Abstract
Dengue (DEN) is the most important mosquito-borne viral disease, with a major impact on global health and economics, caused by four serologically and distinct viruses termed DENV-1 to DENV-4. Currently, there is no licensed vaccine to prevent DEN. We have developed a live attenuated tetravalent DENV vaccine candidate (TDV) (formally known as DENVax) that has shown promise in preclinical and clinical studies and elicits neutralizing antibody responses to all four DENVs. As these responses are lowest to DENV-4 we have used the AG129 mouse model to investigate the immunogenicity of monovalent TDV-4 or tetravalent TDV vaccines, and their efficacy against lethal DENV-4 challenge. Since the common backbone of TDV is based on an attenuated DENV-2 strain (TDV-2) we also tested the efficacy of TDV-2 against DENV-4 challenge. Single doses of the tetravalent or monovalent vaccines elicited neutralizing antibodies, anti-NS1 antibodies, and cellular responses to both envelope and nonstructural proteins. All vaccinated animals were protected against challenge at 60 days post-immunization, whereas all control animals died. Investigation of DENV-4 viremias post-challenge showed that only the control animals had high viremias on day 3 post-challenge, whereas vaccinated mice had no detectable viremia. Overall, these data highlight the excellent immunogenicity and efficacy profile of our candidate dengue vaccine in AG129 mice.
Keywords: Dengue, Vaccines, Immune protection, AG129 mice, Dengue 4
1. Introduction
Dengue disease (DEN) is the most important mosquito-borne viral disease, and a major worldwide public health problem [1]. It is caused by four serologically and distinct viruses or serotypes, termed DENV-1 to DENV-4. The four DENVs are endemic throughout the world's subtropical and tropical regions, especially in Asia and Latin America. In humans, DENV may cause acute febrile illness dengue fever (DF) that is not life threatening. However, DF can progress to a potentially life-threatening dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) [2,3]. It is estimated that these viruses cause approximately 390 million clinical DEN infections annually [1].
The humoral immune response to DENV primarily targets the premembrane (prM) and envelope (E) structural proteins, and is predominately composed of serotype-cross-reactive antibodies [4–6]. In addition, the secreted nonstructural protein 1 (NS1) is highly immunogenic eliciting antibodies that exhibit complement-fixing activity that can trigger the lysis of virus-infected cells, and induces protective immunity in mice against DENV challenge [7–11]. In contrast, cellular immune responses to DENV, predominantly target epitopes located in the NS proteins [12]. Primary infection with any of the four serotypes is believed to confer lifelong protection to the homologous serotype; however, secondary infection with a different DENV serotype is the major risk factor for severe disease. This may be due to “antibody-dependent enhancement” (ADE) of disease where cross-reactive anti-DENV antibodies facilitate entry of DENV into Fcγ receptor-bearing cells [13].
Current vaccine development efforts for DEN are based on inducing simultaneous immune responses to all four DENV serotypes using different technologies. Several of these candidate vaccines are at various stages of preclinical or clinical development [14]. Following successful Phase 1 clinical trials, our candidate live-attenuated tetravalent dengue vaccine (TDV) (formally known as DENVax) is currently being tested in Phase II trials at various endemic geographical locations. This vaccine consists of a molecularly characterized attenuated DENV-2 strain (TDV-2) and three chimeric viruses containing the prM and E protein genes of DENV-1, −3 and −4 expressed in the context of the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4, respectively) [15–19]. In preclinical animal models, and phase I clinical trials this vaccine was shown to elicit tetravalent neutralizing antibody responses, with responses highest to DENV-2, and lowest to DENV-4 ([20,21], Osorio et al., submitted for publication). Type-I & II interferon receptor-deficient (AG129) mice allow efficient replication of DENVs and represent a well-characterized animal model of DENV infection [22]. We used this model to investigate the protective efficacy of TDV-4 vaccine and compared it with that elicited by TDV against a lethal DENV-4 challenge. Since the common backbone of TDV is based on the attenuated TDV-2 virus we also tested the efficacy of this monovalent vaccine against DENV-4 challenge.
2. Materials and methods
2.1. Viruses
The TDV vaccine strains have been previously reported [19]. The human Thai DENV-4 strain 703 was used for the mouse challenge studies (Bourne et al., in preparation). In addition, a number of DENV-4 isolates were used, referred to as; Colombia 2006 (CI/DB039/2006; Indonesian geno-type), Ecuador 1999 (EC/DB041/1999; Indonesian genotype), Micronesia 1995 (MC/DB043/1995; S.E Asian genotype), Mexico 2006 (MX/DB042/2006; Indonesian genotype), Virgin Islands 1994 (SC/DB045/1994; Indonesian genotype, and Thailand 2006 (TH/DB060/2006; S.E. Asian genotype). The viruses were obtained from the Division of Vector-Borne Diseases, Dengue Branch, CDC, San Juan, Puerto Rico.
2.2. Immunizations and virus challenge
On day 0 of the study, groups of 4 week-old AG129 mice (n = 15) were immunized subcutaneously (SC) with 0.2 ml of the tetravalent-TDV (2 × 104, 5 × 104, 1 × 105 and 3 × 105 pfu/ml for TDV 1–4 vaccines, respectively), monovalent TDV-4 (3 × 105 pfu/ml) or monovalent TDV-2 (5 × 104 pfu/ml) vaccines. In addition, a group of mice were injected with × FTA (virus diluent) as a negative control. Mice were bled on day 56 post-immunization to measure neutralizing antibodies against each DENV serotype. On day 60 all animals were challenged intraperitoneally (IP) with ~ 1.5 × 107 pfu of DENV-4 strain 703 in a 0.1 ml volume. Following challenge mice were bled on days 1, 2 and 3 (5 mice from each group/bled day) to measure viremia levels by qRT-PCR. In addition, mice were weighed and monitored for morbidity daily to day 28 post-challenge. Animals exhibiting signs of severe disease or with weight loss greater than 20% of initial body weight were euthanized and counted as being dead on the following day for analysis. At the end of the monitoring period a terminal bleed was collected from all surviving animals to quantify the neutralizing antibody response against DENV-4.
To measure cellular responses, two groups of 4–6 week-old AG129 mice were immunized SC with 0.2 ml TDV (n = 3) or TDV-4 vaccines (n = 2) using the same vaccine doses as described above. Six and seven weeks post-priming, respectively, mice from each group were euthanized and individual spleens were collected for further analysis.
2.3. Measurement of anti-NS1antibodies by ELISA
Purified NS1 antigen from DENV-2 and DENV-4 (abcam, Cambridge, MA) was resuspended in carbonate coating buffer pH 9.6 and coated at 1 ng/μl (50 μl/well) onto 96-well ELISA plates (Corning Polystryrene). Plates were washed with PBS/0.1% Tween 20 (PBST) and blocked with 10% milk in PBST. Sera were serially diluted and incubated at 37 s=degC for 1 h. Following washing with PBST, goat anti-mouse HRP (Jackson Immuno, West Grove, PA) at 1:10,000 in 10% milk/PBST was added, and plates were incubated at 37 s=degC for 1hr. Color reaction was developed by adding 100 μl TMB solution and incubating plates at room temperature in the dark for 6 min. Reaction was stopped by adding 1 N HCl. Absorbance was recorded at 450 nm and 630 nm using a Biotek plate reader. To account for optical interference the A630 was then subtracted from the A450.
2.4. Neutralization test
Vero cells (1.5 × 104 cells/100 μl) were plated into 96-well tissue culture plates in DMEM/10% FBS/1% penicillin/streptomycin and incubated at 37 s=degC with 5% CO2 for 48 h. Heat-inactivated sera were two-fold serially diluted in BA-1 medium, mixed with 2 × virus in an equal volume and incubated at 4 s=degC, overnight. Dengue viruses used are the parent strains to the vaccine viruses (DENV-1; 16007, DENV-2; 16681, DENV-3; 16562, DENV-4; 1036). In addition, we tested the breadth of neutralizing antibody responses elicited by TDV or TDV-4 vaccines against several DENV-4 isolates collected from different geographical locations (see Section 2.1). Next, 30 μl of the serum-virus mixture was added to Vero cell monolayers in triplicate and adsorbed at 37 s=degC for 2 h. Both positive and negative control sera samples were included. At the end of the incubation period, 100 μl/well of 1.2% carboxy-methyl cellulose overlay was added and plates were incubated at 37 s=degC, 5% CO2 for a previously determined time period (plus or minus 3 h) to allow for the formation of detectable foci (DENV-1; 53 h, DENV-2; 72 h, DENV-3; 53 h, DENV4; 48 h). Cells were fixed with 85% ice cold acetone at ambient temperature for 20 min and stored at –20 s=degC. Plates were equilibrated to ambient temperature and washed 3 times with PBS-T (PBS/0.1% Tween 20) to remove residual overlay and then incubated with primary rabbit anti-DENV polyclonal antibody (1:1000 dilution in PBS-T/2.5% milk) at 37 s=degC for 2 h. Plates were washed as before and then incubated with secondary HRP-conjugated anti-rabbit antibody at 37 s=degC for 2 h. Finally, plates were incubated with 100 μl/well of the HRP substrate 3-amino-9-ethylcarbozole until foci were visible. Following washing with water plates were air-dried and foci were quantified on an ELISpot reader. Titers were defined as the reciprocal of the highest serum dilution that reduced the average virus input in the negative control serum by at least 50%.
2.5. Virus quantitation by qRT-PCR
RNA was extracted from sera using the Aurum total RNA isolation kit (Bio-Rad, Hercules, CA) as previously described [23]. Reverse transcription was accomplished using an iScriptTM synthesis kit (Bio-Rad) using the following protocol: 1) 1.5 min, 25 s=degC, 2) 42 s=degC, 30 min, 3) 85 s=degC, 5 min, 4) infinite hold at 4 s=degC. Samples were evaluated using a DENV-4 serotype-specific qRT-PCR [24] utilizing a TaqMan probe (Sigma–Aldrich, St. Louis, MO) to quantify the specific amplification in each reaction. Each 25 μl qRT-PCR reaction contained: 12.5 μl iQ supermixTM (Bio-Rad) and 1 μl (5 μM) of forward and reverse primer and 1.5 μl of (5.0 μM) of the TaqMan probe [24] and 3 μl of cDNA template or nuclease-free water (no template controls). The qPCR was completed in a C1000 thermocycler equipped with a CFXTM optical module (Bio-Rad) using the following parameters: Cycle 1), 95 s=degC, 1.5 min; Cycle 2), Step 1. 95 s=degC, 10 s, Step 2. 62 s=degC, 30 s, repeat 40×. Fluorescent signal data was collected at the end of each annealing/extension step. Starting quantity values were extrapolated from standard curves of plasmids harboring the PCR targets generated in parallel for each run.
2.6. Flow cytometry
Spleens from individual animals were processed into single splenocyte suspension for each vaccine group. After lysis of red blood cells with 1x BD Pharm LyseTM buffer, splenocytes were suspended in RPMI1640 (10% FCS, 100 I.U./ml penicillin, 100 μg/mL streptomycin and 0.14 mM β-mecaptoethanol), and 1 × 106 cells/well were plated onto a 96-well U-bottom plate. Cells were stimulated with pools of peptides encompassing the entire sequence of DENV-1–4 at a final concentration of 20 μg/ml for 16 h in the presence of 1 μg/ml BD GolgiPlug (Bredfeldin A). Cells were stained for surface markers with anti-mouse CD4 FITC (RM4-5), anti-mouse CD8α PerCP (53-6.7) and for intracellular cytokines with monoclonal antibodies for IFN-γ APC (XMG1.2), IL-2 PE (JES6-5H4), TNF-α PE (MP6-XT22). The samples were acquired using a BD FACS Calibur and analyzed with FlowJo v7.6.5 (Tree Star). Background values from medium-treated groups were subtracted from each sample. The frequency of cytokine-positive T cells was presented as the percentage of gated CD4+ or CD8+ T cells.
3. Results
3.1. Immunogenicity of TDV formulations in AG129 mice
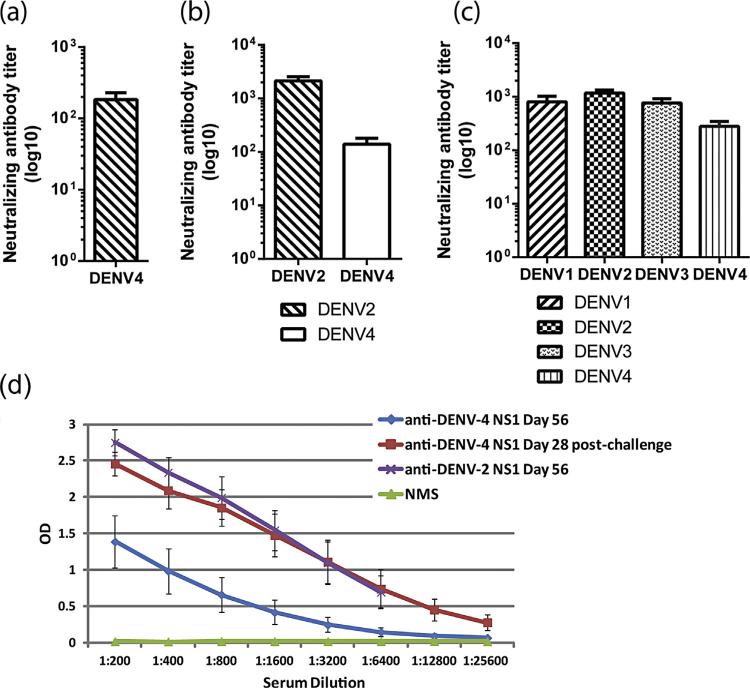
A single immunization with TDV-4 or TDV-2 vaccines was sufficient to elicit neutralizing antibody responses to the homologous viruses (Fig. 1a and b) and antibodies to DENV-2 NS1 protein (Fig. 1d). Further, antibodies elicited by TDV-2 were shown to cross-neutralize DENV-4 (Fig. 1b), and to cross-react with the DENV-4 NS1 protein (Fig. 1d). Animals that received a single immunization of TDV developed neutralizing antibody responses to all four DENV serotypes (Fig. 1c). However, the titers to DENV-4 were the lowest while those induced against DENV-1, DENV-2 and DENV-3 were indistinguishable.
Fig. 1.
Neutralizing and anti-NS1 antibodies. (a–c) Neutralizing antibody responses elicited after a single immunization with TDV-4 (a) or TDV-2 (b) vaccines or tetravalent TDV (c). Individual serum samples collected from each group (day 56 post-primary immunization) were tested for neutralizing activity. Sera from group A were tested for neutralizing activity against DENV-4, sera from group B were tested for neutralizing activity against DENV-2 and DENV-4, and those from group C for tetravalent neutralizing antibody responses. Data present mean of neutralizing antibody titers against each virus ± SE. (d) Titration of immune serum collected from animals vaccinated with TDV on day 56 post-immunization or day 28 post-DENV-4 challenges against DENV-2 and DENV-4 NS1 antigens coated on an ELISA plate. Data represent the mean ± SD of titrations of individual serum for each time point tested against the DENV-2 and DENV-4 NS1 antigens ± SD. Normal mouse serum was tested as negative control (green line).
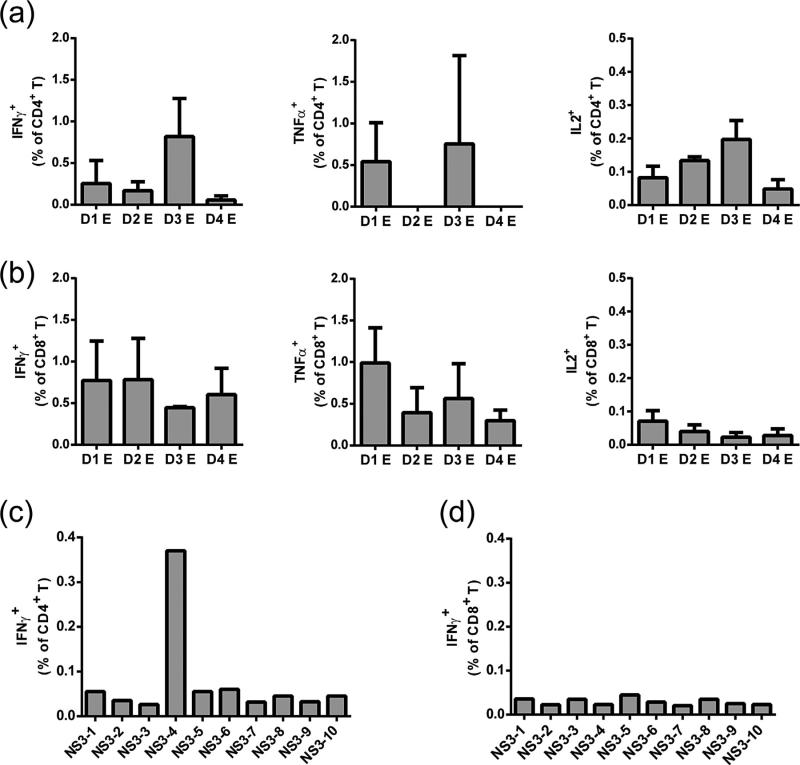
In addition to measuring humoral immune responses we assessed the capacity of TDV to elicit cellular immunity. In vitro restimulation of TDV immune splenocytes with pools of peptides (each pool consisted of the entire peptide array for a given E protein) led to the detection of CD4+ and CD8+ T cells predominantly producing IFN-γ and TNFα (Fig. 2a and b, respectively). Further, since immunized animals were challenged with DENV-4, we also tested whether the TDV-2 backbone elicits cross-reactive T cells recognizing the DENV-4 NS proteins. To this end, TDV-4 immune splenocytes were restimulated in vitro with pools of peptides encompassing the entire sequence of DENV-4 NS1, NS3 or NS5 protein. Although no responses were observed against the NS1 or NS5 proteins (data not shown), pool 4 (representing residues 174–240) from NS3 protein elicited a strong CD4+ IFN-γ producing T cell response (Fig. 2c) but not CD8+ T cells (Fig. 2d).
Fig. 2.
T cell responses to DENV E and NS proteins. (a and b) CD4+ and CD8+ T cell responses to E proteins of each DENV serotype elicited after a single subcutaneous immunization with TDV. Immune splenocytes collected 6 weeks post-primary immunization were restimulated in vitro with pools of peptides encompassing the entire sequence of DENV-1–4 E (each pool consisted of the entire peptide array for a given E protein). Cells were stained for surface markers CD4, CD8 and intracellular cytokines (IFNγ, IL-2 or TNF-α). Samples were analyzed by flow cytometry (FACS Calibur). The percentage of cytokine producing cells in medium treated groups was subtracted from each sample. Data are presented as mean ± SD (n = 3). T cells derived from control FTA group did not produce any significant cytokine response above the medium background (data not shown). Peptide arrays representing the entire sequence of E protein for each serotype and NS3 protein of DENV-4 were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH (http://www.beiresources.org/). For E peptide arrays: Dengue virus type 1, Singapore/S275/1990, NR-4551; Dengue virus type 2, New Guinea C (NGC), NR5-7; Dengue virus type 3, Sleman/1978, NR-511; Dengue type 4, Dominica/814669/1981, NR-512. (c and d) CD4+ and CD8+ T cell responses to the NS3 protein of DENV-4. Immune splenocytes collected seven weeks post-primary immunization with TDV-4 were restimulated in vitro with pools of peptides (10 peptides/pool) encompassing the entire sequence of DENV-4 NS3 protein. Cells were stained for surface markers CD4, CD8 and intracellular cytokines (IFNγ). Samples were analyzed by flow cytometry (FACS Calibur). The percentage of cytokine producing cells in medium treated groups was subtracted from each sample. Data are presented as mean of two individual mice.
3.2. Neutralizing antibody responses against different DENV-4 isolates
Given the fact that the monovalent DENV-4 vaccine induces the weakest neutralizing antibody responses of the four valences in TDV, we further characterized the antibody responses elicited by TDV or TDV-4 vaccines for cross-neutralizing activity against various DENV-4 isolates collected from different geographical locations. Neutralization titers were determined in pooled sera collected on day 56 just prior to DENV-4 challenge. As shown in Table 1, anti-DENV-4 antibody titers elicited by TDV or TDV-4 vaccines exhibited broad neutralizing activity against all DENV-4 isolates tested.
Table 1.
Cross-neutralization of diverse DENV-4 isolates by antibodies induced against TDV and TDV-4 vaccines. Pooled serum samples collected on day 56 post primary immunization from groups of AG129 mice (n = 15) with TDV or TDV-4 were tested for neutralizing activity against each DENV-4 isolate. Control animals (n = 15) were immunized with FTA (virus diluents).
| DENV-4 isolates | Neutralizing antibody titers |
||||||
|---|---|---|---|---|---|---|---|
| 1036 | 1* | 2* | 3* | 4* | 5* | 6* | |
| TDV immune serum | 320 | 320 | 80 | 80 | 160 | 160 | 320 |
| TDV-4 immune serum | 160 | 320 | 160 | 80 | 160 | 160 | 160 |
| FTA control serum | <20 | 40 | <20 | <20 | 20 | <20 | <20 |
1*: CL/DB039/2006; 2*: EC/DB041/1999; 3*: MC/DB043/1995; 4*: MX/DB042/2006; 5*: SC/DB045/1994; 6*: TH/DB060/2006.
3.3. Protective efficacy against DENV-4 challenge
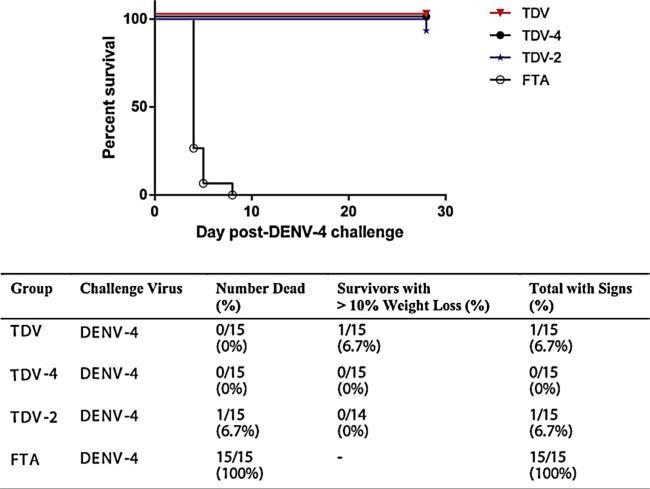
Following challenge with DENV-4 703 strain vaccinated animals were monitored for morbidity and mortality and bled to measure DENV-4 viral RNA. Fig. 3 shows the survival curve at day 28 post-virus challenge and the summarized outcome. Briefly, 100% of the FTA control treated mice succumbed to infection by day 8 post-virus challenge. In contrast, there was no mortality among animals vaccinated with TDV or TDV-4 vaccines, and only a single animal immunized with the TDV-2 vaccine succumbed to infection on day 27 post-virus challenge. Furthermore, among the vaccinated animals only 1 in the tetravalent vaccine group showed >10% weight loss (Fig. 3). Overall, all of the vaccines provided significant protection against mortality and clinical signs as compared with controls (p < 0.0001 each; Fisher's exact test).
Fig. 3.
Impact of immunization on survival following DENV-4 virus challenge. Groups of 15 AG129 mice were immunized with a single dose of TDV-4, TDV-2 or TDV vaccines. Control animals were immunized with FTA (virus diluents). On day 60 post-primary immunization animals were challenged with mouse adapted DENV-4 and morbidity and mortality was monitored over a period of four weeks.
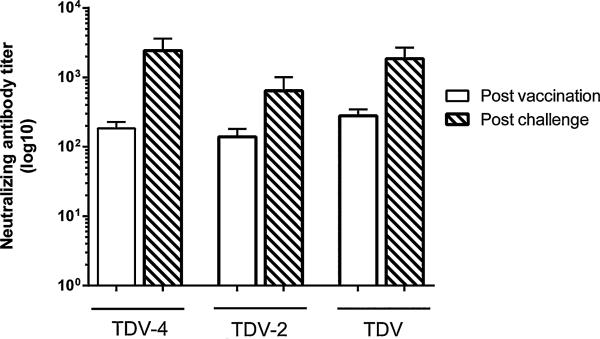
When the anti-DENV-4 neutralizing titers were measured four weeks post-DENV-4 challenge they were found to increase compared with those seen prior to virus challenge (Fig. 4). In particular, anti-DENV-4 titers in groups of animals vaccinated with TDV or monovalent TDV-4 or TDV-2 vaccines increased by 6.6, 13, and 4.6-fold, respectively (Fig. 4).
Fig. 4.
Anti-DENV-4 neutralizing antibody responses at day 56 post-primary vaccination or day 28 post-DENV-4 virus challenge. Groups of 15 AG129 mice were immunized with a single dose of TDV-4, TDV-2 or TDV vaccines. Control animals were immunized with FTA (virus diluents). On day 60 post-primary immunization animals were challenged with mouse adapted DENV-4. Individual sera collected post-vaccination or post-challenge (from all surviving animals) were tested for neutralizing activity against DENV-4. Data represent the mean neutralizing titer from each animal in each group ± SE.
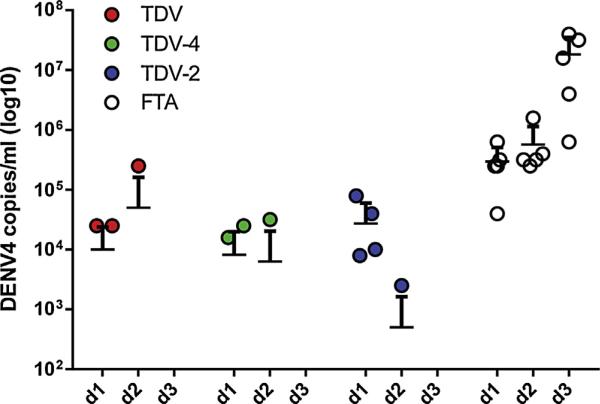
Viral RNA in the serum of challenged mice was quantified by qRT-PCR on days 1, 2 and 3 post-challenge. As shown in Fig. 5, virus was detected in all of the FTA control animals in this study with a high titer viremia ranging from 5 log10 genomes/ml on day 1 increasing to7 log10 genomes/ml on day 3 post-challenge. In comparison, for the TDV formulation viremia was detected in only 3/15 animals in total (p < 0.001) with 2/5 being viremic on day 1, 1/5 mice on day 2, and 0/5 on day 3 post-challenge. A similar picture was seen for animals immunized with TDV-4 with again a total of 3/15 animals having detectable viremia (p < 0.001 vs FTA controls). Again 2/5 animals had detectable viremia on day 1, 1/5 on day 2, and 0/5 on day 3 post-challenge. Immunization with TDV-2 also significantly reduced the number of animals with detectable viremia (p < 0.001 vs FTA control) although the reduction was not as great as that seen with the other two vaccine formulations containing a DENVax-4 component. Specifically, 4/5 animals were viremic on day 1 and 1/5 mice on day 2, and 0/5 on day 3 post-challenge.
Fig. 5.
Viremia data following DENV-4 challenge of animals immunized with TDV-2, TDV-4 or TDV vaccines. Levels of DENV-4 RNA in the serum post-challenge were quantified by qRT-PCR using a Fam labeled 5 prime nuclease assay designed to target the 3 prime non-coding region of dengue virus. Limit of detection is 2.5 log10 genomes/ml.
4. Discussion
In this study a candidate tetravalent dengue vaccine (TDV) and two of its monovalent constituents were evaluated for immunogenicity and efficacy against challenge with DENV-4 in the AG129 mouse model. Although this mouse model is IFN αβγ receptor-deficient it exhibits a normal adaptive immune response to DENVs when compared with the wild-type mice, and has been extensively used for immunogenicity and efficacy studies against DENV-1 and DENV-2 challenge [20,22,25]. However, this is the first use of a lethal DENV-4 challenge mouse model to evaluate candidate vaccines. The findings presented in this study demonstrate that the monovalent TDV-4 and TDV-2 vaccine formulations are immunogenic eliciting homotypic and heterotypic anti-DENV-4 neutralizing antibody responses, respectively, after a single immunization. This is consistent with our previously published observations on the immunogenicity of each monovalent vaccine [20]. In addition, antibodies to these monovalent vaccine formulations recognized the DENV-2 NS1 protein and cross-reacted with the NS1 protein of DENV-4. The NS1 is an important target protein of the humoral immune response to DENVs and anti-NS1 antibodies are cross-reactive due to the close sequence homology between the NS1 proteins of all four serotypes [7–11]. Moreover, TDV was immunogenic inducing tetravalent neutralizing antibody responses. Neutralizing antibodies elicited by TDV-4 or TDV vaccines exhibited broad cross-neutralizing activity against a number of recent DENV-4 isolates from different geographical locations, from the same genotype 2 or against a genotype 3 strain suggesting potential effectiveness in protecting against contemporary DENV-4 strains circulating in endemic areas.
Recent studies have acknowledged the role that T cells play in protection against dengue. In particular, T cells have been shown to contribute toward faster clearance of the virus in the AG129 mouse model ([25,26], Das et al., in preparation), and more recently the association of T cells with protection from dengue virus disease has been highlighted [12]. Therefore, in our studies we tested the capacity of our vaccine to elicit cellular responses. Indeed, following immunization with TDV we observed CD4+ and CD8+ T cells targeting the E protein of each serotype and producing IL-2 and IFN-γ, respectively. In addition, we have observed that the common TDV-2 backbone elicited CD4+ and CD8+ T responses to the homologous NS1, NS3 and NS5 proteins (Das et al.; in preparation) and cross-reactive CD4+ IFN-γ producing T cells upon restimulation with a pool of NS3 peptides from DENV-4. This pool encompasses amino acids that falls within a highly conserved (78%) region of the NS3 protein for all four DENV serotypes containing motifs and charged residues that are essential for helicase activity and virus replication [27,28]. Therefore, one would predict that responses to conserved epitopes would be seen in humans and should be a highly desirable feature of vaccines for broad coverage.
When mice were challenged with DENV-4, all vaccinated animals survived except for 1 of 15 TDV-2 immunized mice. This is consistent with our previous observations demonstrating the short-term protective efficacy of each of the monovalent and TDV vaccines against challenge with mouse adapted DENV-1 and DENV-2 viruses [20]. It is unfortunate that this model cannot be used for long-term immunogenicity and protective efficacy studies since mice become more resistant to challenge virus as they age (data not shown). All protected animals developed an anamnestic neutralizing (Fig. 4), and anti-DENV-4 NS1 (Fig. 1d) antibody response suggesting that some virus replication has occurred sufficient to stimulate memory B cells for rapid activation and differentiation into antibody-secreting plasmoblasts. Indeed, following DENV-4 challenge viral RNA was detectable in the serum of some of the vaccinated animals on day 1 and 2. However, by day 3 no viral RNA was detectable, with levels comparable with control animals. Therefore, it can be argued that under our experimental conditions the TDV vaccine formulations did not prevent infection (sterile immunity) but only disease. Despite the attractive attributes of the AG129 mouse model for vaccine testing [20,22,25] these mice are immunodeficient and permit the rapid replication of DENVs. Given the fact that the virus inoculum used for challenge does not reflect the dose that humans normally get by a mosquito bite we cannot extrapolate these findings to human vaccination. Although the scope of this study was not to address the mechanism(s) involved in protection, neutralizing antibodies are considered to be the main mediators, and recently the role of T cells has been highlighted as being important both in preclinical and clinical studies ([12,25,26], Das et al., in preparation). In addition, anti-NS1 antibodies might have contributed to protection as they have been shown to be efficacious against infection by several Flaviviruses including DENV [7,9,10,29–31]. In future studies we will be addressing the role of anti-TDV-2 NS1 antibodies in protection against homologous or heterologous DENVs by conducting passive transfer studies in AG129 mice. In conclusion, we have shown that our TDV vaccine formulations are immunogenic and confer protection against DENV-4 challenge.
Acknowledgements
This work was supported in part by NIAID contract N01 AI 30065 to A.D.T.B. and N.B.
Footnotes
Conflicts of interest: J.F., S.C.D., H.C., C.D.P., D.T.S., and J.E.O. are affiliated with Takeda Vaccine, Inc.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–81. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29:7221–8. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis. 2011;5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–83. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlesinger JJ, Brandriss MW, Walsh EE. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1987;68:853–7. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- 8.Amorim JH, Diniz MO, Cariri FA, Rodrigues JF, Bizerra RS, Gonçalves AJ, et al. Protective immunity to DENV2 after immunization with a recombinant NS1 protein using a genetically detoxified heat-labile toxin as an adjuvant. Vaccine. 2012;30:837–45. doi: 10.1016/j.vaccine.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Henchal EA, Henchal LS, Schlesinger JJ. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J Gen Virol. 1988;69:2101–7. doi: 10.1099/0022-1317-69-8-2101. [DOI] [PubMed] [Google Scholar]
- 10.Costa SM, Azevedo AS, Paes MV, Sarges FS, Freire MS, Alves AM. DNA vaccines against dengue virus based on the ns1 gene: the influence of different signal sequences on the protein expression and its correlation to the immune response elicited in mice. Virology. 2007;358:413–23. doi: 10.1016/j.virol.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 11.Wan SW, Lu YT, Huang CH, Lin CF, Anderson R, Liu HS, et al. Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS ONE. 2014;9:e92495. doi: 10.1371/journal.pone.0092495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci USA. 2013;110:E2046–53. doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halstead SB. Neutralization and antibody enhancement of dengue viruses. Adv Virus Res. 2003;60:421–67. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–28. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 15.Butrapet S, Huang CY, Pierro DJ, Bhamarapravati N, Gubler DJ, Kinney RM. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. J Virol. 2000;74:3011–9. doi: 10.1128/jvi.74.7.3011-3019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CY-H, Butrapet S, Pierro DJ, Chang G-JJ, Hunt AR, Bhamarapravati N, et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000;74:3020–8. doi: 10.1128/jvi.74.7.3020-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CY-H, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77:11436–47. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinney RM, Butrapet S, Chang GJ, Tsuchiya KR, Roehrig JT, Bhamarapravati N, et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–8. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 19.Osorio JE, Huang CY, Kinney RM, Stinchcomb DT. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine. 2011;29:7251–60. doi: 10.1016/j.vaccine.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brewoo JN, Kinney RM, Powell TD, Arguello JJ, Silengo SJ, Partidos CD, et al. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine. 2012;30:1513–20. doi: 10.1016/j.vaccine.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osorio JE, Brewoo JN, Silengo SJ, Arguello J, Moldovan IR, Tary-Lehmann M, et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in Cynomolgus macaques. Am J Trop Med Hyg. 2011;84:978–87. doi: 10.4269/ajtmh.2011.10-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–6. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi M, Hu F, Joyce M, Saxena V, Welsch C, Chavez D, et al. Evolution of a cell culture-derived genotype 1a hepatitis C virus (H77S.2) during persistent infection with chronic hepatitis in a chimpanzee. J Virol. 2014;88:3678–94. doi: 10.1128/JVI.03540-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houng HS, Chung-Ming Chen R, Vaughn DW, Kanesa-thasan N. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1-4 using conserved and serotype-specific 3′ noncoding sequences. J Virol Methods. 2001;95:19–32. doi: 10.1016/s0166-0934(01)00280-4. [DOI] [PubMed] [Google Scholar]
- 25.Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE. Shresta. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog. 2013;9:e1003723. doi: 10.1371/journal.ppat.1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–73. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matusan AE, Kelley PG, Pryor MJ, Whisstock JC, Davidson AD, Wright PJ. Muta-genesis of the dengue virus type 2 NS3 proteinase and the production of growth-restricted virus. Gen Virol. 2001;82:1647–56. doi: 10.1099/0022-1317-82-7-1647. [DOI] [PubMed] [Google Scholar]
- 28.Matusan AE, Pryor MJ, Davidson AD, Wright PJ. Mutagenesis of the dengue virus type 2 NS3 protein within and outside helicase motifs: effects on enzyme activity and virus replication. J Virol. 2001;75:9633–43. doi: 10.1128/JVI.75.20.9633-9643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin CW, Liu KT, Huang HD, Chen WJ. Protective immunity of E. coli-synthesized NS1 protein of Japanese encephalitis virus. Biotechnol Lett. 2008;30:205–14. doi: 10.1007/s10529-007-9529-9. [DOI] [PubMed] [Google Scholar]
- 30.Chung KM, Nybakken GE, Thompson BS, Engle MJ, Marri A, Fremont DH, et al. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol. 2006;80:1340–51. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung KM, Thompson BS, Fremont DH, Diamond MS. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile Virus-infected cells. J Virol. 2007;81(17):9551–5. doi: 10.1128/JVI.00879-07. [DOI] [PMC free article] [PubMed] [Google Scholar]