Abstract
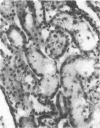
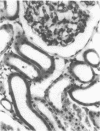
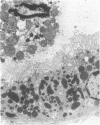
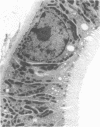
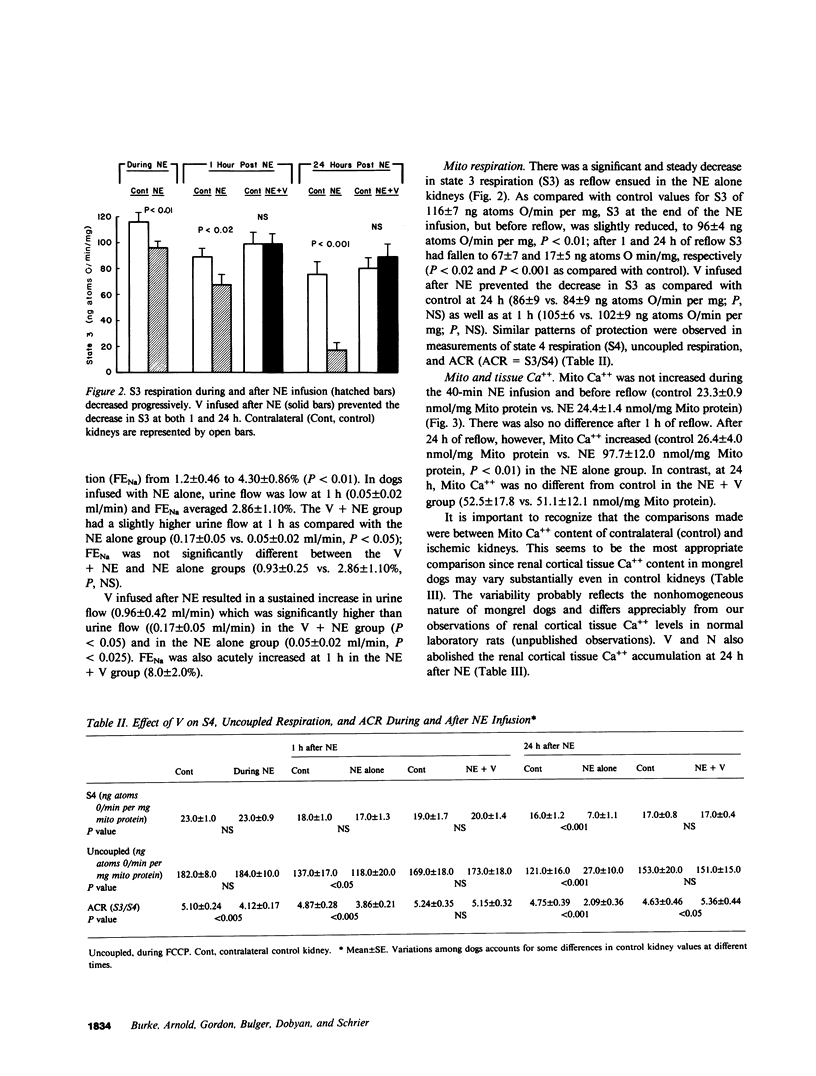
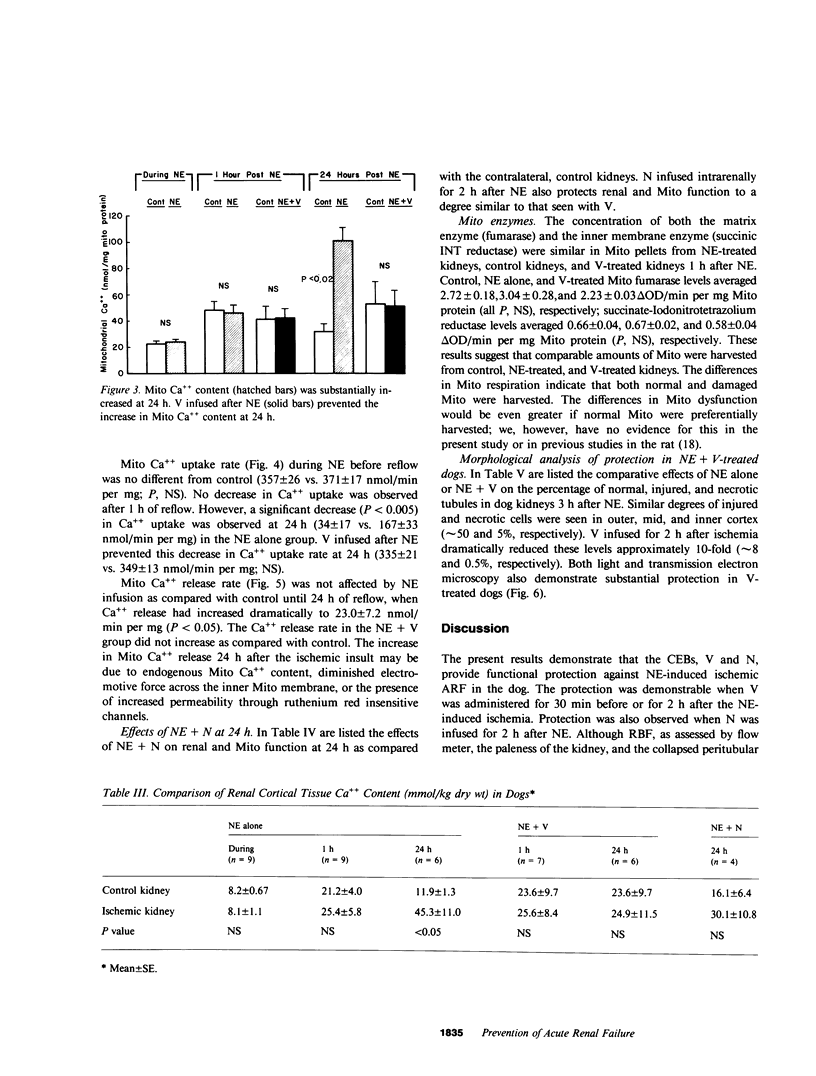
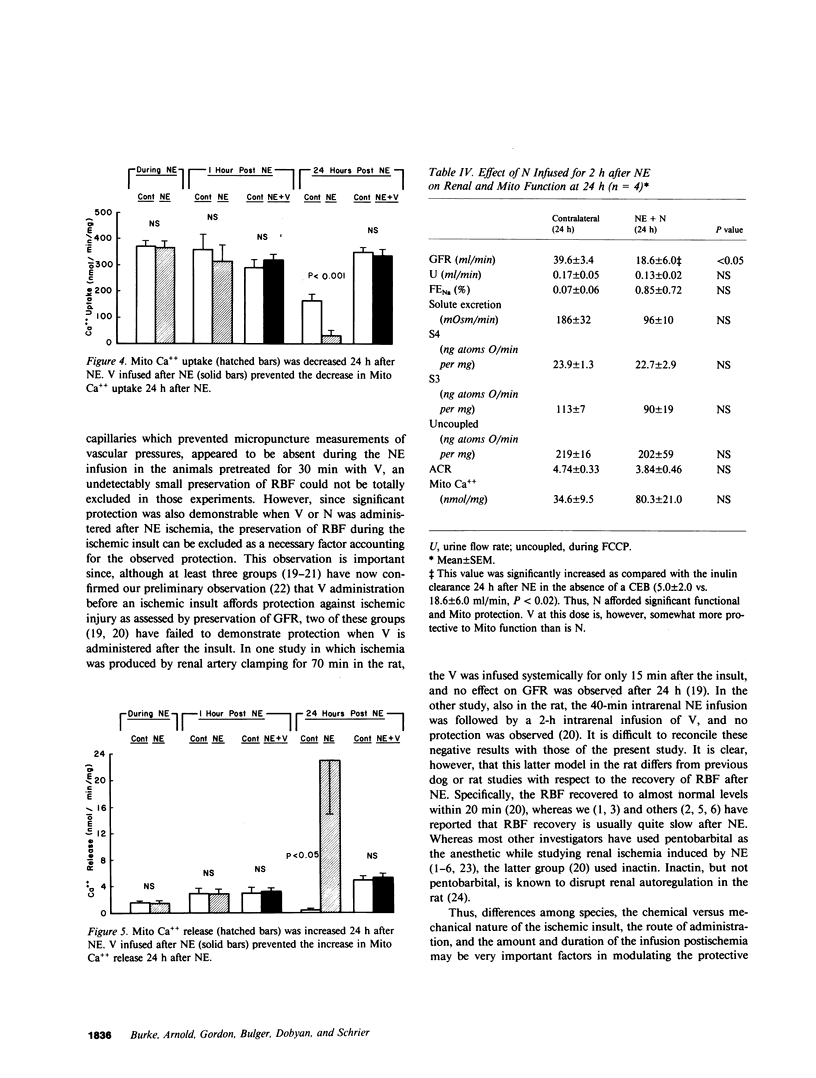
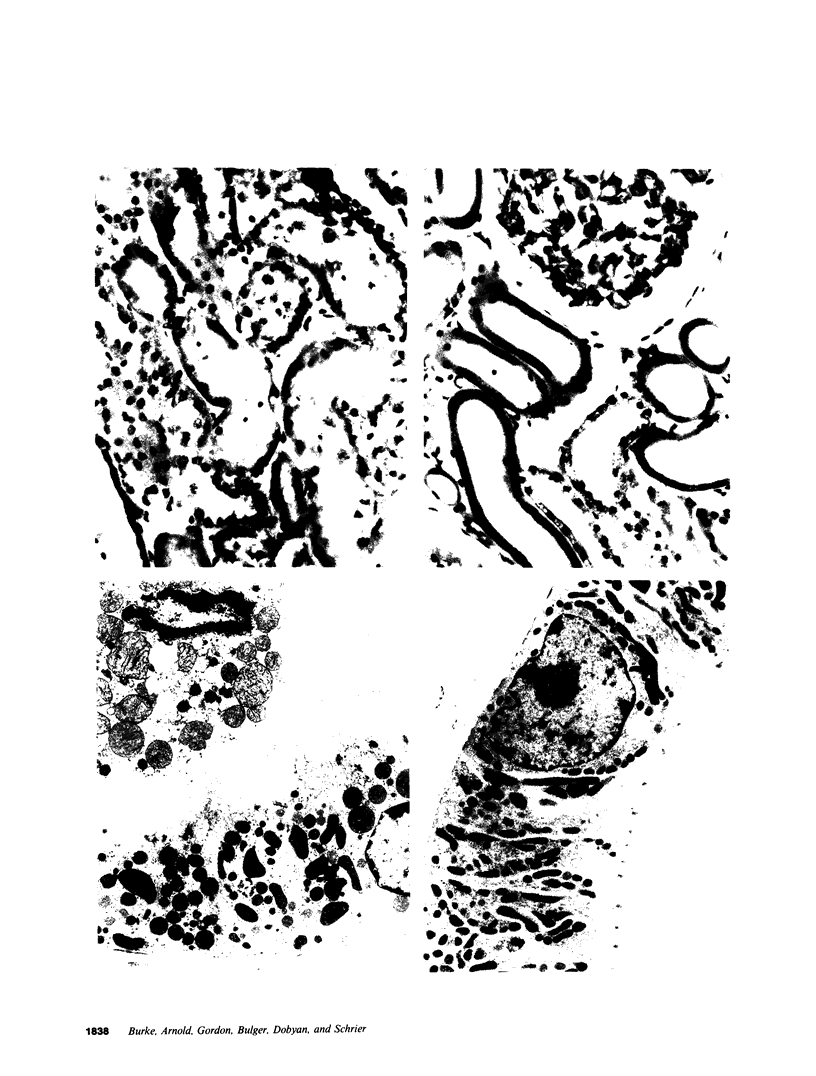
The present study examined whether a pre- or postischemic infusion of verapamil (V) or a postischemic infusion of nifedipine (N), drugs which block calcium (Ca++) influx across plasma membranes, provides protection against ischemic acute renal failure (ARF) in dogs. Renal hemodynamics and excretory function were examined 1 h (initiation phase) and 24 h (maintenance phase) after a 40-min intrarenal infusion of norepinephrine (NE). In each case, the uninfused contralateral kidney served as control. Four groups were studied: (a) dogs receiving NE alone; (b) dogs receiving an intrarenal infusion of V for 30 min before NE (V + NE); (c) dogs in which intrarenal V was infused for 2 h, beginning immediately after completion of NE infusion (NE + V); and (d) dogs in which intrarenal N was infused for 2 h, beginning immediately after completion of NE infusion (NE + N). Glomerular filtration rate (GFR) in the NE kidneys, as assessed by inulin clearance, at 1 and 24 h averaged 2.4 +/- 1.1 and 5.0 +/- 2.0 ml/min, respectively, as compared with control kidney GFRs of 28.0 +/- 3.5 and 43.8 +/- 5.0 ml/min, respectively (both at least P less than 0.01). In the V + NE group, GFR at 1 and 24 h averaged 15.0 +/- 5.5 and 31.0 +/- 4.5 ml/min, respectively, both at least P less than 0.05 as compared with values from NE kidneys. GFRs in the NE + V group averaged 15.0 +/- 2.4 and 16.3 +/- 3.6 ml/min at 1 and 24 h, both at least P less than 0.02 as compared with values from NE kidneys. GFR in the NE + N group averaged 18.6 +/- 6.0 ml/min at 24 h (P less than 0.05 as compared with GFRs in the NE kidneys). In addition, function of cortical mitochondria (Mito) was examined at the end of the 40-min NE infusion and after 1 and 24 h of reperfusion in the NE alone and NE + V groups. Mito respiration, assessed by acceptor control ratios, was reduced at each period in the NE alone kidneys. After 24 h, these Mito had accumulated Ca++ and exhibited reduced Ca++ uptake and increased Ca++ release rates. Mito from NE + V kidneys respired normally, did not accumulate Ca++, and exhibited no alterations in Ca++ uptake or release. Light and electron microscopy also demonstrated morphological protection of V against tubular necrosis and cell injury. Mito from the NE + N kidneys also respired normally and did not accumulate significant amounts of Ca++. The results of the present studies therefore demonstrated that chemically dissimilar calcium entry blockers exert substantial functional, cellular, and morphological protection against experimental ischemic ARF. These findings are compatible with the hypothesis that increased cytosolic Ca++ is critically important in the maintenance of renal vasoconstriction and the development of cellular necrosis with subsequent tubular obstruction in NE-induced ischemic ARF. V or N may provide protection against renal injury by retarding any increase in cytosolic Ca++ in renal vasculature and epithelium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977 May;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Arnold P. E., Schrier R. W. Prevention of ischemic acute renal failure with impermeant solutes. Am J Physiol. 1983 Jun;244(6):F646–F649. doi: 10.1152/ajprenal.1983.244.6.F646. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Cronin R. E., Duchin K. L., Peterson L. N., Schrier R. W. Ischemia and tubule obstruction during acute renal failure in dogs: mannitol in protection. Am J Physiol. 1980 Apr;238(4):F305–F314. doi: 10.1152/ajprenal.1980.238.4.F305. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Bristow M. R., Baim D. S., Schroeder J. S., Jaillon P., Brett P., Harrison D. C. The effects of diltiazem and reduced serum ionized calcium on ischemic ventricular fibrillation in the dog. Circ Res. 1982 Apr;50(4):518–526. doi: 10.1161/01.res.50.4.518. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Burke T. J. Effects of anesthetic agents on autoregulation of renal hemodynamics in the rat and dog. Am J Physiol. 1976 Mar;230(3):652–657. doi: 10.1152/ajplegacy.1976.230.3.652. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Robinette J. B., Guggenheim S. J. Effect of acetylcholine on the early phase of reversible norepinephrine-induced acute renal failure. Kidney Int. 1981 Mar;19(3):399–409. doi: 10.1038/ki.1981.32. [DOI] [PubMed] [Google Scholar]
- Cronin R. E., de Torrente A., Miller P. D., Bulger R. E., Burke T. J., Schrier R. W. Pathogenic mechanisms in early norepinephrine-induced acute renal failure: functional and histological correlates of protection. Kidney Int. 1978 Aug;14(2):115–125. doi: 10.1038/ki.1978.99. [DOI] [PubMed] [Google Scholar]
- De Torrente A., Miller P. D., Cronin R. E., Paulsin P. E., Erickson A. L., Schrier R. W. Effects of furosemide and acetylcholine in norepinephrine-induced acute renal failure. Am J Physiol. 1978 Aug;235(2):F131–F136. doi: 10.1152/ajprenal.1978.235.2.F131. [DOI] [PubMed] [Google Scholar]
- Farber J. L. The role of calcium in cell death. Life Sci. 1981 Sep 28;29(13):1289–1295. doi: 10.1016/0024-3205(81)90670-6. [DOI] [PubMed] [Google Scholar]
- Goldberg J. P., Schrier R. W. Effect of calcium membrane blockers on in vivo vasoconstrictor properties of norepinephrine, angiotensin II and vasopressin. Miner Electrolyte Metab. 1984;10(3):178–183. [PubMed] [Google Scholar]
- Goldfarb D., Iaina A., Serban I., Gavendo S., Kapuler S., Eliahou H. E. Beneficial effect of verapamil in ischemic acute renal failure in the rat. Proc Soc Exp Biol Med. 1983 Mar;172(3):389–392. doi: 10.3181/00379727-172-41576. [DOI] [PubMed] [Google Scholar]
- Griffith L. D., Bulger R. E., Trump B. F. The ultrastructure of the functioning kidney. Lab Invest. 1967 Feb;16(2):220–246. [PubMed] [Google Scholar]
- Humes H. D., Simmons C. F., Jr, Brenner B. M. Effect of verapamil on the hydroosmotic response to antidiuretic hormone in toad urinary bladder. Am J Physiol. 1980 Sep;239(3):F250–F257. doi: 10.1152/ajprenal.1980.239.3.F250. [DOI] [PubMed] [Google Scholar]
- KANAREK L., HILL R. L. THE PREPARATION AND CHARACTERIZATION OF FUMARASE FROM SWINE HEART MUSCLE. J Biol Chem. 1964 Dec;239:4202–4206. [PubMed] [Google Scholar]
- Kohda C., Gemba M. Effect of verapamil on the calcium and magnesium transports of rat kidney cortex mitochondria. Jpn J Pharmacol. 1979 Oct;29(5):745–751. doi: 10.1254/jjp.29.745. [DOI] [PubMed] [Google Scholar]
- Koomen J. M., Schevers J. A., Noordhoek J. Myocardial recovery from global ischemia and reperfusion: effects of pre- and/or post-ischemic perfusion with low-Ca2+. J Mol Cell Cardiol. 1983 Jun;15(6):383–392. doi: 10.1016/0022-2828(83)90322-x. [DOI] [PubMed] [Google Scholar]
- Malis C. D., Cheung J. Y., Leaf A., Bonventre J. V. Effects of verapamil in models of ischemic acute renal failure in the rat. Am J Physiol. 1983 Dec;245(6):F735–F742. doi: 10.1152/ajprenal.1983.245.6.F735. [DOI] [PubMed] [Google Scholar]
- Mauk R. H., Patak R. V., Fadem S. Z., Lifschitz M. D., Stein J. H. Effect of prostaglandin E administration in a nephrotoxic and a vasoconstrictor model of acute renal failure. Kidney Int. 1977 Aug;12(2):122–130. doi: 10.1038/ki.1977.89. [DOI] [PubMed] [Google Scholar]
- Mergner W. J., Smith M. W., Sahaphong S., Trump B. F. Studies on the pathogenesis of ischemic cell injury. VI. Accumulation of calcium by isolated mitochondria of ischemic rat kidney cortex. Virchows Arch B Cell Pathol. 1977 Nov 30;26(1):1–16. [PubMed] [Google Scholar]
- Nayler W. G. The role of calcium in the ischemic myocardium. Am J Pathol. 1981 Feb;102(2):262–270. [PMC free article] [PubMed] [Google Scholar]
- Okamatsu S., Lefer A. M. The protective effects of nifedipine in the isolated cat heart. J Surg Res. 1983 Jul;35(1):35–40. doi: 10.1016/0022-4804(83)90123-3. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patak R. V., Fadem S. Z., Lifschitz M. D., Stein J. H. Study of factors which modify the development of norepinephrine-induced acute renal failure in the dog. Kidney Int. 1979 Mar;15(3):227–237. doi: 10.1038/ki.1979.30. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Laiho K. U., Osornio A. R., Mergner W. J., Smith M. W. The role of calcium in cell injury. A review. Scan Electron Microsc. 1980;(Pt 2):437-62, 492. [PubMed] [Google Scholar]
- Wait R. B., White G., Davis J. H. Beneficial effects of verapamil on postischemic renal failure. Surgery. 1983 Aug;94(2):276–282. [PubMed] [Google Scholar]
- Williams R. H., Thomas C. E., Navar L. G., Evan A. P. Hemodynamic and single nephron function during the maintenance phase of ischemic acute renal failure in the dog. Kidney Int. 1981 Apr;19(4):503–515. doi: 10.1038/ki.1981.48. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Arnold P. E., Burke T. J., Schrier R. W. Mitochondrial calcium accumulation and respiration in ischemic acute renal failure in the rat. Kidney Int. 1984 Mar;25(3):519–526. doi: 10.1038/ki.1984.48. [DOI] [PubMed] [Google Scholar]