Abstract
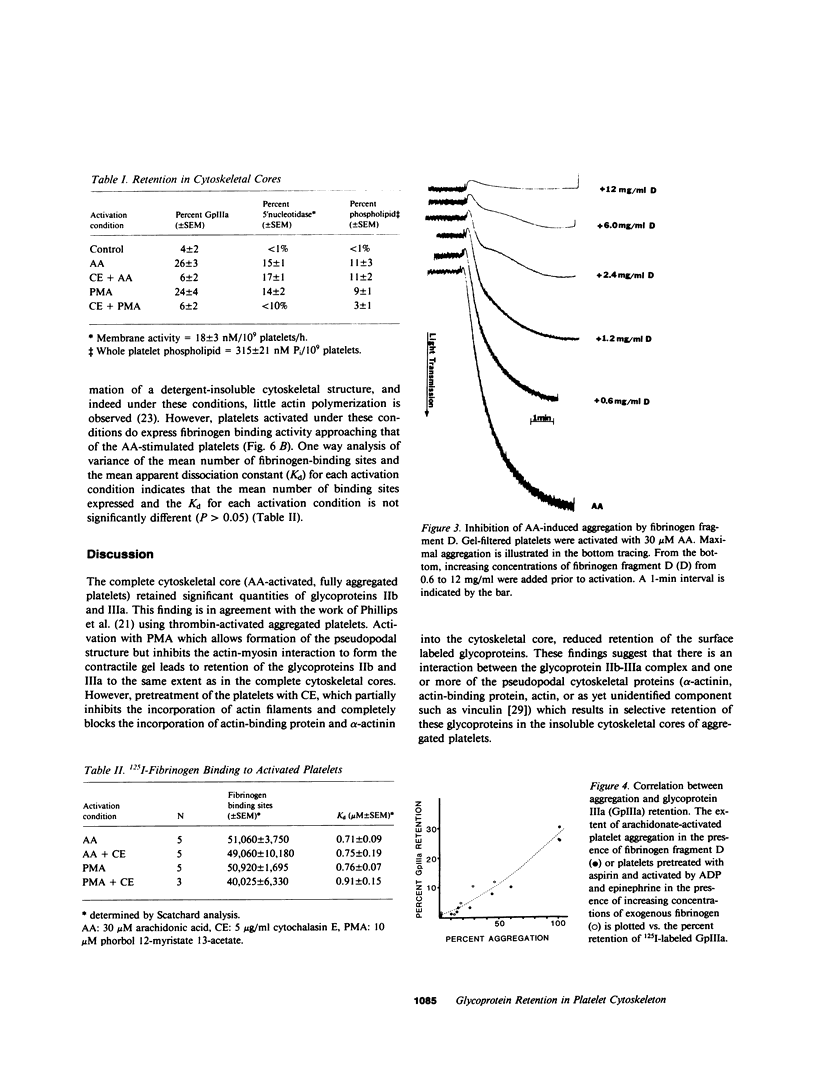
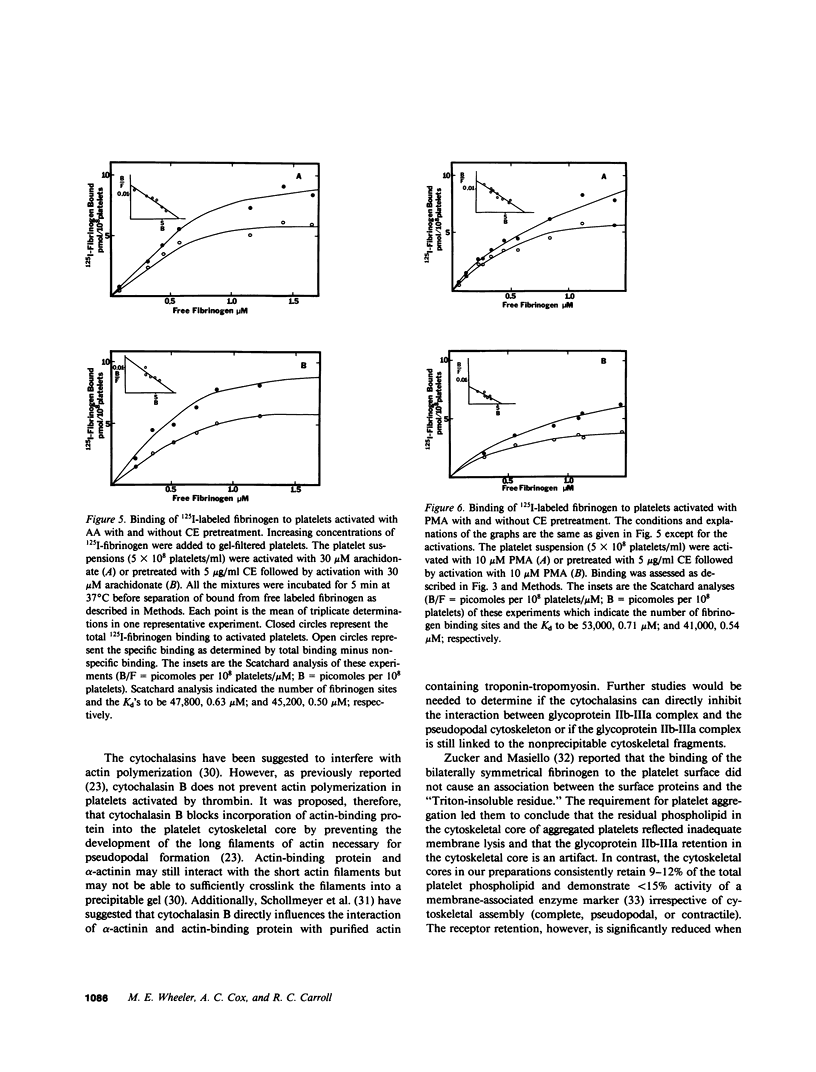
To investigate the association of the putative platelet fibrinogen receptor (glycoprotein IIb-III(a) with the cytoskeleton, 125I-surface labeled human platelets washed by gel-filtration were activated under conditions which allow selective assembly of the platelet cytoskeleton. The four conditions were activation with arachidonate or phorbol 12-myristate 13-acetate (PMA) with and without pretreatment with cytochalasin E. Activation with arachidonate generates a complete cytoskeletal core (pseudopodal and contractile elements) while PMA activation forms only an actin plus actin-binding protein pseudopodal core. Pretreatment with cytochalasin E leads to actomyosin contractile core formation if arachidonate activated, and essentially blocks cytoskeletal development if PMA activated. Cytoskeletal cores from arachidonate or PMA-activated platelets retained 26 (+/- 3%) of the total 125I-IIIa. Pretreatment with cytochalasin E followed by arachidonate or PMA activation reduced the 125I-IIIa retention to near control levels (unactivated platelets: 4 +/- 2%). The role of aggregation vs. receptor occupancy in the retention of IIb-IIIa was assessed by activation of platelets with arachidonate in the presence of fibrinogen fragment D (0.6-12 mg/ml). Aggregation was blocked by increasing concentrations of fragment D reagent while cytoskeletal assembly was not altered. The IIIa retention correlated with extent of aggregation with maximal retention corresponding to full aggregation. To determine if cytoskeletal development is necessary for the expression of the fibrinogen binding site, binding studies were performed with unlabeled platelets and 125I-fibrinogen. The mean number of binding sites and the mean dissociation constant were not significantly different among the four activation conditions. Although the development of a platelet cytoskeletal core is not required for the expression of the fibrinogen binding site, the retention of the glycoprotein IIb-IIIa complex is dependent on fibrinogen-supported aggregation as well as the formation of the pseudopodal cytoskeleton.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Colman R. F., Colman R. W. Localization of human platelet membrane-associated actomyosin using the affinity label 5'-p-fluorosulfonylbenzoyl adenosine. J Biol Chem. 1981 Feb 10;256(3):1185–1190. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie G. N., Baenziger N. L., Chase L. R., Majerus P. W. The effects of thrombin on adenyl cyclase activity and a membrane protein from human platelets. J Clin Invest. 1972 Jan;51(1):81–88. doi: 10.1172/JCI106800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. C., Butler R. G., Morris P. A., Gerrard J. M. Separable assembly of platelet pseudopodal and contractile cytoskeletons. Cell. 1982 Sep;30(2):385–393. doi: 10.1016/0092-8674(82)90236-7. [DOI] [PubMed] [Google Scholar]
- Cox A. C., Carroll R. C., White J. G., Rao G. H. Recycling of platelet phosphorylation and cytoskeletal assembly. J Cell Biol. 1984 Jan;98(1):8–15. doi: 10.1083/jcb.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Schollmeyer J. V., Phillips D. R., White J. G. alpha-Actinin deficiency in thrombasthenia: possible identity of alpha-actinin and glycoprotein III. Am J Pathol. 1979 Mar;94(3):509–528. [PMC free article] [PubMed] [Google Scholar]
- Glastris B., Pfeiffer S. E. Mammalian membrane marker enzymes: sensitive assay for 5'-nucleotidase and assay for mammalian 2',3'-cyclic-nucleotide-3'-phosphohydrolase. Methods Enzymol. 1974;32:124–131. doi: 10.1016/0076-6879(74)32015-0. [DOI] [PubMed] [Google Scholar]
- Gogstad G. O., Brosstad F., Krutnes M. B., Hagen I., Solum N. O. Fibrinogen-binding properties of the human platelet glycoprotein IIb-=IIIa complex: a study using crossed-radioimmunoelectrophoresis. Blood. 1982 Sep;60(3):663–671. [PubMed] [Google Scholar]
- Hagen I., Nurden A., Bjerrum O. J., Solum N. O., Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980 Mar;65(3):722–731. doi: 10.1172/JCI109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Howard L., Shulman S., Sadanandan S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. The major antigen consists of a complex of glycoproteins, GPIIb and GPIIIa, held together by Ca2+ and missing in Glanzmann's thrombasthenia. J Biol Chem. 1982 Jul 25;257(14):8331–8336. [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Keenan J. P., Solum N. O. Quantitative studies on the release of platelet fibrinogen by thrombin. Br J Haematol. 1972 Oct;23(4):461–466. doi: 10.1111/j.1365-2141.1972.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Langer B. G., Gonnella P. A., Nachmias V. T. alpha-Actinin and vinculin in normal and thrombasthenic platelets. Blood. 1984 Mar;63(3):606–614. [PubMed] [Google Scholar]
- Marguerie G. A., Ardaillou N., Cherel G., Plow E. F. The binding of fibrinogen to its platelet receptor. J Biol Chem. 1982 Oct 25;257(20):11872–11875. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Marguerie G. A., Thomas-Maison N., Ginsberg M. H., Plow E. F. The platelet-fibrinogen interaction. Evidence for proximity of the A alpha chain of fibrinogen to platelet membrane glycoproteins IIb/III. Eur J Biochem. 1984 Feb 15;139(1):5–11. doi: 10.1111/j.1432-1033.1984.tb07968.x. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Mihalyi E. Physicochemical studies of bovine fibrinogen. IV. Ultraviolet absorption and its relation to the structure of the molecule. Biochemistry. 1968 Jan;7(1):208–223. doi: 10.1021/bi00841a026. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Kinlough-Rathbone R. L., Packham M. A., Perry D. W., Harfenist E. J., Pai K. R. Comparison of fibrinogen association with normal and thrombasthenic platelets on exposure to ADP or chymotrypsin. Blood. 1979 Nov;54(5):987–993. [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B. Relationship of ADP-induced fibrinogen binding to platelet shape change and aggregation elucidated by use of colchicine and cytochalasin B. Thromb Haemost. 1980 Feb 29;43(1):58–60. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Thrombin substrates and the proteolytic site of thrombin action on human-platelet plasma membranes. Biochim Biophys Acta. 1974 Jun 13;352(2):218–227. doi: 10.1016/0005-2736(74)90213-2. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Schollmeyer J. V., Rao G. H., White J. G. An actin-binding protein in human platelets. Interactions with alpha-actinin on gelatin of actin and the influence of cytochalasin B. Am J Pathol. 1978 Nov;93(2):433–446. [PMC free article] [PubMed] [Google Scholar]
- Sheterline P., Hopkins C. R. Transmembrane linkage between surface glycoproteins and components of the cytoplasm in neutrophil leukocytes. J Cell Biol. 1981 Sep;90(3):743–754. doi: 10.1083/jcb.90.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Ash J. F., Carroll R. C., Heggeness M. H., Vogt P. K. On the biochemistry of transformation by Rous sarcoma virus. Prog Clin Biol Res. 1980;41:765–772. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus P. W. Inhibition of human platelet aggregation by monovalent antifibrinogen antibody fragments. J Clin Invest. 1975 Jun;55(6):1259–1268. doi: 10.1172/JCI108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Masiello N. C. The Triton X-100-insoluble residue ("cytoskeleton") of aggregated platelets contains increased lipid phosphorus as well as 125I-labeled glycoproteins. Blood. 1983 Apr;61(4):676–683. [PubMed] [Google Scholar]




