Abstract
INTRODUCTION
The Prouts Neck Meetings on Prostate Cancer began in 1985 through the efforts of the Organ Systems Branch of the National Cancer Institute to stimulate new research and focused around specific questions in prostate tumorigenesis and therapy.
METHODS
These meetings were think tanks, composed of around 75 individuals, and divided equally between young investigators and senior investigators. Over the years, many new concepts related to prostate cancer resulted from these meetings and the prostate cancer community has sorely missed them since the last one in 2007.
RESULTS
We report here the first of a new series of meetings. The 2013 meeting focused on defining how the field of treatment for metastatic prostate cancer needs to evolve to impact survival and was entitled: “Beyond AR: New Approaches to Treating Metastatic Prostate Cancer.” As castrate resistant prostate cancers escape second generation anti-androgen agents, three phenotypes/genotypes of CRPC appear to be increasing in prevalence and remain resistant to treatment: NeuroEndocrine Prostate Cancer, Persistent AR—Dependent Prostate Cancer, and Androgen Receptor Pathway Independent Prostate Cancer.
DISCUSSION
It is clear that new treatment paradigms need to be developed for this diverse group of diseases. The Prouts Neck 2013 Meeting on Prostate Cancer helped to frame the current state of the field and jumpstart ideas for new avenues of treatment. Prostate 74:314–320, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: tumor microenvironment, metastases, diagnostics, therapeutics, treatment resistance
INTRODUCTION
After a barren two decades when the only new drug approved for the treatment of castration resistant prostate cancer was docetaxel, the last 3 years has seen a bounty of new agents to help extend the life of men with this lethal disease (see Table1) 1–10. Prostate cancer cell addiction to the androgen receptor (AR) forms the basis both for initial androgen deprivation therapy as well as the new second-generation androgen ablative agents (abiraterone and enzalutamide). Unfortunately, prostate cancers escape these second generation agents and castration resistant prostate cancer (CRPC) remains an incurable disease. Three phenotypes/genotypes of CRPC after treatment with second-generation agents appear to be increasing in prevalence and remain resistant to treatment: NeuroEndocrine Prostate Cancer (NEPC), Persistent AR—Dependent Prostate Cancer (PADPC), and Androgen Receptor Pathway Independent Prostate Cancer (APIPC) 11–14. It is clear that new treatment paradigms need to be developed for this diverse group of diseases.
I.
Approved Agents for the Treatment of Castrate Resistant Prostate Cancer
| Agent | Target | Year approved | Reference |
| Estramustine | Estrogen mimetic | 1981 | 1 |
| Mitoxantrone | Type II topoisomerase | 1996 | 2 |
| Zoledronic acid | Osteoclast inhibition (adjunctive) | 2002 | 3 |
| Docetaxel | Microtubules | 2004 | 4 |
| Sipuleucel T | Immunomodulation | 2010 | 5 |
| Cabazitaxel | Microtubules | 2010 | 6 |
| Denosumab | RANK ligand (adjunctive) | 2010 | 7 |
| Abiraterone | Androgen synthesis | 2011 | 8 |
| Enzalutamide | Androgen receptor | 2012 | 9 |
| Radium-223 | Calcium mimetic | 2013 | 10 |
The Prouts Neck Meetings on Prostate Cancer began in 1985 through the efforts of the Organ Systems Branch of the National Cancer Institute to stimulate new research and focused around specific questions in prostate tumorigenesis and therapy 15,16. These meetings were unique on many fronts. First, the meetings were relatively small think tanks, composed of around 75 individuals, and divided equally between young investigators and senior investigators. The meeting was organized with short presentations and lengthy discussion times. Over the years, many new concepts related to prostate cancer have resulted from these meetings and the prostate cancer community has sorely missed them since the last one in 2007. Through the support of the Prostate Cancer Foundation, it was decided to re-initiate the Prouts Neck meetings to drive the prostate cancer field forward. The first meeting focused on defining how the field of treatment for metastatic prostate cancer needs to evolve to impact survival and was entitled: “Beyond AR: New Approaches to Treating Metastatic Prostate Cancer”. The meeting focused on delineating some of the current “Big Questions” that need to be addressed by the prostate cancer community as submitted prior to the meeting by the participants.
What Ultimately Kills People With Metastatic CRPC?
Despite multiple autopsy studies as well as observations by many experienced clinicians the cause of death from metastatic castrate resistant prostate cancer remains poorly defined 17. Although reasons for death such as cancer cachexia and thrombotic events have been documented to account for many deaths, the underlying mediators of these syndromes has not been defined. It is believed that these and other cancer related syndromes are the result of cytokine over production 17–20. Cancer, as it evolves in the host patient over time, acts as multiple endocrine organs, producing local and systemic effects (See Fig. 1) 21–23. A key question is whether identifying and targeting this slurry of soluble factors could decrease the morbidity of cancer.
Fig I.
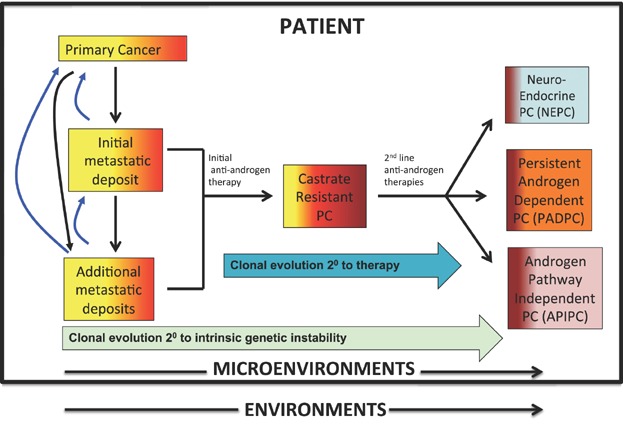
The systems pathobiology of prostate cancer. Prostate cancer develops and evolves as a complex adaptive system in a dynamic manner over time. Different cancer clones (tumor cell heterogeneity) evolve through inherent genomic and epigenomic instability as well as in response to therapeutic pressure. It appears that multiple host cells, including hematopoietic stem cells, mesenchymal stem cells, endothelial progenitors, cancer-associated fibroblasts, and inflammatory mononuclear cells (T-, B-, and monocytes) not only contribute to the pathogenesis of CRPC within the primary and metastatic microenvironments, but also traffic freely between tumor sites 16,17. Three phenotypes/genotypes of CRPC after treatment with second-generation agents appear to be increasing in prevalence and remain resistant to treatment: NeuroEndocrine Prostate Cancer (NEPC), Persistent AR—Dependent Prostate Cancer (PADPC), and Androgen Receptor Pathway Independent Prostate Cancer (APIPC) (9,9a). It is clear that new treatment paradigms, taking into account cancer cell genetic and epigenetic pathways, contributing factors within the microenvironment, and the macroenvironment of the host/patient need to be developed for this diverse group of diseases.
How Do We Apply Principles of Precision Oncology to the Treatment of CRPC?
Prostate cancer, within a patient, evolves over time as a result of intrinsic genetic instability and as a result of therapeutic pressure (Fig. 1) 16,24–28. While NEPC can arise as an early phenotype independent of castration therapy, all three prostate cancer phenotypes, NEPC, PADPC, and APIPC appear to be increasing in prevalence after treatment with first and second line anti-androgens 16. Identifying these pathways, as well as others, will be dependent on “personalized medicine” or “precision oncology” approaches. Tumor sequencing and other characterization assays will be required to identify the essential combinations of pathways that drive castration resistant prostate cancer. Identifying driver versus passenger mutations will be critical. Serial biopsies of patients (through sequential tissue biopsies or “liquid” circulating tumor cell characterization) will be required to identify the best sequence and/or combined treatment approaches for individual patients.
It is clear, however, that personalized oncology approaches for patients with CRPC are hampered by intra- and inter-tumoral heterogeneity 28–31. Clinical experience shows that within individual patients, some lesions can respond to systemic therapies while others stabilize or progress. Clinically validated biomarkers need to be developed that evaluate this heterogeneity to help prioritize actionable targets for individual patients. Similarly, Can heterogeneity be monitored in real time to more quickly identify emerging cancer clones responsible for therapeutic resistance? It remains unclear if this is possible with currently available technology.
What Is the Relevance of AR Splice Variants?
Androgen Receptor (AR) splice variants have now been identified and are present in many patients, increasing in frequency after second line anti-androgen therapy 32–36. The importance of these variants, which exhibit loss of the ligand-binding domain, in disease progression, remains undefined. It is likely that these variants contribute to the pathogenesis of PADPC. New agents that target the N-terminal domain of the AR are being developed but their clinical efficacy remains unknown.
What Is the Role of the Prostate Cancer Stem/Progenitor Cell in Tumor Progression and Resistance?
Identifying a prostate cancer stem cell in mice and men remains elusive 37–39. Several studies have pointed to a basal cell origin for mouse and human prostate cancer but others, at least in the mouse, have demonstrated that both the basal and luminal layers can initiate prostate cancer 38. To date, the most common markers in human to delineate progenitor cells have been a phenotype that includes CD44+/CD133+/ABCG2+/CD24−. Cancer stem cells appear to be rare in primary cancers, in xenografts, and in tissue culture but their number and their role in disease progression remain a mystery 37–40. The continued survival and self-renewal of stem cells/progenitors remains a potential explanation for disease dissemination as well as therapeutic resistance to castration and chemotherapies. The field continues to isolate, characterize, and study how these cells may be generated intrinsically with the cancer cell population as well as how the changing microenvironments of primary and metastatic cancer may influence their behavior.
How Relevant Is The Tumor Microenvironment in Modulating Cancer Growth and Resistance?
That the tumor microenvironment contributes to tumorigenesis and therapeutic resistance is now widely accepted 41–44. Reactive stroma, including fibroblasts, endothelial cells, osteoblasts, and osteoclasts (in bone), and mesenchymal stem cells all contribute to prostate cancer development and tumorigenesis 22,41–45. Several studies have demonstrated that the stroma can protect cancer cells from radiation and chemotherapy. Since host cells do not exhibit high levels of genetic instability, targeting these facilitative cells present an attractive option for adjunctive therapy 44. Targeting osteoclast function with bisphosphonates is a prototypical example of this in prostate cancer metastasis to bone 42–44. Development of further therapies that target other tumor—host interactions are underway and need to be clinically tested 22,41–44.
What Is the Evolving Role of Immunotherapy in CRPC?
While in general, the enthusiasm for immunotherapy for prostate cancer utilizing Sipuleucel-T is mixed at best, the recent case report of a patient with metastatic CRPC who achieved a complete and durable biochemical response after treatment with sipuleucel-T while on enzalutamide has been met with much interest 46. In addition, documented responses utilizing the CTLA-4 blocking antibody ipilimumab in prostate cancer as well as melanoma has caught the imagination of the field and suggest that immunotherapy for CRPC is coming of age. Trials need to be performed in combination with antigen presentation strategies such as radiotherapy and cryotherapy 47–49. Moving these therapies into combination studies with androgen ablation or chemotherapy are being tested and to explore the immunologic basis for such a response 47–50. Multiple newer agents, such as those targeting PD-1hold even more promise, however, how these agents provide clinical benefit are not completely understood and biomarkers to predict and monitor response are desperately needed.
What Is The Evolving Role Of Molecular Imaging in CRPC?
There is wide consensus that the field requires better imaging modalities for metastatic disease, especially in the areas of detecting minimal disease and quantitative responses to therapy 51–53. Bone scans remain qualitative tools and many patients have disease beyond the limits of detection of CT and MRI 54. Several new agents are being developed but the path to approval is difficult and costly. The application of functional imaging to risk-adapted therapy, selection of optimal combination therapies, and prognosis in metastatic prostate cancer does not appear to be likely in the near future. In addition, linking functional imaging as a surrogate biomarker for specific genetic or signaling pathway aberrations is under development but is far from being available for wide use in animal prostate cancer models and in humans. The rational incorporation of novel PET radiotracer(s) and other functional imaging modalities to accelerate and improve therapeutic development is desperately needed.
Should We Classify Prostate Cancer Progression Based on Molecular Underpinnings of the Disease?
As our understanding of prostate cancer evolution during progression grows, the challenge is to effectively sequence and combine our growing armamentarium of therapeutic agents for maximal patient benefit- the right drugs, in the right combinations, given at the right time; especially by anticipating the need for therapy before it is clinically apparent; that is, to move beyond anatomically-based clinical decisions and prognostication to biologically (marker)-driven therapy prediction. Logothetis et al. suggest a molecular classification into four distinct phases of evolution of the disease, based on the underlying molecular mechanisms 55. Stage I, dihydrotestosterone (DHT)-dependent disease that responds to treatment with inhibitors such as dutasteride and finasteride. A subset of these cancers progress to the endocrine-driven stage, or stage II where the tumors are driven by androgens derived from the testes and the gonads, and respond to androgen ablation. Upon androgen deprivation therapy, cancers develop treatment resistance and progress to Stage III, the paracrine, microenvironment-dependent stage of the disease, where factors from the tumor microenvironment in addition to cellular changes from within the tumors drive disease progression. Tumors largely remain androgen signaling driven and respond to next-generation anti-androgens (e.g., abiraterone acetate) and AR inhibitors (e.g., enzalutamide). Cancers that stop responding to these therapies have usually exited the microenvironment-dependent phase and progressed to Stage IV, the Tumor Cell Autonomous phase, the precise mechanisms of development of which aren't clearly understood, however, are likely represented by NEPC, PADPC, and APIPC (Fig. 1).
What Are the Next Targets for CRPC?
As our knowledge of how CRPC evolves over time continues to grow, new therapeutic targets are being discovered and their value defined (Fig. 1) 56–68. Agents that target DNA repair, altered kinase pathways, and epigenetic pathways are all in development. Agents that target supporting / facilitating host cells of the tumor microenvironment are moving forward. A holistic and integrated approach of altered critical biologic pathways is serving as an impetus for developing new therapeutics and repurposing agents from other diseases 60. Three challenges that continue to stymie progress are the lack of good animal models to test new agents, good adaptive strategies that utilize the current agents, and the lack of our ability to rapidly test combinations of agents in preclinical models, and more importantly in patients 60–63 As our understanding of prostate cancer evolution during progression grows, applying the armamentarium of therapeutic agents in the right sequences in the right combinations at the right time is a major goal in prostate cancer treatment 55.
What Can We Crowdsource?
The presentations and questions catalyzed the desire for collaborative, transdisciplinary research efforts that will accelerate the search for a cure. One idea that was presented was to gather the unpublished cell line and cell xenograft models that are being utilized within individual labs that could be utilized by others if they simply knew they existed. As a result of the meeting, these resources are being collected and will be publicized through a manuscript as well as the web.
CONCLUSIONS
The overall consensus was that the meeting, bringing young investigators as well investigators from disparate areas together, resulted in a successful and stimulating exchange of ideas and information. Future investigations on mechanisms of treatment resistance; the role of field cancerization and tumor microenvironment; and determining heterogeneity and its impact on precision medicine are important and warranted. The androgen receptor signaling axis remains a crucial driver of prostate cancer progression and treatment resistance, and newer ways of targeting this axis, as well as PADPC and APIPC, are needed. The vigorous open discussion of unclear and controversial topics helped give everyone a better sense and appreciation of the big unanswered questions and issues in prostate cancer. The 2014 conference will be on the topic: Beyond immune checkpoint blockade: “New Approaches to targeting Host—Tumor Interactions in Prostate Cancer.”
Acknowledgments
The authors thank all presenters for their valuable contributions (Supplementary Information, Agenda).
REFERENCES
- 1.Soloway MS, deKernion JB, Gibbons RP, Johnson DE, Loening SA, Pontes JE, Prout GR, Jr, Schmidt JD, Scott WW, Chu TM, Gaeta JF, Slack NH, Murphy GP. Comparison of estramustine phosphate and vincristine alone or in combination for patients with advanced, hormone refractory, previously irradiated carcinoma of the prostate. J Urol. 1981;125(5):664–667. doi: 10.1016/s0022-5347(17)55156-3. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, Armitage GR, Wilson JJ, Venner PM, Coppin CM, Murphy KC. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 10.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall'Oglio M, Franzén L, Coleman R, Vogelzang NJ, O'Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 11.Nelson PS. Molecular states underlying androgen receptor activation: A framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012;30:644–646. doi: 10.1200/JCO.2011.39.1300. [DOI] [PubMed] [Google Scholar]
- 12.Lapuk AV, Wu C, Wyatt AW, McPherson A, McConeghy BJ, Brahmbhatt S, Mo F, Zoubeidi A, Anderson S, Bell RH, Haegert A, Shukin R, Wang Y, Fazli L, Hurtado-Coll A, Jones EC, Hach F, Hormozdiari F, Hajirasouliha I, Boutros PC, Bristow RG, Zhao Y, Marra MA, Fanjul A, Maher CA, Chinnaiyan AM, Rubin MA, Beltran H, Sahinalp SC, Gleave ME, Volik SV, Collins CC. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J Pathol. 2012;227(3):286–297. doi: 10.1002/path.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, Blattner M, Lonard DM, Demichelis F, Coarfa C, Rubin MA, Zhou P, O'Malley BW, Mitsiades N. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci USA. 2013;110(17):6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues DN, Butler LM, Estelles DL, de Bono JS. Molecular pathology and prostate cancer therapeutics: From biology to bedside. J Pathol. 2013 doi: 10.1002/path.4272. DOI: 10.1002/path.4272. [DOI] [PubMed] [Google Scholar]
- 15.Current concepts and approaches to the study of prostate cancer. Proceedings of a meeting. Prouts Neck, Maine, October 18–20, 1985. Prog Clin Biol Res. 1987;239:1–820. [PubMed] [Google Scholar]
- 16.Keller ET, Rowley DR, Tomlins SA, Drake CG, Kantoff PW, Pienta KJ, Montie JE, Carter HB, Hruszkewicz AM, Gomez J, Mohla S, Getzenberg RH. Eleventh Prouts Neck Meeting on Prostate Cancer: Emerging strategies in prostate cancer therapy. Cancer Res. 2007;67(20):9613–9615. doi: 10.1158/0008-5472.CAN-07-1529. [DOI] [PubMed] [Google Scholar]
- 17.Loberg RD, Bradley DA, Tomlins SA, Chinnaiyan AM, Pienta KJ. The lethal phenotype of cancer: The molecular basis of death due to malignancy. CA Cancer J Clin. 2007;57(4):225–241. doi: 10.3322/canjclin.57.4.225. [DOI] [PubMed] [Google Scholar]
- 18.Chou E, Simons JW. The molecular biology of prostate cancer morbidity and mortality: Accelerated death from ejaculate poisoning. Urol Oncol. 1997;3(3):79–84. doi: 10.1016/s1078-1439(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 19.Twillie DA, Eisenberger MA, Carducci MA, Hseih WS, Kim WY, Simons JW. Interleukin-6: A candidate mediator of human prostate cancer morbidity. Urology. 1995;45(3):542–549. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 20.Kanat O, Cubukcu E, Avci N, Budak F, Ercan I, Canhoroz M, Olmez F. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori. 2013;99(2):229–233. doi: 10.1177/030089161309900218. [DOI] [PubMed] [Google Scholar]
- 21.Pienta KJ, Robertson B, Coffey D, Taichman RS. The Cancer Diaspora: Metastasis beyond the seed and soil hypothesis. Clin Cancer Res. 2013;19(21):5849–5855. doi: 10.1158/1078-0432.CCR-13-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camacho DF, Pienta KJ. Disrupting the networks of cancer. Clin Cancer Res. 2012;18(10):2801–2808. doi: 10.1158/1078-0432.CCR-12-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eferl R. CCL2 at the crossroad of cancer metastasis. JAKSTAT. 2013;2(2):e23816. doi: 10.4161/jkst.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letouzé E, Allory Y, Bollet MA, Radvanyi F, Guyon F. Analysis of the copy number profiles of several tumor samples from the same patient reveals the successive steps in tumorigenesis. Genome Biol. 2010;11(7):R76. doi: 10.1186/gb-2010-11-7-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aryee MJ, Liu W, Engelmann JC, Nuhn P, Gurel M, Haffner MC, Esopi D, Irizarry RA, Getzenberg RH, Nelson WG, Luo J, Xu J, Isaacs WB, Bova GS, Yegnasubramanian S. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci Transl Med. 2013;5(169):169ra10. doi: 10.1126/scitranslmed.3005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson WG, Yegnasubramanian S. Resistance emerges to second-generation antiandrogens in prostate cancer. Cancer Discov. 2013;3(9):971–974. doi: 10.1158/2159-8290.CD-13-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roychowdhury S, Chinnaiyan AM. Advancing precision medicine for prostate cancer through genomics. J Clin Oncol. 2013;31(15):1866–1873. doi: 10.1200/JCO.2012.45.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hieronymus H, Sawyers CL. Traversing the genomic landscape of prostate cancer from diagnosis to death. Nat Genet. 2012;44(6):613–614. doi: 10.1038/ng.2301. [DOI] [PubMed] [Google Scholar]
- 29.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, Mehra R, Prensner JR, Palanisamy N, Ryslik GA, Vandin F, Raphael BJ, Kunju LP, Rhodes DR, Pienta KJ, Chinnaiyan AM, Tomlins SA. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, Park K, Kitabayashi N, MacDonald TY, Ghandi M, Van Allen E, Kryukov GV, Sboner A, Theurillat JP, Soong TD, Nickerson E, Auclair D, Tewari A, Beltran H, Onofrio RC, Boysen G, Guiducci C, Barbieri CE, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Ramos AH, Winckler W, Cipicchio M, Ardlie K, Kantoff PW, Berger MF, Gabriel SB, Golub TR, Meyerson M, Lander ES, Elemento O, Getz G, Demichelis F, Rubin MA, Garraway LA. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, Coleman I, Ng SB, Salipante SJ, Rieder MJ, Nickerson DA, Corey E, Lange PH, Morrissey C, Vessella RL, Nelson PS, Shendure J. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci USA. 2011;108(41):17087–17092. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez F, Vessella RL, Plymate SR. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE. 2011;6(11):e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2013:1–11. doi: 10.1038/onc.2013.284. DOI: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2013 doi: 10.1038/onc.2013.235. doi: 10.1038/onc.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsiades N. A road map to comprehensive androgen receptor axis targeting for castration-resistant prostate cancer. Cancer Res. 2013;73(15):4599–4605. doi: 10.1158/0008-5472.CAN-12-4414. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe B, Beresford M, Bowen R, Mitchard J, Chalmers AD. Searching for prostate cancer stem cells: Markers and methods. Stem Cell Rev. 2013;9(5):721–730. doi: 10.1007/s12015-013-9453-4. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein AS, Witte ON. Does the microenvironment influence the cell types of origin for prostate cancer. Genes Dev. 2013;27(14):1539–1544. doi: 10.1101/gad.222380.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S, Principessa L, Isaacs JT. Human prostate cancer initiating cells isolated directly from localized cancer do not form prostaspheres in primary culture. Prostate. 2012;72(13):1478–1489. doi: 10.1002/pros.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo C, Zhang B, Garraway IP. Isolation and characterization of human prostate stem/progenitor cells. Methods Mol Biol. 2012;879:315–326. doi: 10.1007/978-1-61779-815-3_18. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson SE, Furic L, Buchanan G, Larsson O, Pedersen J, Frydenberg M, Risbridger GP, Taylor RA. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate. 2013;73(16):1810–1823. doi: 10.1002/pros.22720. [DOI] [PubMed] [Google Scholar]
- 42.Brennen WN, Denmeade SR, Isaacs JT. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr Relat Cancer. 2013;20(5):R269–R290. doi: 10.1530/ERC-13-0151. DOI: 10.1530/ERC-13-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer. 2012;19(6):R187–R204. doi: 10.1530/ERC-12-0085. DOI: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Msaouel P, Nandikolla G, Pneumaticos SG, Koutsilieris M. Bone microenvironment-targeted manipulations for the treatment of osteoblastic metastasis in castration-resistant prostate cancer. Expert Opin Investig Drugs. 2013;22(11):1385–1400. doi: 10.1517/13543784.2013.824422. [DOI] [PubMed] [Google Scholar]
- 45.Graff JN, Drake CG, Beer TM. Complete biochemical (prostate-specific antigen) response to sipuleucel-T with enzalutamide in castration-resistant prostate cancer: A case report with implications for future research. Urology. 2013;81(2):381–383. doi: 10.1016/j.urology.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wada S, Jackson CM, Yoshimura K, Yen HR, Getnet D, Harris TJ, Goldberg MV, Bruno TC, Grosso JF, Durham N, Netto GJ, Pardoll DM, Drake CG. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89. doi: 10.1186/1479-5876-11-89. DOI: 10.1186/1479-5876-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waitz R, Solomon SB, Petre EN, Trumble AE, Fassò M, Norton L, Allison JP. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72(2):430–439. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonarakis ES, Drake CG. Combining immunological and androgen-directed approaches: An emerging concept in prostate cancer immunotherapy. Curr Opin Oncol. 2012;24(3):258–265. doi: 10.1097/CCO.0b013e32835205a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jadvar H. Molecular imaging of prostate cancer with PET. J Nucl Med. 2013;54(10):1685–1688. doi: 10.2967/jnumed.113.126094. [DOI] [PubMed] [Google Scholar]
- 51.Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ, Dannals RF, Sgouros G, Lodge M, Eisenberger MA, Rodriguez R, Carducci MA, Rojas C, Slusher BS, Kozikowski AP, Pomper MG. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53(12):1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris MJ, Autio KA, Basch EM, Danila DC, Larson S, Scher HI. Monitoring the clinical outcomes in advanced prostate cancer: What imaging modalities and other markers are reliable. Semin Oncol. 2013;40(3):375–392. doi: 10.1053/j.seminoncol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Thomas MA, Nagarajan R, Huda A, Margolis D, Sarma MK, Sheng K, Reiter RE, Raman SS. Multidimensional MR spectroscopic imaging of prostate cancer in vivo. NMR Biomed. 2013 doi: 10.1002/nbm.2991. doi: 10.1002/nbm.2991. [DOI] [PubMed] [Google Scholar]
- 54.Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, Patel S, Wang X, Liang H, Yu J, Palanisamy N, Siddiqui J, Yan W, Cao X, Mehra R, Sabolch A, Basrur V, Lonigro RJ, Yang J, Tomlins SA, Maher CA, Elenitoba-Johnson KS, Hussain M, Navone NM, Pienta KJ, Varambally S, Feng FY, Chinnaiyan AM. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19(5):664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas C, Lamoureux F, Crafter C, Davies BR, Beralidi E, Fazli L, Kim S, Thaper D, Gleave ME, Zoubeidi A. Synergistic targeting of PI3K/AKT-pathway and androgen-receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther. 2013;12(11):2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- 56.Beltran H, Rubin MA. New strategies in prostate cancer: Translating genomics into the clinic. Clin Cancer Res. 2013;19(3):517–523. doi: 10.1158/1078-0432.CCR-12-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J, Graeber TG, Witte ON. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci USA. 2012;109(5):1643–1648. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoenborn JR, Nelson P, Fang M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clin Cancer Res. 2013;19(15):4058–4066. doi: 10.1158/1078-0432.CCR-12-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuczynski EA, Sargent DJ, Grothey A, Kerbel RS. Drug rechallenge and treatment beyond progression-implications for drug resistance. Nat Rev Clin Oncol. 2013;10(10):571–587. doi: 10.1038/nrclinonc.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pritchard JR, Bruno PM, Gilbert LA, Capron KL, Lauffenburger DA, Hemann MT. Defining principles of combination drug mechanisms of action. Proc Natl Acad Sci USA. 2013;110(2):E170–E179. doi: 10.1073/pnas.1210419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin D, Gout PW, Wang Y. Lessons from in-vivo models of castration-resistant prostate cancer. Curr Opin Urol. 2013;23(3):214–219. doi: 10.1097/MOU.0b013e32835e9f07. [DOI] [PubMed] [Google Scholar]
- 62.Michiel Sedelaar JP, Dalrymple SS, Isaacs JT. Of mice and men—Warning: Intact versus castrated adult male mice as xenograft hosts are equivalent to hypogonadal versus abiraterone treated aging human males, respectively. Prostate. 2013;73(12):1316–1325. doi: 10.1002/pros.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Logothetis CJ, Gallick GE, Maity SN, Kim J, Aparicio A, Efstathiou E, Lin SH. Molecular classification of prostate cancer progression: Foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3(8):849–861. doi: 10.1158/2159-8290.CD-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallick GE, Corn PG, Zurita AJ, Lin SH. Small-molecule protein tyrosine kinase inhibitors for the treatment of metastatic prostate cancer. Future Med Chem. 2012;4(1):107–119. doi: 10.4155/fmc.11.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Comstock CE, Augello MA, Goodwin JF, de Leeuw R, Schiewer MJ, Ostrander WF, Jr, Burkhart RA, McClendon AK, McCue PA, Trabulsi EJ, Lallas CD, Gomella LG, Centenera MM, Brody JR, Butler LM, Tilley WD, Knudsen KE. Targeting cell cycle and hormone receptor pathways in cancer. Oncogene. 2013 doi: 10.1038/onc.2013.83. doi: 10.1038/onc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim AC, Attard G. Improved therapeutic targeting of the androgen receptor: Rational drug design improves survival in castration-resistant prostate cancer. Curr Drug Targets. 2013;14(4):408–419. doi: 10.2174/1389450111314040003. [DOI] [PubMed] [Google Scholar]
- 67.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu J, McEachern D, Sun H, Bai L, Peng Y, Qiu S, Miller R, Liao J, Yi H, Liu M, Bellail A, Hao C, Sun SY, Ting AT, Wang S. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, Smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol Cancer Ther. 2011;10(5):902–914. doi: 10.1158/1535-7163.MCT-10-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]


