Abstract
Objective
Quantitative T1ρ MRI has been suggested as a promising tool to detect changes in cartilage composition that are characteristic of cartilage damage and degeneration. The objective of this study was to evaluate the capability of MR T1ρ to detect cartilage lesions as evaluated by arthroscopy in acutely ACL-injured knees and to compare with the Whole-Organ Magnetic Resonance Imaging Score (WORMS) using clinical standard MRI.
Method
Ten healthy controls (mean age 35) with no ACL injury or history of osteoarthritis (OA) and 10 patients with acute ACL injuries (mean age 39) were scanned at 3 Tesla (3T). ACL patients underwent ACL reconstruction, where focal lesions were graded according to an Outerbridge grading system during arthroscopic evaluation. Normalized MR T1ρ values (T1ρ z-scores normalized to control values in matched regions) in full thickness, and superficial and deep layers of cartilage were compared between defined sub-compartments with and without focal lesions. Intraclass (ICC) correlation and the root mean square coefficient of variation (RMS-CV) were performed to evaluate the inter-observer reproducibility of T1ρ quantification. Sub-compartments of cartilage were also evaluated using WORMS scoring and compared to their Outerbridge score respectively.
Results
The inter-observer ICC and the RMS-CV of the sub-compartment T1ρ quantification were 0.961 and 3.9%, respectively. The average T1ρ z-scores were significantly increased in sub-compartments with focal lesions compared to those without focal lesions and to the control cohort (p < 0.05).
Conclusion
Our results indicate that T1ρ provided a better diagnostic capability than clinical standard MRI grading in detecting focal cartilage abnormalities after acute injuries. Quantitative MRI may have great potential in detecting cartilage abnormalities and degeneration non-invasively, which are occult with standard morphological MRI.
Keywords: cartilage degeneration, magnetic resonance imaging, T1ρ quantification, arthroscopy, Outerbridge, ACL injuries
INTRODUCTION
Anterior cruciate ligament (ACL) rupture is a common and severe knee injury. Studies have demonstrated that approximately fifty percent of people that have undergone ACL reconstruction display characteristics of post-traumatic osteoarthritis (OA) ten to fifteen years after surgery [1–3]. Furthermore, these patients are much younger than patients diagnosed with primary osteoarthritis [4].
General advances in quantitative MR have allowed for the detection of changes in cartilage composition that are characteristic of cartilage damage and degeneration. These advances include T2, T1ρ, dGEMRIC, and sodium imaging [5–9]. Among these different imaging methods, T1ρ (T1rho) is a promising technique that detects changes in the proteoglycan and collagen matrix [10,11]. T1ρ mapping uses parameters that describe the spin-lattice relaxation time in a rotating frame by relating energy changes between proton spins and the environment surrounding it [12]. This technique describes how motion-restricted water molecules interact with the environment around them, such as the extracellular matrix in articular cartilage. Therefore, changes in the extracellular matrix can be reflected through T1ρ relaxation measurements [13]. Many studies have shown that MR T1ρ quantification mapping can be utilized as a non-invasive technique to detect cartilage matrix degeneration [14–17], where patients with OA obtain higher T1ρ relaxation times of cartilage compared to healthy controls [18,19].
Despite promising results, only one study has been documented to correlate quantitative MR T1ρ with clinical evaluations of cartilage damage and degeneration using arthroscopy [20]. Thus, the purpose of this study was to evaluate the capability of MR T1ρ to detect cartilage lesions as evaluated by arthroscopy and to compare this with clinical standard MRI. We performed the study in knees that have suffered ACL injuries and had arthroscopic evaluation available during ACL reconstruction. We hypothesize that cartilage T1ρ will be significantly elevated in regions with arthroscopically confirmed focal lesions and will be a better indicator of cartilage damage than standard clinical imaging with FSE.
MATERIALS AND METHODS
Subjects
This study was approved by the Committee for Human Research at our institution, and informed consent was obtained from all subjects after the nature of the examinations had been fully explained. The study was Health Insurance Portability and Accountability Act (HIPAA) compliant.
Twenty subjects, 10 patient subjects with acute ACL injuries (5 male, 5 female, mean age = 39 years, range = 28–51 years), and 10 control subjects (8 male, 2 female, mean age = 35 years, range = 29–53 years) were recruited for this study. A t-test was performed to compare gender, age, and body mass index (BMI) between the two groups, resulting in p=0.178, 0.717, and 0.583 respectively, indicating no significant differences between both cohorts. Patient subjects were defined as having an acute ACL rupture and in need of ACL reconstruction. Control subjects were defined as having no knee pain at the time of MRI examination and no history of osteoarthritis or trauma of the knee.
Magnetic Resonance Imaging Protocol
Patients with acute ACL-injured knees were scanned with T1ρ MR imaging techniques before their surgeries. This imaging protocol was performed separate from the usual standard of care imaging. Patients were scanned with a 3T MR scanner (HDx, GE Healthcare, Milwaukee, WI) using a transmit/receive quadrature knee coil (Clinical MR Solutions, Brookfield, WI). The imaging protocol included: sagittal fat-saturated intermediate-weighted fast spin-echo (FSE) images (TR/TE = 4300/51 ms, field of view (FOV) = 14 cm, matrix size = 512 × 256, slice thickness (ST) = 2.5 mm, gap = 0.5 mm), sagittal 3D water excitation high-resolution spoiled gradient-echo (SPGR) images (TR/TE = 15/6.7 ms, flip angle = 12°, FOV = 14 cm, matrix = 512 × 512, slice thickness = 1 mm), 3D T1ρ quantification based on spin-lock and SPGR sequences (TR/TE = 9.3/3.7 ms; FOV = 14 cm, matrix size = 256 × 128, slice thickness = 4 mm, view per segment = 64, time of recovery = 1.5 s, time of spin-lock (TSL)= 0, 10, 40, 80 ms, frequency of spin-lock = 500 Hz). Patients were scanned prior to their ACL reconstruction. The average time between the date of injury and the scan was 67.8 days with a standard deviation of 31.1. The average time between the date of injury and ACL reconstruction was 81.0 days with a standard deviation of 43.1.
Arthroscopic Assessment of Cartilage
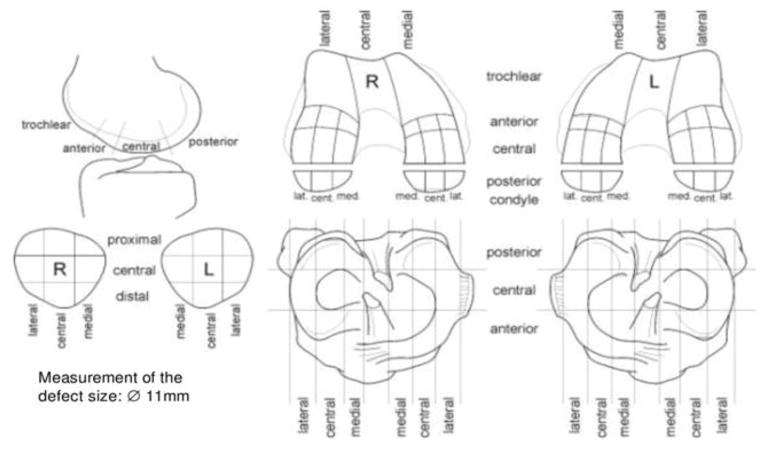
During ACL reconstruction, cartilage sub-compartments of the knee were evaluated using arthroscopy by an orthopedic surgeon at UCSF (C.B.M., with 8 years of experience) and then re-evaluated by the same surgeon post-surgery based on the video taken in the surgery room. A total number of 49 sub-compartments were defined, with 9, 18, 18, and 4 sub-compartments defined in the patella, tibia, femoral condyle, and trochlea, respectively (Figure 1). The sub-compartments were defined as seen in Figure 1, which comes from the ICRS Evaluation Package. The Outerbridge grading score was used to grade the cartilage in each defined sub-compartment (Figure 1): Grade 0 – normal; Grade I - cartilage with softening and swelling; Grade II - a partial-thickness defect with fissures on the surface that do not reach subchondral bone or exceed 1.5 cm in diameter; Grade III - fissuring to the level of subchondral bone in an area with a diameter more than 1.5 cm; Grade IV - exposed subchondral bone [21].
Figure 1.
The ICRS Outerbridge grading sheet. A total number of 49 sub-compartments were defined, with 9, 18, 18, and 4 sub-compartments defined in the patella, tibia, femoral condyle, and trochlea, respectively. The Outerbridge grading score was used to grade the cartilage in each defined sub-compartment.
MR Images and Clinical Gradings of Cartilage
Modified sub-scores of the Whole-Organ Magnetic Resonance Imaging Score (WORMS) system were obtained by two experienced musculoskeletal radiologists (T.M.L. and W.V.) using intermediate-weighted fast spin-echo (FSE) images. FSE images were used as the primary images for WORMS grading as these images provide the optimal contrast for any intrasubstance lesions within cartilage. SPGR images were also used in regards to assess cartilage thickness. The cartilage was graded using a modified WORMS score of the knee as follows: 0 – normal thickness and signal; 1 – normal thickness but increased signal on the T2-weighted FSE image; 2 – partial thickness focal defect less than 1.0 cm in width; 3 – multiple areas of partial thickness defects intermixed with areas of normal thickness or a Grade 2 defect wider than 1.0 cm but in less than 75% of the region; 4 – diffuse (greater than or equal to 75% of the region) partial thickness loss; 5 – multiple areas of full thickness loss or a grade 2.5 lesion wider than 1.0 cm but in less than 75% of the region; 6 – diffuse (greater than 75% of the region) full-thickness loss [22].
Image Processing
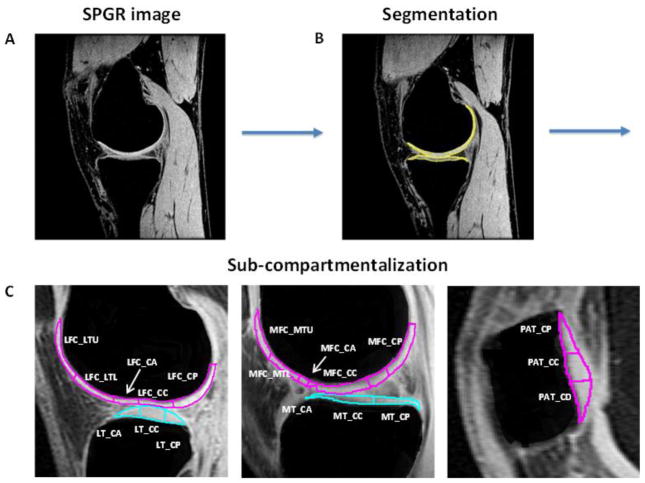
All baseline MR images were transferred to a Sun Workstation (Sun Microsystems, Palo Alto, CA) for data processing and quantification of T1ρ relaxation measurements. Cartilage compartments were segmented semi-automatically in high resolution SPGR images (Figure 2A) through IPP, an in-house software developed with MATLAB (Mathworks, Natick, MA, USA) [23]. Cartilage of the lateral/medial femoral condyles (LFC/MFC), lateral/medial tibia (LT/MT), and the patella (P) were defined (Figure 2B).
Figure 2.
Process of defining sub-compartment ROIs analogous to the ICRS Outerbridge grading sub-compartments as shown in Figure 1. The SPGR image (A) is segmented (B) with an in-house software through MATLAB. 3-D T1ρ maps are generated on top of the cartilage segmentation. Sub-compartments are defined according to the ICRS Outerbridge grading and anatomical markers, such as the meniscus between joints. All sub-compartments are then labeled according to facet, side, and orientation (C). Labeling is as follows: LFC_LTU/LTL: lateral femoral condyle lateral upper/lower trochlear regions; LFC_CA/CC/CP: lateral femoral condyle central anterior/central/posterior region; LT_CA/CC/CP: lateral tibial central anterior/central/posterior region; MFC_MTU/MTL: medial femoral condyle medial upper/lower trochlear region; MFC_CA/CC/CP: medial femoral condyle central anterior/central/posterior region; MT_CA/CC/CP: medial tibial central anterior/central/posterior region; PAT_CP/CC/CD: patellar central proximal/central/distal region.
The cartilage in the LFC/MFC, the LT/MT, and the patella were regarded as regions of interest (ROIs), and were further divided manually into sub-compartments analogous to the Outerbridge compartments. These were labeled accoring to facet (LFC, MFC, LT, MT, or P), side (lateral, central, medial), and orientation (anterior, central, posterior), with a total of 49 sub-compartments in every ACL and control subject (Figure 2C) as in arthroscopy. The sub-compartments were divided on a slice-by-slice basis and by anatomical markers, specifically by the meniscus of the knee. For example, considering cartilage in a patella that was nine slices thick, three slices would represent the lateral side, three would represent the central side, and three would represent the medial side. Then, taking each of these three sides individually, the orientation (proximal, central, distal) would be manually divided in-plane into three equivalent portions. A slice of a sub-compartmentalized knee is seen in Figure 2C.
T1ρ maps were generated on a voxel-by-voxel basis using a Levenberg-Marquardt algorithm that was developed by an in-house program. The equation Eq. (1) was of the form:
| (1) |
where TSL is the time of spin lock, S is the signal intensity in a T1ρ-weighted image with a certain TSL, and S0 is the signal intensity in that image when TSL = 0 ms. T1ρ maps were registered rigidly to SPGR images using the Visualization Toolkit Computational Science Group (VTK CISG) Registration Toolkit [24].
T1ρ relaxation times were quantified in each of the 49 sub-compartments of all twenty subjects. To reduce artifacts caused by synovial fluid, relaxation times above 130 ms for T1ρ-weighted images were manually removed from data. These average relaxation times were designated as “full thickness” values.
In order to evaluate inter-observer reproducibility of cartilage T1ρ quantification in these manually defined sub-compartments, sub-compartmentalization was performed independently by two observers (R.G. and F.S.) on 4 ACL subjects and 4 control subjects. The intraclass (ICC) correlation for these 8 subjects was determined through the software R (www.r-project.org). The root mean square (RMS) CV% (coefficient of variation) was calculated using Eq. (2):
| (2) |
T1ρ cartilage laminar analysis was performed on a slice-by-slice basis using IPP. Laminar analysis automatically divides cartilage into two layers of equal thickness termed as deep and superficial [25]. The deep layer is defined as the layer closest to the bone-cartilage interface. The superficial layer is defined as the layer closest to the articular surface. Even though histologically, cartilage is defined with three layers, only two layers were defined in this study due to the limited image resolution and to minimize partial volume effects between layers. T1ρ relaxation times in both layers were quantified in the 49 sub-compartments of all subjects.
Statistical Analysis
The mean T1ρ values in each sub-compartment for full thickness, superficial, and deep layers were statistically normalized to remove spatial variation between sub-compartments using a z-score with the equation Eq. (3):
| (3) |
where Zi represents the z-score of T1ρ in a sub-compartment i, T1ρi represents the value of T1ρ in sub-compartment i, and Meani and SDi are the mean and standard deviation of the control T1ρ in sub-compartment i. The T1ρ z-scores (superficial layer, deep layer, and full thickness, respectively) of the sub-compartments with focal lesions (an Outerbridge scoring greater than 0) were compared to T1ρ z-scores of the sub-compartments without focal lesions (an Outerbridge scoring of 0) using a Wilcoxon signed-rank test. The T1ρ z-scores for the full thickness of the sub-compartments of the patient cohort was compared to the T1ρ z-scores for full thickness of all sub-compartments in the control cohort. T1ρ z-scores for sub-compartments with and without focal lesions were also compared solely in the 90 ROIs of the LT (9 ROIs for 10 patients). The LT was specifically chosen because this was the region with the most number of Outerbridge and FSE lesions. All statistical analyses were considered significant at p < 0.05.
RESULTS
Arthroscopic Findings
Nine out of ten ACL subjects showed cartilage lesions during arthroscopic evaluation, resulting in a total number of 64 lesions (Outerbridge Grade I: 54 lesions; Grade II: 8 lesions; Grade III: 2 lesions). The most common site of Outerbridge lesions occurred in the lateral tibia (LT) (n = 26 from eight patients), followed by the patella (n = 20 from seven patients). Table 1 shows the number of lesions in each defined compartment (LFC, LT, MFC, MT, and Patella) based on the Outerbridge scoring (and MRI WORMS scoring as detailed below).
Table 1.
Total number of lesions in each compartment of the knee for each grade in Outerbridge and modified WORMS scoring.
| LFC | LT | MFC | MT | Patella | ||
|---|---|---|---|---|---|---|
| Outerbridge (64 lesions) | Total | 1 | 26 | 11 | 6 | 20 |
| Grade 1 | 1 | 24 | 6 | 6 | 17 | |
| Grade 2 | 0 | 2 | 4 | 0 | 2 | |
| Grade 3 | 0 | 0 | 1 | 0 | 1 | |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | |
| WORMS (37 lesions) | Total | 3 | 15 | 6 | 0 | 13 |
| Grade 1 | 0 | 14 | 2 | 0 | 1 | |
| Grade 2 | 0 | 0 | 3 | 0 | 5 | |
| Grade 2.5 | 0 | 1 | 0 | 0 | 0 | |
| Grade 3 | 1 | 0 | 0 | 0 | 7 | |
| Grade 4 | 0 | 0 | 1 | 0 | 0 | |
| Grade 5 | 2 | 0 | 0 | 0 | 0 | |
| Grade 6 | 0 | 0 | 0 | 0 | 0 |
MRI Clinical Gradings
Based on MRI WORMS, all ten patients showed cartilage lesions in the FSE images (cartilage WORMS > 0), with a total number of 37 lesions (WORMS Grade 1: 17 lesions; Grade 2: 8 lesions; Grade 2.5: 1 lesion; Grade 3: 8 lesions; Grade 4: 1 lesion; Grade 5: 2 lesions), as seen in Table 1. Similarly to the arthroscopic findings, the most common site of FSE lesions occurred in the LT (n = 15 from six patients), followed by the patella (n = 13 from six patients).
T1ρ Quantification of Superficial, Deep, and Full-Thickness Cartilage
The RMS-CV for T1ρ quantification between two observers in a total of 392 sub-compartments from 8 subjects (4 from the control cohort and 4 from the patient cohort, 49 sub-compartments in each subject) was 3.9%. The inter-observer ICC was 0.961, indicating excellent reproducibility.
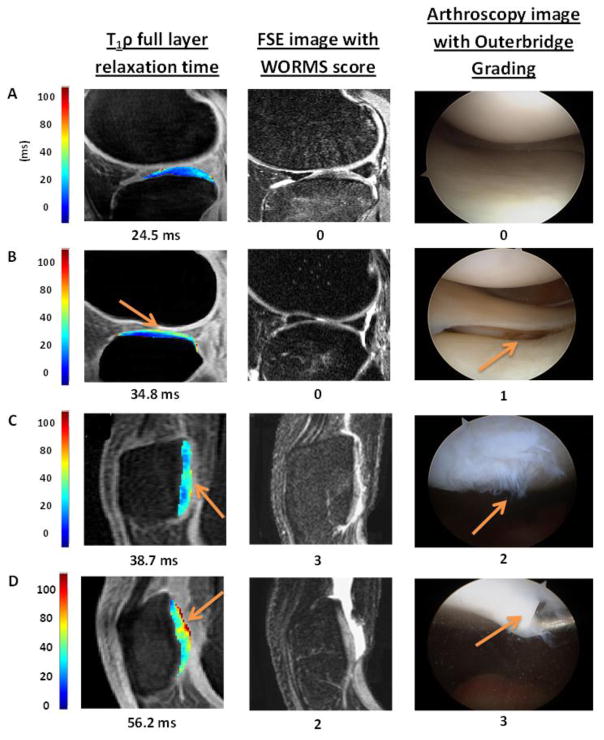
Table 2a summarizes the results of T1ρ z-score values (mean ± SD) for superficial, deep, and full thickness for normal cartilage (Outerbridge = 0) and for cartilage with focal lesions (Outerbridge > 0) in the whole knee and in the LT only. Significantly elevated T1ρ z-scores were observed in cartilage with focal lesions compared to normal cartilage in full-thickness cartilage and both superficial and deep layers. Comparing full thickness T1ρ z-scores of all sub-compartments in the patient and control cohort resulted in a very small p-value (p < 0.01). Furthermore, in order to assess the capability of T1ρ to evaluate cartilage damage with low grade, we compared T1ρ values in regions with Outerbridge Grade I (cartilage with softening and swelling) to Grade 0, and similarly, significantly elevated T1ρ z-scores were observed in cartilage with focal lesions compared to normal cartilage in full-thickness cartilage and both superficial and deep layers (Table 2b). There is an increasing trend of T1ρ z-scores from Outerbridge grade 1 to grade 3 (Table 3), but no statistical analysis was performed due to the small number of lesions with high grades. Figure 3 shows T1ρ color maps, FSE images, and arthroscopic images of example patients who had cartilage focal lesions with Outerbridge grades from 0 to 3 corresponding with increasing T1ρ relaxation times.
Table 2a.
Combined mean T1ρ z-scores, SD, and p-values of all 49 sub-compartments of the whole knee and of the LT only in each of the three layers. Normal corresponds to sub-compartments that received an Outerbridge grade = 0 and lesion corresponds to sub-compartments that received an Outerbridge grade > 0.
| Normal | Lesion | p-value | |
|---|---|---|---|
| Whole Knee | |||
| Superficial | 0.3 ± 1.2 | 0.7 ± 1.2 | 0.03 |
| Deep | 0.04 ± 1.1 | 0.3 ± 1.1 | 0.04 |
| Full | 0.2 ± 1.3 | 0.6 ± 1.3 | 0.02 |
| LT | |||
| Superficial | −0.3 ± 1.0 | 0.4 ± 0.9 | 0.01 |
| Deep | 0.09 ± 0.8 | 0.6 ± 0.8 | 0.003 |
| Full | −0.03 ± 1.2 | 0.9 ± 1.1 | 0.004 |
Table 2b.
T-test comparison of T1ρ z-scores between Outerbridge grade 0 and 1 in the whole knee and the LT only in each of the three layers.
| Whole Knee | LT | |
|---|---|---|
| Superficial | 0.03 | 0.01 |
| Deep | 0.06 | 0.006 |
| Full | 0.02 | 0.01 |
Table 3.
Mean ± SD T1ρ z-scores for Outerbridge grades 1, 2, and 3 in each layer. There is an increasing trend of the T1ρ z-score as the Outerbridge grade increases that is seen each layer.
| Superficial | Deep | Full | |
|---|---|---|---|
| Grade 1 | 0.6 ± 0.9 | 0.2 ± 0.9 | 0.6 ± 1.2 |
| Grade 2 | 0.8 ± 2.2 | 0.4 ± 1.8 | 0.8 ± 2.1 |
| Grade 3 | 1.6 ± 1.9 | 0.8 ± 1.2 | 1.7 ± 2.2 |
Figure 3.
Color maps of T1ρ relaxation times alongside registered FSE images and arthroscopy images of the same region. Increased T1ρ relaxation times are observed in conjunction with the increased Outerbridge grading of 0 to 3. Elevated T1ρ values were observed in regions with arthroscopically confirmed focal lesions (as indicated by arrows) in Figure B–D.
DISCUSSION AND CONCLUSIONS
The present study aimed to evaluate the capability of MR T1ρ relaxation times for detecting cartilage damage using arthroscopic evaluation as a standard of reference. Knees with acute ACL injuries were studied post-injury/prior to ACL reconstruction with a 3T MRI, and were evaluated for cartilage focal lesions using arthroscopy during ACL reconstruction. We have demonstrated that MRI T1ρ z-scores were significantly increased in cartilage with focal lesions (as defined with arthroscopic Outerbridge grading) compared to normal cartilage. T1ρ was also shown by ROC analysis to be a better indicator of cartilage focal lesions than standard clinical MR images with WORMS grading.
Our results indicated that patients with acute ACL tears showed the most number of arthroscopically confirmed focal lesions in the LT. This is consistent with previous studies [26,27], as bone bruises that accompany ACL ruptures are almost always seen in the LFC and the LT. Such damages to the lateral side are thought to be a result from translational impact during ACL rupture, where the anterolateral femur impacts the posterolateral tibia [28,29]. This is the so-called kissing contusion, with cartilage lesions most commonly found on the LFC and LT [30]. However, surprisingly, not many lesions were found in the LFC based on Outerbridge or WORMS scoring. The different number of lesions between LT and LFC may suggest different response and recovery mechanisms of the tibial and femoral cartilage from the injury during ACL rupture.
Interestingly, in this study, we observed that the second most common location for cartilage focal lesions was the patella. The cohort recruited in this study was composed with young and active subjects, although the study is limited as no data was collected regarding the specific activity level of the patients. Previous studies have suggested that physically active subjects have a high prevalence of focal cartilage abnormalities particularly at the femoral-patellar joint [31], suggesting a high mechanical burden at this location. Therefore, the lesions found in the patella in the present study may indicate pre-existing cartilage lesions in these subjects. Despite the potential different mechanisms that cause cartilage focal lesions in different compartments, the results from the present study suggest that MRI T1ρ is sensitive for detecting changes within the cartilage matrix.
In a previous study, Witschey et al. [20] reported elevated post-arthroscopic MRI T1ρ in cartilage with arthroscopic Outerbridge grades of 3 and 4, while agreement between MRI T1ρ and arthroscopic evaluation was only modest in the cases of grade 1 or 2 damage when averaging across compartments (medial or lateral: patella, femur, and tibia). The authors demonstrated that calculating T1ρ values within manually defined ROIs, instead of the whole compartments, will improve the agreement between MR T1ρ and arthroscopic evaluation. The definition of the lesion ROIs, however, can be very subjective and is prone to location errors, especially for small lesions.
In this study, a total number of 49 sub-compartments were defined within the patella-femoral-tibial joint based on anatomical markers. Both Outerbridge scores and mean T1ρ values were evaluated in each sub-compartment. Calculating the mean T1ρ values within these well-defined and small sub-compartments provided a trade-off between the sensitivity of T1ρ detecting focal lesions (as compared to using mean T1ρ values of the large compartment) and the subjectivity of using manually defined ROIs. Variation of the T1ρ quantification of these small sub-compartments between different observers was evaluated in a subset of the subjects and the results demonstrated excellent inter-observer reproducibility. In addition, previous studies suggest spatial variation of T1ρ values in different regions of the joint [32]. T1ρ z-scores, which normalized the T1ρ based on healthy controls in matching regions, were applied in this study to account for the spatial variation of T1ρ values. Therefore, the T1ρ values can be compared directly between sub-compartments with and without focal lesions.
In this study, significantly elevated mean T1ρ z-scores were observed in sub-compartments with cartilage focal lesions compared to those without focal lesions. The focal lesions in this cohort are dominated by Outerbridge grade 1 lesions. Elevation of T1ρ in these lesions indicated the capability of T1ρ detecting biochemical changes within the cartilage matrix. Previous studies have demonstrated that T1ρ values are correlated significantly with proteoglycan changes in both digested bovine cartilage [33] and in human osteoarthritic cartilage [34]. In particular, with the techniques of spin-lock, the residual dipolar interaction is reduced and the chemical exchange between water and proteoglycan protons may contribute to the relaxation mechanism [35,36].
Furthermore, T1ρ quantification showed a better capability of detecting focal lesions compared to the current clinical standard MRI, specifically the WORMS grading using T2-weighted fast-spin-echo images. These results are consistent with our previous case report [37] and were illustrated in an example patient in Figure 3B, where, in regions with arthroscopically confirmed focal cartilage lesions, the standard MRI appeared to be normal (given a grade 0), while the T1ρ values were significantly elevated (34.8 ms). These results suggest that quantitative MRI provides a higher sensitivity of detecting cartilage degeneration compared to the standard MRI.
Using laminar analysis, we observed a significant elevation in T1ρ z-scores both in the superficial and deep layers in sub-compartments with focal lesions compared to those without focal lesions. The capabilities of detecting cartilage lesions were comparable between the superficial and deep layers in this cohort. This result suggests that there might be a mixture of the degeneration mechanism of these focal lesions with regard to if the degeneration starts from the superficial layer or the deep layer.
There were several limitations to this study. First, the study had a small cohort size (10 healthy subjects and 10 ACL patients); a larger cohort should be acquired in order to confirm the findings and to better understand the results obtained. Second, although the Outerbridge grading system is considered the “gold standard” for evaluating focal lesions, there is subjectivity in defining the scores; demonstrating that the surgeon inter-operatively scored the same regions would be ideal. Third, only two layers, termed as superficial and deep, were studied instead of the histologically defined three layers (superficial, transitional, and deep layers) due to the limited image resolution. Fourth, axial PD or SPGR imaging would be ideal for a qualitative evaluation of articular cartilage in the patellofemoral region, but axial sequences were not performed due to the overall scan time limitation of the study; therefore, the patellofemoral cartilage was evaluated using sagittal FSE and SPGR images. In addition, all twenty subjects were studied only at baseline; T1ρ quantification should also be evaluated in longitudinal studies. Furthermore, we are aware that the lesions observed in this study can be a mixture of lesions caused by the acute ACL injury as well as other chronic chondral pathologies. Although we focused our statistical analysis on the lateral tibia, as we observed the most number of lesions in this area that are most likely a result of the acute injury, other regions of the knee can contain lesions of mixed pathology, and we have included all lesions observed in the patients in the study. Lastly, this study was cross-sectional; it would be very interesting to follow up the cartilage focal lesions that were observed and examine their longitudinal progression in future studies with a larger cohort and a longer follow-up period.
In summary, this study demonstrated the capability of T1ρ quantification for detecting cartilage focal lesions using arthroscopic evaluation as a standard of reference. Significantly elevated T1ρ values were observed in sub-compartments with cartilage focal lesions compared to those without focal lesions in acutely ACL-injured knees. Micro-instability of the joint after ACL reconstruction is also a risk factor for developing post-traumatic OA, and patients without discernable cartilage injury may also be prone to post-traumatic OA development; however, this does not exclude the potential contributing role of the initial ACL injury to OA development in these joints. Therefore, future follow up studies are warranted to evaluate if the lesions and their elevated T1ρ values observed in the study will contribute to future OA development in these joints. Nonetheless, MR T1ρ provided a better diagnostic capability than standard clinical MRI to detect cartilage lesions in these joints. Quantitative MRI has great potential to detect damage in cartilage that occurs prior to morphologic changes non-invasively. Such capability of early diagnosis may allow early intervention, in acutely injured joints for example, and can help evaluate treatment efficacy of new therapeutic strategies for osteoarthritis.
Acknowledgments
The authors thank Fei Liang, Dr. Keerthi Shet, Joseph Schooler, and Paran Yap for their technical support.
Role of Funding Source
This research was supported by NIH K25 AR053633, K25AR053633-S1, R01 AR46905, P50AR060752, and the Berkeley Undergraduate Research Apprentice Program (URAP).
Footnotes
Author Contributions
All authors have made substantial contributions to the conception and design, study execution, manuscript preparation, and final approval of the submitted article.
RG: analyzed data and drafted the manuscript; WV: performed clinical grading of the cartilage lesions; DK: collected in-vivo MRI data and arthroscopic grading data; FS: analyzed data; TL: provided supervision of clinical grading and clinical input on data interpretation; BM: overall study design, patient management, provided arthroscopic grading and clinical input on data interpretation; XL: overall study design, provided supervision on data analysis and interpretation.
Competing Interest Statement
The authors have no conflict of interest to disclose in regard to this research or manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Riti Gupta, Email: ritigupta11@gmail.com.
Warapat Virayavanich, Email: kaooat7@hotmail.com.
Daniel Kuo, Email: dankuo@gmail.com.
Favian Su, Email: faviansu@gmail.com.
Thomas Link, Email: thomas.link@ucsf.edu.
Benjamin Ma, Email: maben@orthosurg.ucsf.edu.
References
- 1.Ferretti A, Conteduca F, De Carli A, Fontana M, Mariani PP. Osteoarthritis of the knee after ACL reconstruction. Int Orthop. 1991;15(4):367–371. doi: 10.1007/BF00186881. [DOI] [PubMed] [Google Scholar]
- 2.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the in uence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 5.Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. 1999;41(5):857–65. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49(3):488–92. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 9.Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19(7):781–821. doi: 10.1002/nbm.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makela HI, Grohn OH, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289(4):813–8. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 11.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–53. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 12.Redfield AG. Nuclear spin thermodynamics in the rotating frame. Science. 1969;164:1015–1023. doi: 10.1126/science.164.3883.1015. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Ma BC, Link TM, Castillo D, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 15.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 16.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 17.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Han E, Ma BC, Link TM, Newitt D, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–936. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 19.Duvvuri U, Charagundla SR, Kudchodkar SB, Kaufman JH, Kneeland JB, Rizi R, et al. Human knee: in vivo T1(rho)-weighted MR imaging at 1.5 T-preliminary experience. Radiology. 2001;220:822–826. doi: 10.1148/radiol.2203001662. [DOI] [PubMed] [Google Scholar]
- 20.Witschey WR, Borthakur A, Fenty M, Kneeland BJ, Lonner JH, McArdle EL, et al. T1rho MRI quantification of arthroscopically confirmed cartilage degeneration. Magn Reson Med. 2010;63:1376–1382. doi: 10.1002/mrm.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31:83–86. doi: 10.1177/03635465030310012601. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, Majumdar S. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 25.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65:1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 26.Mair SD, Schlegel TF, Gill TJ, Hawkins RJ, Steadman JR. Incidence and location of bone bruises after acute posterior cruciate ligament injury. Am J Sports Med. 2004;32:1681–1687. doi: 10.1177/0363546504266481. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26:409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 28.Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17:445–449. doi: 10.1053/jars.2001.23581. [DOI] [PubMed] [Google Scholar]
- 29.Speer KP, Spritzer CE, Bassett FH, 3rd, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20:382–389. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 30.Terzidis IP, Christodoulou AG, Ploumis AL, Metsovitis SR, Koimtzis M, Givissis P. The appearance of kissing contusion in the acutely injured knee in the athletes. Br J Sports Med. 2004;38:592–596. doi: 10.1136/bjsm.2003.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl R, Luke A, Li X, Carballido-Gamio J, Ma BC, Majumdar S, Link TM. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link TM, Ma BC, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akella SV, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med. 2004;52:1103–1109. doi: 10.1002/mrm.20241. [DOI] [PubMed] [Google Scholar]
- 36.Duvvuri U, Goldberg AD, Kranz JK, Hoang L, Reddy R, Wehrli FW, et al. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci U S A. 2001;98:12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma BC. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349–1352. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]