinoin may be caused by the thin and low barrier function of the nonkeratinized epithelium22.
The human skin is rich in sebaceous glands, and the sebum excreted by these glands is primarily composed of tryglycerides, wax esters, and squalene23. Sebum analysis demonstrates that the major components of sebum differ from those of EFA24. Human skin is not able to biosynthesize GLA from the precursor linoleic acid (LA, 18:2ω6), or arachidonic acid (20:4ω6) from dihomogamma-linolenic acid (20:3ω6), because of the absence of the enzymes delta 6- and delta 5-desaturase19,25. Therefore, EFA metabolites have to be synthesized in the liver and transported to the skin through the bloodstream. Because EFAs come strictly from the diet24, dietary supplementation with GLA-containing EPO skips the metabolic step of 6-desaturation of LA to form GLA26. Such a supplementation should compensate for the lack of EFA in patients treated with isotretinoin27.
GLA and its synthetic derivatives serve important and diverse roles in the structural maintenance and immunologic balance of the epidermis28,29,30. Therefore, GLA might aid in the prevention of cheilitis by the following mechanisms: (i) maintenance of the stratum corneum permeability barrier and (ii) maturation and differentiation of the stratum corneum.
According to the patients' satisfaction level, the difference in clinical efficacy against xerosis between the experimental and control groups was a peak at 4 weeks after treatment, which gradually decreased. This means that the most uncomfortable period of cheilitis is at about 4 weeks after taking isotretinoin. Thereafter, the patients adapted themselves to the xerotic change. We observed that the use of oral EPO did not influence the efficacy of oral isotretinoin treatment and improved xerotic cheilitis and the sensation of dryness. It seemed that acne patients had better compliance with the treatment with EPO because of its fewer mucocutaneous adverse effects.
EPO was found to induce an improvement in the cutaneous barrier function of the lips in acne patients taking isotretinoin, as reflected in their TEWL. The addition of EPO prevented retinoid-induced adverse effects without any complications. The statistical correlation between EPO and xerotic cheilitis is relatively weak. On the basis of these results, additional studies are required before we can have a complete understanding of the role of EPO on xerotic cheilitis in acne patients being treated with oral isotretinoin.
ACKNOWLEDGMENT
This work was supported by the Infrastructure Program for New-growth Industries (10044186; Development of Smart Beauty Devices Technology and Establishment of Commercialization Support Center) funded by the Ministry of Trade, Industry and Energy (MI, Korea).
References
- 1.Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162–169. doi: 10.4161/derm.1.3.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haider A, Shaw JC. Treatment of acne vulgaris. JAMA. 2004;292:726–735. doi: 10.1001/jama.292.6.726. [DOI] [PubMed] [Google Scholar]
- 3.Brito Mde F, SantAnna IP, Galindo JC, Rosendo LH, Santos JB. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331–337. doi: 10.1590/s0365-05962010000300006. [DOI] [PubMed] [Google Scholar]
- 4.Herane MI, Fuenzalida H, Zegpi E, De Pablo C, Espadas MJ, Trullás C, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase--a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181–185. doi: 10.1111/j.1473-2165.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 5.McLane J. Analysis of common side effects of isotretinoin. J Am Acad Dermatol. 2001;45:S188–S194. doi: 10.1067/mjd.2001.113719. [DOI] [PubMed] [Google Scholar]
- 6.Nykiforuk CL, Shewmaker C, Harry I, Yurchenko OP, Zhang M, Reed C, et al. High level accumulation of gamma linolenic acid (C18:3△6.9,12 cis) in transgenic safflower (Carthamus tinctorius) seeds. Transgenic Res. 2012;21:367–381. doi: 10.1007/s11248-011-9543-5. [DOI] [PubMed] [Google Scholar]
- 7.Muggli R. Systemic evening primrose oil improves the biophysical skin parameters of healthy adults. Int J Cosmet Sci. 2005;27:243–249. doi: 10.1111/j.1467-2494.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 8.Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2008;74:447–452. doi: 10.4103/0378-6323.42645. [DOI] [PubMed] [Google Scholar]
- 9.Vahlquist A, Saurat JH. Retinoid. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill; 2012. pp. 2759–2766. [Google Scholar]
- 10.Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53:358–388. doi: 10.2165/00003495-199753030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(1 Suppl):S1–S37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 12.Thielitz A, Krautheim A, Gollnick H. Update in retinoid therapy of acne. Dermatol Ther. 2006;19:272–279. doi: 10.1111/j.1529-8019.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 13.Cunliffe WJ, van de, Caputo R, Cavicchini S, Cooper A, Fyrand OL, et al. Roaccutane treatment guidelines: results of an international survey. Dermatology. 1997;194:351–357. doi: 10.1159/000246134. [DOI] [PubMed] [Google Scholar]
- 14.Ellis CN, Krach KJ. Uses and complications of isotretinoin therapy. J Am Acad Dermatol. 2001;45:S150–S157. doi: 10.1067/mjd.2001.113717. [DOI] [PubMed] [Google Scholar]
- 15.Almond-Roesler B, Blume-Peytavi U, Bisson S, Krahn M, Rohloff E, Orfanos CE. Monitoring of isotretinoin therapy by measuring the plasma levels of isotretinoin and 4-oxo-isotretinoin. A useful tool for management of severe acne. Dermatology. 1998;196:176–181. doi: 10.1159/000017856. [DOI] [PubMed] [Google Scholar]
- 16.Wauquier F, Barquissau V, Léotoing L, Davicco MJ, Lebecque P, Mercier S, et al. Borage and fish oils lifelong supplementation decreases inflammation and improves bone health in a murine model of senile osteoporosis. Bone. 2012;50:553–561. doi: 10.1016/j.bone.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Yoo TH, Lee SH, Kang HY, Nam BY, Kwak SJ, et al. Gamma linolenic acid exerts anti-inflammatory and anti-fibrotic effects in diabetic nephropathy. Yonsei Med J. 2012;53:1165–1175. doi: 10.3349/ymj.2012.53.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CS, Sun HL, Lii CK, Chen HW, Chen PY, Liu KL. Gamma-linolenic acid inhibits inflammatory responses by regulating NF-kappaB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation. 2010;33:46–57. doi: 10.1007/s10753-009-9157-8. [DOI] [PubMed] [Google Scholar]
- 19.Chapkin RS, Ziboh VA. Inability of skin enzyme preparations to biosynthesize arachidonic acid from linoleic acid. Biochem Biophys Res Commun. 1984;124:784–792. doi: 10.1016/0006-291x(84)91026-x. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura A, Ooyama K, Kojima K, Kachi H, Abe T, Amano K, et al. Dietary supplementation of gamma-linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J Oleo Sci. 2011;60:597–607. doi: 10.5650/jos.60.597. [DOI] [PubMed] [Google Scholar]
- 21.Chung S, Kong S, Seong K, Cho Y. Gamma-linolenic acid in borage oil reverses epidermal hyperproliferation in guinea pigs. J Nutr. 2002;132:3090–3097. doi: 10.1093/jn/131.10.3090. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Tagami H. Functional properties of the surface of the vermilion border of the lips are distinct from those of the facial skin. Br J Dermatol. 2004;150:563–567. doi: 10.1046/j.1365-2133.2003.05741.x. [DOI] [PubMed] [Google Scholar]
- 23.Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinol. 2009;1:68–71. doi: 10.4161/derm.1.2.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappas A. Epidermal surface lipids. Dermatoendocrinol. 2009;1:72–76. doi: 10.4161/derm.1.2.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapkin RS, Ziboh VA, Marcelo CL, Voorhees JJ. Metabolism of essential fatty acids by human epidermal enzyme preparations: evidence of chain elongation. J Lipid Res. 1986;27:945–954. [PubMed] [Google Scholar]
- 26.Brenner RR. Nutritional and hormonal factors influencing desaturation of essential fatty acids. Prog Lipid Res. 1981;20:41–47. doi: 10.1016/0163-7827(81)90012-6. [DOI] [PubMed] [Google Scholar]
- 27.Proksch E, Holleran WM, Menon GK, Elias PM, Feingold KR. Barrier function regulates epidermal lipid and DNA synthesis. Br J Dermatol. 1993;128:473–482. doi: 10.1111/j.1365-2133.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 28.Ziboh VA, Cho Y, Mani I, Xi S. Biological significance of essential fatty acids/prostanoids/lipoxygenase-derived monohydroxy fatty acids in the skin. Arch Pharm Res. 2002;25:747–758. doi: 10.1007/BF02976988. [DOI] [PubMed] [Google Scholar]
- 29.Brosche T, Platt D. Effect of borage oil consumption on fatty acid metabolism, transepidermal water loss and skin parameters in elderly people. Arch Gerontol Geriatr. 2000;30:139–150. doi: 10.1016/s0167-4943(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 30.De Spirt S, Stahl W, Tronnier H, Sies H, Bejot M, Maurette JM, et al. Intervention with flaxseed and borage oil supplements modulates skin condition in women. Br J Nutr. 2009;101:440–445. doi: 10.1017/S0007114508020321. [DOI] [PubMed] [Google Scholar]


