Abstract
Activation of frontal and parietal brain regions is associated with attentional control during visual search. We used fMRI to characterize age-related differences in frontoparietal activation in a highly efficient feature search task, detection of a shape singleton. On half of the trials, a salient distractor (a color singleton) was present in the display. The hypothesis was that frontoparietal activation mediated the relation between age and attentional capture by the salient distractor. Participants were healthy, community-dwelling individuals, 21 younger adults (19 – 29 years of age) and 21 older adults (60 – 87 years of age). Top-down attention, in the form of target predictability, was associated with an improvement in search performance that was comparable for younger and older adults. The increase in search reaction time (RT) associated with the salient distractor (attentional capture), standardized to correct for generalized age-related slowing, was greater for older adults than for younger adults. On trials with a color singleton distractor, search RT increased as a function of increasing activation in frontal regions, for both age groups combined, suggesting increased task difficulty. Mediational analyses disconfirmed the hypothesized model, in which frontal activation mediated the age-related increase in attentional capture, but supported an alternative model in which age was a mediator of the relation between frontal activation and capture.
Keywords: Attention, Aging, fMRI, Neuroimaging, Reaction Time, Perception
Introduction
Behavioral investigations of cognitive aging have established that age-related decline occurs in some aspects of attention, including those related to executive function (Craik and Bialystok, 2006; Kramer and Madden, 2008; Wecker et al., 2000). One aspect of this decline is decreased ability to inhibit irrelevant information, for older adults, relative to younger adults, which may in turn lead to an age-related increase in distraction (Hasher and Zacks, 1988; Healey et al., 2008; Lustig et al., 2007; Rabbitt, 1979). In the context of visual search and discrimination tasks (Eckstein, 2011; Wolfe, 1998, 2007), age-related decline in attentional functioning is reflected in a disproportionate slowing of older adults’ conjunction search, in which the target and nontarget (distractor) items share visual features (e.g., a red vertical bar among red horizontal and green vertical bars), relative to feature search, in which the target has a feature not shared with the distractors (e.g., a red vertical bar among green vertical bars; Hommel et al., 2004; Madden, 2007; Madden and Whiting, 2004; Plude and Doussard-Roosevelt, 1989). Similarly, older adults appear to be more vulnerable than younger adults to attentional capture by salient distractors (Cashdollar et al., 2013; Pratt and Bellomo, 1999; Tsvetanov et al., 2013).
The age-related increase in attentional capture, however, varies across task context and is most pronounced when perceptual load is low (Maylor and Lavie, 1998), and the source of distraction is both visually salient (Porter et al., 2012) and known to be irrelevant to task goals (Kramer et al., 2000). Age-related differences in distraction and attentional capture thus do not appear to reflect a general failure of all inhibitory processes (Kramer et al., 1994; Madden and Plude, 1993), but rather result from an interaction of bottom-up (stimulus-driven) and top-down (goal-driven) attentional selection within specific task demands (Ludwig and Gilchrist, 2002; Yantis, 1998). In addition, some aspects of attention are resistant to age-related decline. When top-down attentional guidance is available, in terms of the observer’s knowledge of the task goals or target predictability, older adults often exhibit improvements in search performance that are at least as pronounced as those of younger adults (Humphrey and Kramer, 1997; Madden, 1987; Madden et al., 2004; Whiting et al., 2007; Whiting et al., 2005; Whiting et al., 2014). Age-related decline in the efficiency of visual sensory functioning and perceptual motor speed are well established (Madden et al., 1999; Salthouse, 1985; Salthouse and Madden, 2007; Schneider and Pichora-Fuller, 2000; Scialfa, 2002), and thus older adults may place greater emphasis on top-down attention as a compensatory mechanism for decline in bottom-up processing (Madden et al., 2007a; McAvinue et al., 2012; Porter et al., 2012).
The neural bases of age-related differences and constancies in visual attention are not yet well defined. Extensive neuroimaging research suggests that, for healthy younger adults, performance in visual search tasks is mediated by a widely distributed frontoparietal network with dorsal and ventral components (Corbetta et al., 2008; Corbetta and Shulman, 2002; Hopfinger et al., 2000; Shulman et al., 2004; Vossel et al., 2013). The dorsal component of this network comprises the middle frontal gyrus (MFG), frontal eye field (FEF), superior parietal lobule (SPL), and intraparietal sulcus (IPS), bilaterally. These regions support the voluntary, top-down allocation of attention (Anderson et al., 2007; Donner et al., 2002; Egner et al., 2008; Pollmann et al., 2003; Shulman et al., 2003) and active generation of a salience map or template of features defining the search target (de Fockert et al., 2004; Hodsoll et al., 2009; Kusunoki et al., 2000; Mevorach et al., 2009). The ventral component of the frontoparietal network, comprising the temporoparietal junction (TPJ) and ventral frontal cortex, particularly in the right hemisphere, operates in a more sensory-driven, bottom-up manner and is active in the detection of infrequently occurring targets (Astafiev et al., 2006; Kincade et al., 2005; Shulman et al., 2003).
One theme across several functional magnetic resonance imaging (fMRI) studies is that older adults exhibit higher levels of activation than younger adults (Dennis and Cabeza, 2008; Grady et al., 2010; Madden et al., 2005; Nielson et al., 2002), particularly within the dorsal frontoparietal regions. Madden et al. (2007b), for example, reported that older adults exhibited higher frontoparietal activation, specifically in the FEF and SPL than younger adults, during visual search with multi-letter displays. The behavioral effect of visual salience (from a color singleton in the display) was correlated with the frontoparietal activation for older adults, whereas for younger adults the corresponding relation between activation and salience occurred in more ventral (fusiform) cortical regions. In addition, these activation-performance correlations occurred only when the salient display item was predictive of target location, consistent with an age-related increase in top-down attention, perhaps as a compensatory response to decline in the efficiency of bottom-up processing (Davis et al., 2008). Schmitz et al. (2010) reported a related pattern, with a perceptually demanding task involving identification of superimposed face/place images. These authors found that older adults exhibited less suppression, relative to younger adults, of parahippocampal activation by the task-irrelevant place images but increased activation in left MFG.
Nearly all previous neuroimaging research on age-related differences in visual attention has relied on tasks with substantial demands on either perceptual discrimination (Madden et al., 2007b; Schmitz et al., 2010), working memory (Campbell et al., 2012; Gazzaley and D’Esposito, 2007), or response selection (Nielson et al., 2002). The Madden et al. (2007b) study, for example, reported an age-related difference in activation associated with visual salience, but the salient display item (a color singleton) could be either a target or a distractor, and consequently the effects of salience, and the interaction with top-down attention, combined facilitatory and inhibitory influences. Thus, the neural basis of age-related differences in attentional processes such as inhibition, particularly at the early perceptual level related to visual salience, have not been clearly isolated.
In this experiment we therefore investigated younger and older adults’ fMRI activation by a highly salient but irrelevant display item, in the context of a feature search task: detection of a shape singleton (e.g., a vertical bar among four triangles). Further, the salient distractor, a color singleton, could never be the target. The combination of low perceptual load and designation of the salient distractor as irrelevant should thus maximize older adults’ vulnerability to attentional capture (Kramer et al., 2000; Maylor and Lavie, 1998; Porter et al., 2012) and allow for the isolation of inhibitory effects. We also included separate task conditions of feature search that differed in the availability of top-down attention, in terms of the predictability of a particular shape occurring as a target. Although the visual salience of the display items is a critical determinant of feature search performance (Theeuwes, 2004, 2010; Theeuwes et al., 2006), top-down attention, in the form of target predictability, can also lead to improvement in feature search (Bravo and Nakayama, 1992; Wolfe et al., 2003). Under some conditions, target predictability may reduce or eliminate attentional capture by salient distractors (Bacon and Egeth, 1994; Leber and Egeth, 2006). Thus, a feature search task with color singleton distractors should provide an optimal context in which to assess age-related differences, not only in attentional capture by a salient distractor, but also in the ability of top-down attention to reduce the magnitude of distraction.
We predicted that, at the behavioral level, the magnitude of attentional capture by the color singleton would be greater for older adults than for younger adults (Hasher and Zacks, 1988; Lustig et al., 2007; Tsvetanov et al., 2013), but that both younger and older adults would exhibit an overall improvement in target detection with the availability of top-down attention (Madden, 2007; Whiting et al., 2014). If top-down attention can reduce the magnitude of attentional capture by a salient distractor (Bacon and Egeth, 1994; Leber and Egeth, 2006), then top-down reduction in attention capture should also be evident for both younger and older adults.
At the neural level, we expected an age-related increase in the magnitude of frontoparietal activation, particularly in response to attentional capture by salient distractors (Madden et al., 2007b; Schmitz et al., 2010). More critically, we sought to characterize the relation between age-related differences in activation and behavioral performance. If age-related increases in capture-related activation are compensatory (Davis et al., 2008), then we would expect that, particularly for older adults, increasing frontoparietal activation would be associated with decreasing reaction time (RT; i.e., better performance). Alternatively, increasing activation may reflect the increased number or complexity of information processing operations (i.e., task difficulty) and would thus be associated with increasing RT (Anderson et al., 2007; Donner et al., 2002).
Finally, we used mediation analyses to determine the causal relations among the variables representing age group, activation, and search performance (Baron and Kenny, 1986; Mackinnon and Fairchild, 2009; Preacher and Hayes, 2008; Salthouse, 1992a). Typically, neuroimaging studies assume that variables related to brain structure and function are mediators, in the sense that they directly influence the relation between other variables such as age and cognitive performance. As Salthouse (2011) has pointed out, however, the evaluation of mediation requires the assessment of different models of the relations among the relevant variables. We compared two models, one in which brain activation mediates the relation between age and search performance, and an alternative model in which age is the mediator of the relation between activation and performance.
Material and methods
Participants
The participants were 21 younger adults between 19 and 29 years of age (12 women) and 21 older adults between 60 and 87 years of age (11 women). Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. During a screening session conducted up to two weeks before the fMRI testing, participants completed several psychometric tests and a practice version (120 trials) of the visual search task to be used during scanning (described in the following section, Visual Search Task). By self-report, all participants were right-handed, free from significant health problems (including atherosclerosis, neurological and psychiatric disorders), and none reported taking medications known to affect cognitive function or cerebral blood flow (including antihypertensive agents). Participants scored a minimum of 27 points on the Mini Mental State Exam (Folstein et al., 1975), a maximum of 9 on the Beck Depression Inventory (Beck, 1978), and had normal color vision with a score of at least 12 (out of 14) on the Dvorine color plates (Dvorine, 1963). Vocabulary and years of education were comparable for the two age groups (Table 1).
Table 1.
Participant Characteristics by Age Group
| Younger | Older | |
|---|---|---|
| Age (years) | 21.67a (2.89) | 69.241b (5.62) |
| Education (years) | 14.95a (1.39) | 16.19a (2.64) |
| Vocabulary | 63.09a (3.69) | 64.38a (3.15) |
| Digit Symbol Acc | 97.48a (2.22) | 95.77a (2.86) |
| Digit Symbol RT (ms) | 1241.33a (243.06) | 1841.38b (275.73) |
| Visual Acuity (log MAR) | −0.11a (0.13) | 0.04 b (0.15) |
Note. n = 21 per age group. Values are means, with standard deviations in parentheses. Vocabulary = raw score (maximum of 70) on the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981); Digit Symbol Acc and Digit Symbol RT = percentage correct and reaction time (ms), respectively, on a computer test of digit-symbol coding (Salthouse, 1992b). Visual acuity = logarithm of the minimum angle of resolution (MAR), for the Freiburg Visual Acuity Test (FRACT; Bach, 1996). Log MAR of 0 corresponds to Snellen 20/20, with negative values corresponding to better resolution. Means in the same row that do not share subscripts differ by t-test at p < .05.
Visual Search Task
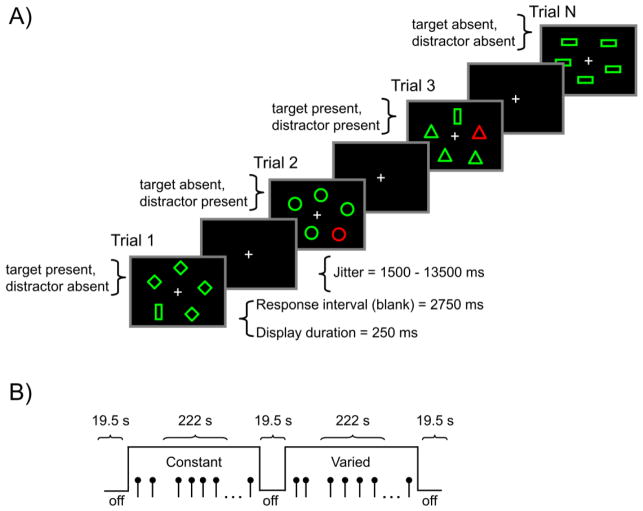
The behavioral task during fMRI scanning was a version of visual search in which, on each trial, participants made a yes/no decision regarding whether one target shape (hexagon, trapezoid, or vertical bar) was present among nontarget shapes (diamonds, horizontal bars, ovals, circles, or triangles). Thus, this was a feature search task in which the shape of the target was always different from that of the nontargets (i.e., the target was a shape singleton), and all of the nontargets had the same shape (Figure 1, Panel A). The target and nontargets were green outline shapes on a black background. There was an equal number of target-present and target-absent trials. On half of the trials (both target-present and target-absent), one of the nontargets was red (i.e., a color singleton), thus making it a salient distractor. The color singleton distractor never corresponded to the target, and participants were instructed to try to ignore this distractor when it occurred. Display size was constant at five items, either one target within four distractors, or five distractors, and the five display locations were approximately equidistant from fixation.
Figure 1.
Panel A = Sequence of events on each trial. The task required a present/absent response regarding the presence of a target shape singleton. In the example, the target is a vertical bar. In the constant search condition, the target was known in advance and remained constant during all trials, and was never a distractor. In the varied search condition, the target was also a shape singleton but varied unpredictably among hexagon, trapezoid, and vertical bar shapes. In the experiment, the target and nontarget items were green outline shapes against a black background. On half of the trials (both target-present and target-absent) within each search condition, there was a red color singleton distractor, illustrated in the figure as the darker outlined shapes. The color singleton was never the target. Panel B = fMRI task design. Each of five runs contained two on-task periods, one constant and one varied search condition, each containing 32 trials.
Two search conditions, varied and constant, differed in the degree to which knowledge of the task structure could influence the ability to ignore the distractor. In both conditions, the shapes assigned to target and distractor status remained distinct (i.e., consistent mapping; Schneider and Shiffrin, 1977). Also, for both search conditions, the distractor shapes (diamonds, horizontal bars, ovals, circles, or triangles) were homogeneous within the display on each trial but varied across trials. Varied and constant conditions differed regarding the predictability of the target: In the varied condition, the target was not predictable and varied among the three possible target shapes (hexagon, trapezoid and vertical bar), whereas in the constant condition, the shape of the target was pre-defined at the beginning of the task and remained constant for each participant. Thus, in this latter condition, top-down attention was available, in the form of target predictability.
Within the scanner, participants completed two blocks of 32 practice trials, one for constant search and one for varied search. These practice trials were followed by five functional imaging runs, each comprising two on-task periods (one constant and one varied). Each on-task period comprised 32 trials, yielding a total of 64 trials per run, for a total of 320 trials across the five runs (Figure 1, Panel B). Within each run, three off-task periods were interleaved with the on-task periods (i.e., an off-task period occurred at both the beginning and end of each scanner run, as well as between the two on-task periods). Within an on-task period, the 32 trials represented eight instances for each combination of target status (present, absent) and distractor status (present, absent), distributed pseudorandomly, yielding a total of 40 trials for each task condition, per participant. Target location was balanced approximately equally across the display locations. The shape assigned as the target in the constant condition was balanced across participants. Two run orders, alternated across participants, started with either a constant or varied search condition. Participants held an fMRI-compatible response box and used the left and right index fingers to respond to the presence or absence of the target shape. Participants were instructed to respond as quickly as possible without sacrificing accuracy, and the assignment of target presence to the response buttons was balanced across participants within each age group.
An instruction screen at the beginning of the on-task period informed participants as to whether the target would be constant or varied. Following the instruction screen, each trial started with stimuli displayed for 250 ms, followed by a 2750 ms post-display response period, during which the display was black. We measured the RT from display onset, but the screen remained black during the 2750 ms response period regardless of when the participant responded. Following the response period, a white center fixation cross was displayed on the black screen, for a variable time period (jitter). Nine jitter durations between 1500 ms and 13500 ms were defined by multiples of the fMRI repetition time (TR) value (1500 ms). The jitters were distributed randomly within on-task periods, but the shorter durations more heavily sampled, so that the overall frequency distribution of individual jitter values was approximately exponential. No feedback regarding response accuracy was provided. During the off-task periods, three white center fixation crosses remained onscreen for 19.5 s. Participants were instructed to fixate the three crosses during the off-task period, in preparation for the upcoming task.
MRI Data Acquisition
Imaging was conducted on a 3 T GE Signa Excite MRI scanner (GE Healthcare, Waukesha, WI) with an eight-channel head coil. Head motion was minimized with foam pads and headband, and participants wore earplugs to reduce scanner noise. The imaging sequence included a three-plane localizer, followed by T1-weighted anatomical images and five runs of T2*-weighted (functional) imaging. Slice orientation was near-axial, parallel to the anterior-posterior commissure (AC-PC) plane.
The T1-weighted anatomical images were 60 contiguous slices acquired with a 3D fast inverse-recovery-prepared spoiled gradient recalled (SPGR) sequence, with TR = 7.3 ms, echo time (TE) = 2.97 ms, inversion recovery time (TI) = 450 ms, field of view (FOV) = 256 mm, slice thickness = 2 mm, flip angle = 12°, voxel size = 2 × 2 × 2 mm, 128 × 128 matrix, and a parallel imaging with a selection factor of 2.
The T2*-weighted echo-planar, functional images were sensitive to the blood oxygen level dependent (BOLD) signal. These were 30 contiguous slices acquired using an inverse spiral sequence, with TR = 1500 ms, TE = 30 ms, FOV = 256 mm, slice thickness = 4 mm, flip angle = 60°, voxel size = 4 × 4 × 4 mm, and 64 × 64 matrix.
fMRI Analyses
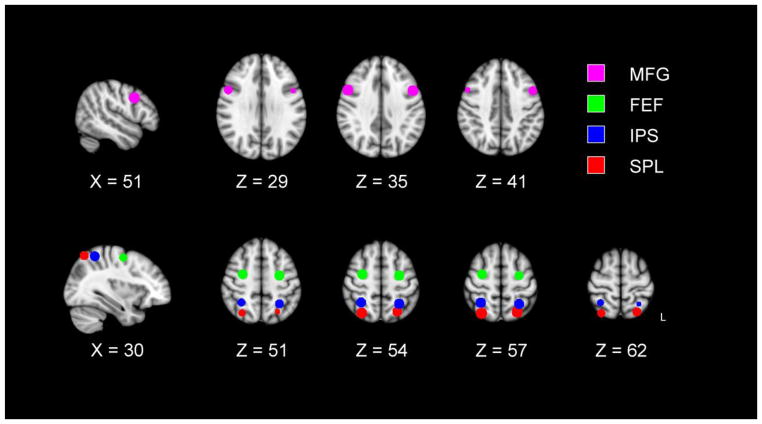
To maintain the independence of activation magnitude and region selection (Kriegeskorte et al., 2009), we adopted a region of interest (ROI) approach, with eight ROIs selected from two previous studies of visual search that focused on issues related to top-down attention, salience, and distraction (de Fockert et al., 2004; Shulman et al., 2003). The ROIs are illustrated in Figure 2 and the coordinate locations are listed in Table 2. The first of the previous studies, de Fockert et al. (2004), reported activation related to the presence of a color singleton distractor during visual search, in the left MFG, and SPL bilaterally. We thus used the coordinates, in standardized space, for these local maxima reported by de Fockert et al. in their Table 1, adding a right MFG ROI homologous to their left hemisphere value. The second of the previous studies, Shulman et al. (2003), found that both the IPS and FEF maintained activity during visual search (digit detection and motion detection), consistent with top-down attentional control, whereas the IPS also exhibited sensitivity to the sensory properties of the display (i.e., salience). We defined ROIs from the IPS and FEF coordinates listed in Table 2 of the Shulman et al. article. The complete set of eight dorsal frontoparietal ROIs also represent regions that tend to exhibit higher activation for older adults than for younger adults (Dennis and Cabeza, 2008; Grady et al., 2010; Madden et al., 2005; Nielson et al., 2002).
Figure 2.
Regions of interest (ROIs) were selected from local maxima of event-related activation in previous neuroimaging studies of visual search. The left middle frontal gyrus (MFG) and superior parietal lobule (SPL) ROIs were selected from de Fockert (2004). The frontal eye field (FEF) and intraparietal sulcus (IPS) ROIs were selected from Shulman et al. (2003). Further information is provided in Table 2.
Table 2.
Regions of Interest
| Location | BA | Source | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Left MFG | −48 | 9 | 36 | 6 | de Fockert et al. (2004) |
| Right MFG | 51 | 10 | 34 | 6 | de Fockert et al. (2004) |
| Left FEF | −27 | −9 | 53 | 6 | Shulman et al. (2003) |
| Right FEF | 29 | −7 | 54 | 6 | Shulman et al. (2003) |
| Left SPL | −24 | −64 | 58 | 7 | de Fockert et al. (2004) |
| Right SPL | 30 | −66 | 57 | 7 | de Fockert et al. (2004) |
| Left IPS | −27 | −52 | 55 | 7 | Shulman et al. (2003) |
| Right IPS | 31 | −50 | 56 | 7 | Shulman et al. (2003) |
Note. MFG = middle frontal gyrus; FEF = frontal eye field; SPL = superior parietal lobule; IPS = intraparietal sulcus. Location = center of mass in x, y, z coordinates of Montreal Neurological Institute (MNI) standard space; BA = Brodmann area.
For all of the ROIs, we used the coordinates of the reported local maxima, converting from Talairach space, as reported in the original articles, to Montreal Neurological Institute (MNI) space. We then used these coordinates as the centers of 8 mm diameter ROI spheres. Because the IPS and SPL ROIs are spatially proximate, the spheres for these two regions overlapped slightly in the left hemisphere, with 116 voxels shared by the two ROIs. These overlapping voxels were eliminated from the analyses, reducing the size of each of these ROIs by 5.8%. Thus, no voxel contributed to more than one ROI.
We used Statistical Parametric Mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) along with locally developed Matlab scripts (Mathworks, Natick, MA) and the MarsBar toolbox (http://marsbar.sourceforge.net/) to estimate the magnitude of activation within the pre-selected ROIs. The initial six volumes of each run, which always occurred during an off-task period, were discarded. Images were corrected for slice-timing and head motion, spatially normalized to the MNI template, and then spatially smoothed with an 8 mm Gaussian kernel. No participant moved more than 3 mm in any direction, either within or across runs, and a high-pass filter was included in every model to correct for scanner drift.
At the first level, we conducted voxelwise analyses within the general linear model of SPM5 (Friston et al., 1995). Because the constant- versus varied-search manipulation was a block-level effect, with each search condition associated with an on-task period, we used a mixed blocked/event-related design, which incorporated both event-related (transient) and blocked (sustained) regressors for the hemodynamic response function (Donaldson et al., 2001; Visscher et al., 2003). The participant-specific SPM models contained both event-related and sustained regressors. Event-related regressors were coded as a stick function (delta) convolved with the hemodynamic response + time basis function (Miezin et al., 2000), with the onset corresponding to the onset of the display. We modeled activation for each event-related regressor (i.e., trial type), for each participant, versus the implicit baseline (the jittered inter-trial interval from the on-task periods). For each search condition (constant, varied), four trial types were modeled including the orthogonal representation of target present/absent, and distractor present/absent, resulting in a total of eight trial types. To estimate sustained activation across the on-task period, sustained regressors were modeled as a fixed response (boxcar) waveform, with onset corresponding to the onset of the first display and lasting the duration of the on-task period. The 19.5 s off-task periods were modeled explicitly as a baseline for analysis of sustained effects. Trials with either an incorrect response (accuracy rates presented in Table 3) or no response (< 2.1% of trials per age group) were modeled as a nuisance regressor. Head motion and scanner drift were treated as covariates of no interest.
Table 3.
Visual Search Performance as a Function of Age Group, Search Condition, Target Presence, and Distractor Presence
| Mean RT | Accuracy | Scaled RT | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Condition | Target | Distractor | Younger | Older | Younger | Older | Younger | Older |
| Constant | Abs | Abs | 743 (112) | 804 (123) | 0.960 (0.046) | 0.943 (0.069) | −0.059 (0.218) | −0.197 (0.225) |
| Constant | Abs | Pres | 774 (155) | 900 (169) | 0.960 (0.039) | 0.905 (0.113) | 0.059 (0.211) | 0.166 (0.338) |
| Constant | Pres | Abs | 729 (142) | 829 (124) | 0.954 (0.049) | 0.933 (0.072) | −0.123 (0.243) | −0.165 (0.210) |
| Constant | Pres | Pres | 752 (154) | 906 (161) | 0.927 (0.059) | 0.897 (0.101) | −0.060 (0.250) | 0.191 (0.356) |
| Varied | Abs | Abs | 761 (126) | 832 (140) | 0.964 (0.039) | 0.913 (0.079) | −0.012 (0.174) | −0.094 (0.258) |
| Varied | Abs | Pres | 793 (141) | 922 (156) | 0.947 (0.039) | 0.892 (0.097) | 0.211 (0.299) | 0.352 (0.321) |
| Varied | Pres | Abs | 756 (139) | 836 (127) | 0.931 (0.060) | 0.919 (0.045) | −0.020 (0.243) | −0.113 (0.169) |
| Varied | Pres | Pres | 781 (134) | 903 (166) | 0.942 (0.045) | 0.905 (0.067) | 0.109 (0.230) | 0.170 (0.246) |
Note. n = 21 for each age group; values are means, with standard deviations in parentheses; RT = reaction time; Abs = Absent; Pres = Present; mean RT values are milliseconds, for correct responses; Accuracy = percentage of correct responses; Scaled RT = standardized reaction time, for each participant, divided by accuracy (see Methods).
From the first-level SPM models (for both the event-related and sustained data), we used the MarsBar toolbox to extract the average fMRI activation effect size (beta value), at the individual participant level, for each task condition, across all voxels within each of the eight ROIs. We conducted the hypothesis testing with the ROI beta values, using multivariate and univariate analysis of variance (ANOVA) and linear regression. Statistical methods are described further in Results.
Additional SPM analyses with a voxelwise approach confirmed that these eight ROIs captured areas of task-relevant activation. To estimate the overall pattern of task-related activation, we entered the first-level SPM contrast images for each of the eight task conditions, for each participant, in a second-level analysis with participants as a random effect. The contrast compared all task conditions to the implicit baseline (p < 0.0001, FWE corrected with an extent threshold of 1,000 voxels), using age group as a covariate of no interest. The results of this contrast (Figure 3) yielded widespread activation in the dorsal frontal and parietal regions used as the basis for the selected ROIs. When we superimposed the 8 mm-diameter ROI spheres (Figure 2) on the overall task-related activation (Figure 3), the FEF, SPL, and IPS ROIs all exhibited > 96% overlap with the SPM activation, and the MFG ROIs exhibited 73% overlap.
Figure 3.
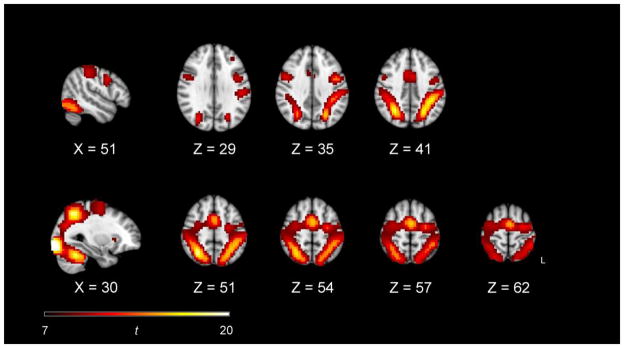
Voxelwise SPM analysis of the overall pattern of task-related activation, for both age groups combined. The contrast compared all eight task conditions to the implicit baseline (p < 0.0001, FWE corrected with an extent threshold of 1,000 voxels), using age group as a covariate of no interest.
Voxelwise SPM analyses of task-related and age group effects, described in Supplementary Material, also confirmed that activation occurred within frontoparietal regions, consistent with our ROI analyses reported in the following section (fMRI Results). Specifically, these voxelwise analyses detected activation for distractor presence (relative to distractor absence), for target presence (relative to target absence), and for older adults (relative to younger adults). The voxelwise analyses did not detect any regions of decreased activation for older adults relative to younger adults.
Statistical Power
With 21 participants in each of the two age groups, power was 0.89 to detect within-subjects (task condition) main effects and associated interactions, with an ANOVA effect size (f) of 0.25, at an alpha level of p < .05 (Faul et al., 2007). Power was 0.84 to detect age group main effects with an effect size of 0.35, at an alpha level of p < .05. Using Cohen’s (1988) effect size guidelines of f = 0.10 as small, f = 0.25 as medium, and f = 0.40 as large, our sample size was sufficient to detect medium effect sizes.
Results
Visual search performance
Individual trials on which one of the assigned response keys was not pressed within 3000 ms were eliminated from the RT analyses. These failures to respond, as a percentage of all trials, did not differ significantly for younger adults (2.10%) relative to older adults (0.95%). Three additional trials (one for an older adult, two for younger adults), with responses < 200 ms were also eliminated. For each participant, we obtained the median RT for correct responses in each task condition, and the means of these values, across participants, are presented in Table 3. Mean accuracy was high overall, but was higher for younger adults (94.81%) than for older adults (91.34%), F(1, 40) = 7.20, p < .01. In addition, across the eight task conditions listed in Table 3, RT was unrelated to accuracy for younger adults (r = −0.064), but RT and accuracy were highly related for older adults (r = −0.922). Thus, whereas younger adults maintained a relatively constant level of accuracy across the task conditions, older adults exhibited slower responses as accuracy decreased. That is, older adults did not exhibit a speed-accuracy tradeoff, but rather responded more slowly in those conditions in which they made more errors.
In addition to the age-related differences in accuracy, mean RT was 105 ms higher for older adults than for younger adults, which complicates age group comparisons because statistical interactions between age group and task condition may reflect a proportional age-related increase in RT rather than a specific effect of the task conditions (i.e., generalized slowing; Salthouse, 1985; Salthouse and Madden, 2007). To take into account generalized slowing as well as the age-related differences in accuracy, for analyzing visual search performance, we used a scaled RT measure that both standardized RT across participants and adjusted for accuracy. First, for each participant, across the complete distribution of correct responses within the 320 total trials, we recoded each RT value as a standardized z value, using the mean and standard deviation of the participant’s distribution (Faust et al., 1999). We then obtained the mean z value across trials within each task condition, for each participant, and adjusted this standardized RT measure (mean z) for accuracy by either dividing by accuracy (for positive values) or multiplying by accuracy (for negative values). Tsvetanov (2013) used a similar approach, based on methods developed by Townsend and Ashby (1983) and Horowitz and Wolfe (2003). The RT standardization provides a common metric for the two age groups, as the accuracy adjustment moves the z value higher (i.e., towards slower responding) when accuracy decreases. For example, for two identical RT values, the one associated with lower accuracy will be adjusted upward. The mean scaled RT values are presented in Table 3.
A univariate ANOVA of the scaled RT values included age group (younger, older) as a between-subjects variable; search condition (constant, varied), target status (present, absent), and distractor status (present, absent) were within-subjects variables. The ANOVA yielded significant main effects for age group, F(1, 40) = 5.51, p < .05, search condition, F(1, 40) = 9.04, p < .01, and distractor presence, F(1, 40) = 97.14, p < .001. These main effects represented higher scaled RT values for older adults relative to younger adults,1 for the varied condition relative to the constant condition, and for the presence of the color singleton distractor relative to its absence.
The only interaction that was significant in the scaled RT data was the Age Group x Distractor effect, F(1, 40) = 20.69, p < .0001 (Figure 4). This interaction represents an age-related increase in attentional capture by the color singleton: The increase in scaled RT for distractor-present trials, as compared to distractor-absent trials, was greater for older adults than for younger adults. The effect of distractor presence was significant for both age groups, F(1, 40) = 36.0, p < .001, in each case, and the age-related difference in the distractor effect remained significant when covaried for best-corrected visual acuity (Table 1), F(1, 39) = 17.29, p < .001.
Figure 4.
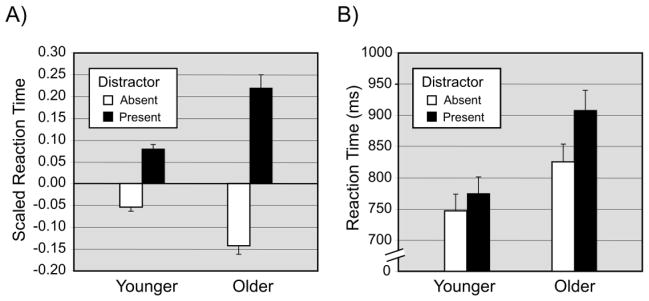
The Age Group x Distractor Presence interaction in visual search performance. Panel A = Scaled reaction time (RT). For each participant, RT was scaled by first standardizing to the mean of all correct responses (for that participant) and then adjusting for accuracy. Illustrated values are mean scaled RT values across participants (+1 SE). Panel B = untransformed, mean RT. For comparison, the Age Group x Distractor Presence interaction is also presented in mean RT (+1 SE), untransformed. For both the scaled and untransformed RT data, the increased slowing of responses associated with the presence of the singleton distractor, relative to its absence, was greater in magnitude for older adults than for younger adults.
fmRI Results
Event-related activation
We analyzed the event-related activation in a multivariate analysis of variance (MANOVA), with age group as a between-subjects independent variable, and search condition, target presence, and distractor presence as within-subjects independent variables. The eight ROIs were multiple dependent variables. Univariate tests were conducted when the corresponding multivariate effect was significant. The univariate tests were Bonferroni-corrected by the number of ROIs (eight).
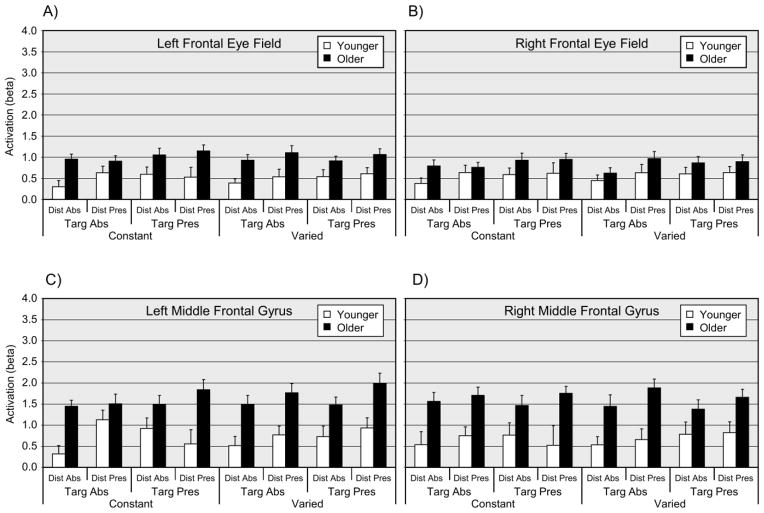
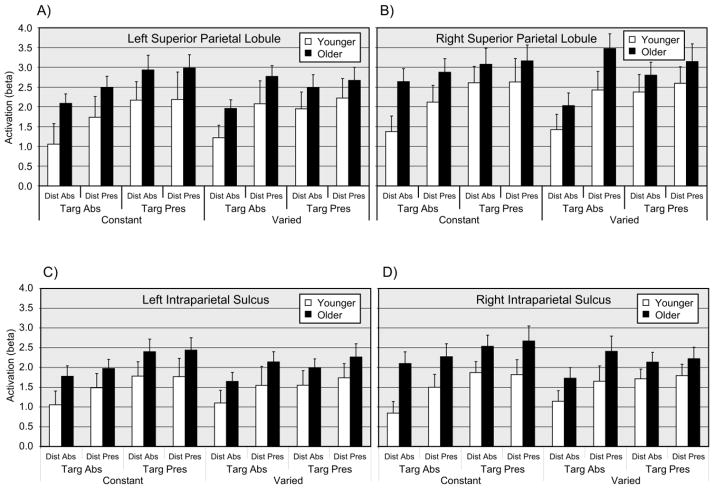
The MANOVA main effect of age group was significant, F(8, 33) = 3.83, p < .01, with corresponding univariate tests significant for the left MFG, right MFG, and left FEF, with F(1, 40) > 11.0, p < .05 (corrected) in each case. For all three ROIs with significant univariate age group effects, mean activation was higher for older adults than for younger adults (Figure 5). The MANOVA main effect of target presence was significant, F(8, 33) = 4.69, p < .01, with corresponding univariate effects significant for the left SPL, right SPL, left IPS, and right IPS, with F(1, 40) > 9.0, p < .05 (corrected) in each case (Figure 6). The MANOVA main effect of distractor presence was significant, F(8, 33) = 3.47, p < .01, (Figure 6), with the corresponding univariate test significant for the right SPL, F(1, 40) = 11.53, p < .05 (corrected). For both the target presence and distractor presence effects, the univariate effects reflected relatively higher activation for the presence of either a target or a distractor. No interaction terms were significant in the MANOVA analysis.2
Figure 5.
Event-related activation (beta values) for frontal regions of interest, as a function of age group, search condition, target presence, and distractor presence. Panel A = Left frontal eye field; Panel B = Right frontal eye field; Panel C = Left middle frontal gyrus; Panel D = Right middle frontal gyrus. Dist = Distractor; Targ = Target; Abs = Absent; Pres = Present. Values are means (+1 SE).
Figure 6.
Event-related activation (beta values) for parietal regions of interest, as a function of age group, search condition, target presence, and distractor presence. Panel A = Left superior parietal lobule (SPL); Panel B = Right SPL; Panel C = Left intraparietal sulcus (IPS); Panel D = Right IPS. Dist = Distractor; Targ = Target; Abs = Absent; Pres = Present. Values are means (+1 SE).
Sustained activation
The first-level SPM models (see the previous section, fMRI Analyses), included a regressor representing activation sustained for the duration of the on-task period, relative to the off-task baseline, as well as the event-related regressors, in a mixed blocked/event-related design (Donaldson et al., 2001; Visscher et al., 2003). The estimated activation associated specifically with the sustained component was small in magnitude. Within each combination of age group, and constant/varied search condition, none of the ROI mean beta values for the sustained activation (Table 4) differed significantly from zero at p < .05 (corrected), with the event-related regressors included. A MANOVA of the sustained effects, with age group as a within-subjects variable, search condition as a within-subjects variable, and the eight ROIs as dependent variables, did not yield any significant effects.
Table 4.
Sustained Activation as a Function of Age Group, Search Condition, and Region of Interest
| Younger | Older | |||
|---|---|---|---|---|
|
| ||||
| Constant | Varied | Constant | Varied | |
| Left MFG | 0.078 (0.606) | 0.103 (0.432) | 0.143 (0.720) | −0.013 (0.508) |
| Right MFG | −0.029 (0.364) | 0.024 (0.488) | 0.010 (0.341) | 0.039 (0.456) |
| Left FEF | 0.019 (0.287) | −0.027 (0.284) | 0.091 (0.527) | 0.036 (0.279) |
| Right FEF | −0.069 (0.244) | −0.095 (0.304) | 0.082 (0.321) | 0.042 (0.309) |
| Left SPL | −0.160 (0.767) | −0.089 (0.466) | 0.004 (0.513) | 0.111 (0.492) |
| Right SPL | −0.136 (0.790) | −0.002 (0.477) | 0.077 (0.701) | 0.234 (0.763) |
| Left IPS | −0.192 (0.630) | −0.152 (0.314) | −0.082 (0.509) | 0.028 (0.513) |
| Right IPS | −0.137 (0.504) | −0.095 (0.353) | −0.090 (0.581) | 0.023 (0.553) |
Note. n = 21 for each age group; data are mean beta values, with standard deviations in parentheses. MFG = middle frontal gyrus; FEF = frontal eye field; SPL = superior parietal lobule; IPS = intraparietal sulcus.
The mixed blocked/event-related design assumes that the raw time series fMRI data have a boxcar shape in which the off-task periods have a low-amplitude signal that is relatively constant across time (Figure 1, Panel B). To test this assumption, we conducted further analyses of the fMRI blood oxygen-level dependent (BOLD) signal across the time course of the imaging volumes (TRs) within each imaging run (Supplementary Material). These analyses suggested that the data did not conform well to the assumptions of the mixed design model. Specifically, the off-task period signal varied significantly over the TRs and was not substantially lower than the on-task period signal, even when the initial five TRs of the off-task periods were removed to reduce transient signal fluctuations.
Correlation between event-related activation and search performance
The analyses of the behavioral data (see the previous section, Visual search performance) demonstrated that the presence of the color distractor led to a greater slowing of search performance for older adults than for younger adults. This age-related difference in attentional capture, in the RT data, did not interact with either search condition or target presence. We thus calculated Pearson r correlations for the relation between mean activation in each ROI, and scaled RT, for each combination of age group and distractor presence, as well as for both age groups combined. The alpha level p = 0.05 was Bonferroni-corrected within each level of distractor presence by the number of ROIs (eight).
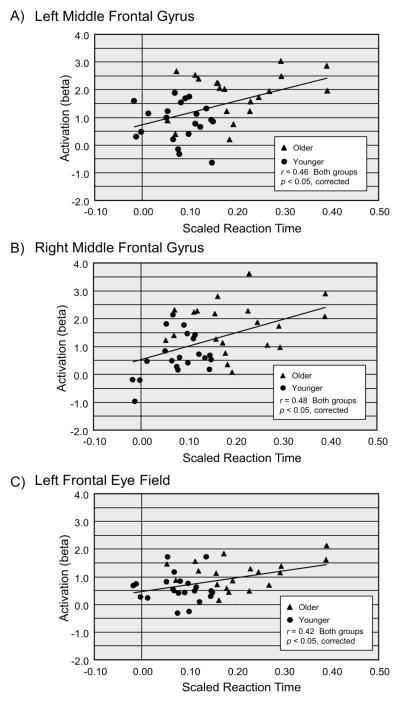
Preliminary analyses indicated the presence of one outlier, with a studentized residual of 4.98, in the correlation data, which was removed. For both age groups combined, the correlation between activation and scaled RT was significant at p < 0.05 (corrected), on distractor-present trials, for the MFG bilaterally, and for the left FEF (Table 5). For both age groups combined, comparison of the correlations for the distractor-present and distractor-absent trials, with Steiger’s Z (1980), demonstrated that the activation-RT correlation was significantly higher for distractor presence, relative to distractor absence, for the left MFG, Z = 2.82, p < .005, right MFG, Z = 2.76, p < .006, and left FEF, Z = 4.65, p < .001. All correlations were positive, indicating that, in the presence of a distractor, increasing scaled RT was associated with increasing activation. Within each of these three ROIs, we compared the two age groups’ activation-RT correlations, for the distractor-present trials, with the Fisher r to z transformation. None of these age group comparisons, however, was significant, and as is evident in Figure 7 the two age groups’ data comprise a single linear function. Exploratory analyses did not yield any reliable modulation of these correlations by target presence or search condition.
Table 5.
Correlation between Event-Related Activation and Scaled Reaction Time, as a Function of Distractor Presence and Region of Interest
| Distractor
|
||
|---|---|---|
| Absent | Present | |
| Left MFG | −0.158 | 0.465* |
| Right MFG | −0.125 | 0.481* |
| Left FEF | −0.169 | 0.421* |
| Right FEF | −0.182 | 0.316 |
| Left SPL | −0.159 | 0.393 |
| Right SPL | −0.037 | 0.160 |
| Left IPS | −0.183 | 0.336 |
| Right IPS | −0.169 | 0.369 |
Note. n = 21 younger adults and 20 older adults, following removal of one outlier (see Results). Values are Pearson r correlations. MFG = middle frontal gyrus; FEF = frontal eye field; SPL = superior parietal lobule; IPS = intraparietal sulcus.
p < .05, corrected
Figure 7.
Correlation between event-related activation (beta values) and visual search performance, on distractor-present trials. Panel A = left middle frontal gyrus (MFG); Panel B = right MFG; Panel C = left frontal eye field (FEF). Scaled reaction time (RT) is standardized at the individual participant level and adjusted for accuracy (see Methods).
Mediation of search performance
For the three regions exhibiting a significant correlation between event-related activation and attentional capture (Figure 7), we examined the degree to which individual differences in activation accounted for age-related variance of scaled RT on distractor-present trials. Because years of age was not distributed continuously, we used contrast coding (-1, 1) to represent the difference between the two age groups. Considered separately, the age group variable accounted for 39.20% of the variance in scaled RT on distractor-present trials (Table 6, Model 1). Activation in the three prefrontal ROIs from Figure 7 shared a moderate amount of the age-related variance in this scaled RT measure. When left MFG activation was entered before age group, the variance associated specifically with age decreased (i.e., attenuated) by 50.0%, indicating shared variance between left MFG activation and age, though age group remained a significant predictor (Table 6, Model 2). Similar regression models, conducted separately for the activation in the right MFG and left FEF (Table 6, Models 3 and 4), demonstrated that entering either of these variables before age group in the regression model attenuated the age-related variance in scaled RT by 52% and 38%, respectively, though age group remained a significant predictor in each case.
Table 6.
Hierarchical Regression Analyses of Scaled RT on Distractor-Present Trials
| B | SE B | F | Δr2 | r2 | Attenuation | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Age Group | −0.06 | 0.012 | 25.10*** | 0.392 | 0.392 | |
| Model 2 | ||||||
| Left MFG | 0.018 | 0.016 | 1.33 | 0.216 | 0.216 | |
| Age Group | −0.051 | 0.014 | 12.68** | 0.196 | 0.412 | 50.00 |
| Model 3 | ||||||
| Right MFG | 0.020 | 0.015 | 1.86 | 0.232 | 0.232 | |
| Age Group | −0.049 | 0.014 | 12.35** | 0.188 | 0.420 | 52.04 |
| Model 4 | ||||||
| Left FEF | 0.031 | 0.023 | 1.75 | 0.177 | 0.177 | |
| Age Group | −0.052 | 0.013 | 15.77*** | 0.241 | 0.418 | 38.52 |
Note. n = 21 younger adults and 20 older adults, following removal of one outlier (see Results). For each model, the dependent variable is scaled RT (standardized RT adjusted for accuracy) on the distractor-present trials. B = regression model parameter estimate for predictor variable; SE B = standard error of parameter estimate; F = F value for parameter estimate; Δr2 = unique effect of predictor; r2 = cumulative r2 for current and preceding steps; Attenuation = percentage attenuation of age-related variance (r2 for age group alone minus r2 for age group following predictor) / (r2 for age group alone). Predictor variables are beta values for activation within regions. MFG = middle frontal gyrus; FEF = frontal eye field.
p < 0.05;
p < 0.01;
p < .0001.
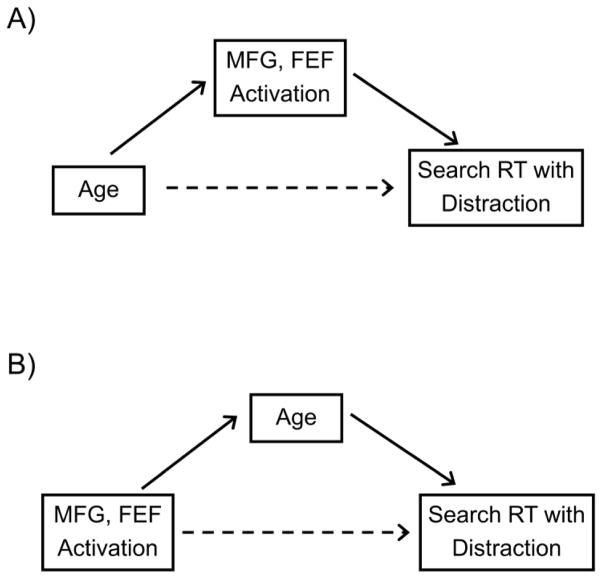
We used mediation analyses to determine whether the shared variance between age and prefrontal activation, in scaled RT on distractor-present trials, represents a specifically mediating effect, in the sense that the relation between two variables is not entirely direct but instead influenced (i.e., mediated) by the effect of a third variable (Baron and Kenny, 1986; Mackinnon and Fairchild, 2009; Preacher and Hayes, 2008; Salthouse, 1992a). We investigated two models of the relations among the variables (Salthouse, 2011), one in which activation is a mediator of the relation between age and search performance (Figure 8, Panel A), and an alternative model in which age is a mediator of the relation between activation and performance (Figure 8, Panel B). We used bootstrap analyses (Mackinnon and Fairchild, 2009; Preacher and Hayes, 2008) based on a resampling procedure to obtain a point estimate of the beta value for the mediating effect. The original data were resampled 20,000 times, with replacement, providing a sampling distribution (and 95% confidence interval) of the estimated beta for the mediating effect. Thus, mediation was considered to be significant when the confidence intervals for the parameter estimate of the mediator variable did not include zero.
Figure 8.
Alternative models of the relation among age, activation in either left or right middle frontal gyrus (MFG) or left frontal eye field (FEF) ROIs, and visual search reaction time (RT) on distractor-present trials. Panel A = Activation as a mediator of the relation between age and visual search performance. Panel B = Age as a mediator of the relation between activation and visual search performance. The solid arrows represent an indirect effect between variables through a mediator, and the dotted arrows represents a direct effect, controlling for the mediator variable.
The bootstrap results did not support the model in which activation is a mediator of the age-RT relation (Figure 8, Panel A). The confidence intervals associated with the parameter estimates for both MFG ROIs, and the left FEF, all included zero (Table 7, Models 1–3). The tests of the alternative model, however, were significant for all three of the ROIs (Table 7, Models 4–6) and support a model in which age is the mediator between activation and RT on the distractor-present trials (Figure 8, Panel B).
Table 7.
Mediation Analyses of Scaled RT on Distractor-Present Trials
| PE | SE | Lower CI | Upper CI | |
|---|---|---|---|---|
| Event-related Activation as mediator
| ||||
| Model 1: Age → Left MFG → Scaled RT | −0.009 | 0.009 | −0.031 | 0.005 |
| Model 2: Age → Right MFG → Scaled RT | −0.010 | 0.008 | −0.033 | 0.001 |
| Model 3: Age → Left FEF → Scaled RT | −0.008 | 0.008 | −0.032 | 0.002 |
|
| ||||
| Age as mediator
| ||||
| Model 4: Left MFG → Age → Scaled RT | 0.031 | 0.011 | 0.014 | 0.054* |
| Model 5: Right MFG → Age → Scaled RT | 0.028 | 0.008 | 0.016 | 0.047* |
| Model 6: Left FEF → Age → Scaled RT | 0.041 | 0.015 | 0.015 | 0.073* |
Note. n = 21 younger adults and 20 older adults, following removal of one outlier (see Results). For each regression model, the outcome variable was scaled reaction time for distractor-present trials. Potential mediator variable listed in bold font. PE = point estimate of regression coefficient for the mediator variable, defined as the mean of the estimated regression coefficients for the indirect (mediating) effect, across 20,000 bootstrap samples; SE = bootstrap derived estimate of the standard error of the indirect effect point estimate. Lower CI / Upper CI = lower / upper bounds of 95% bias-corrected and accelerated bootstrap confidence interval for indirect effect. MFG = middle frontal gyrus; FEF = frontal eye field.
p < .05 for regression coefficient of mediator variable, as defined by 95% confidence interval that does not include 0.
Discussion
Visual search performance
At the behavioral level, we extend previous findings by demonstrating a contribution of top-down attention to feature search (shape singleton detection) for both older and younger adults, although top-down attention was not successful in preventing attentional capture from a salient distractor for either age group. The Age Group x Distractor interaction for scaled RT (Figure 4) confirmed our prediction that attentional capture by a salient distractor was greater for older adults than for younger adults, consistent with previous reports of age-related decline in inhibitory functioning (Hasher and Zacks, 1988; Healey et al., 2008; Lustig et al., 2007; Rabbitt, 1979). Previous studies of age-related differences in attentional capture, however, have used tasks with more complex attentional demands, in which the distractor is a response-incompatible target in an irrelevant display location (Maylor and Lavie, 1998; Porter et al., 2012), or the response is a two-choice visual discrimination combined with target detection (Pratt and Bellomo, 1999; Whiting et al., 2007). In the present experiment, we used a highly efficient, feature search task requiring a target present/absent response regarding a shape singleton (i.e., target detection). Our results thus provide novel evidence that visual salience per se is a critical component of the age-related increase in attentional capture, even when perceptual load is low and the distractor is defined as an irrelevant item to be ignored. The age-related difference in search performance remained significant when covaried for acuity, suggesting that this age-related effect does not reflect a deficit in the ability to discriminate target and distractor items visually but instead reflects the allocation of attention to the salient distractor.
The behavioral data also support our hypothesis of age-related preservation of top-down attention. The predictability of the target, in the constant search condition, led to a decrease in scaled RT, relative to the varied search condition, which did not vary significantly across the age groups. This pattern is consistent with several previous findings in visual search tasks, indicating that older adults use some forms of top-down attention as efficiently as younger adults (Humphrey and Kramer, 1997; Madden, 1987, 2007; Madden et al., 2004; McAvinue et al., 2012; Whiting et al., 2005). Target detection in this feature search task required the relatively simple recognition that one different shape was present among four other homogeneous shapes. The improved search performance in the constant condition confirms that, even within a feature search task with minimal attentional demands, top-down attention can facilitate target detection (Bravo and Nakayama, 1992; Wolfe et al., 2003). The constant search condition includes more repetition priming of target features, relative to the varied condition, which would also allow a greater contribution from bottom-up attentional guidance in the constant condition (Geyer et al., 2010; Kristjansson et al., 2002; Maljkovic and Nakayama, 1994). Target feature priming, however, would contribute more to target-present trials than to target-absent trials, and the improvement in search performance in the constant condition did not vary significantly in relation to target presence, as predicted by a priming explanation. Assuming that target detection occurs when the activation of target-relevant features, within the internal representation of the display (i.e., target template), exceeds a threshold value (Eckstein, 2011; Wolfe, 1998, 2007), the age-constancy of the search condition effect indicates that top-down attentional guidance within the target template is available to older as well as younger adults.
Finally, the behavioral results demonstrate that the beneficial effects of target predictability do not include protection from attentional capture by a salient distractor. Contrary to our initial hypothesis, top-down attention was not associated with a reduction in attentional capture for either age group. Bacon and Egeth (1994) and Leber and Egeth (2006) have reported that, in some forms of feature search, target predictability may enable a singleton detection mode, based on top-down attention, that can override attentional capture. Theeuwes and colleagues, however, have proposed that attentional capture is determined entirely by the bottom-up variables associated with visual salience (Theeuwes, 2004, 2010; Theeuwes et al., 2006). In the present form of feature search for a shape singleton, with homogeneous distractors and separation of target and distractor sets, target predictability leads to enhanced guidance within the target template, but consistent with the findings of Theeuwes and colleagues, this contribution of top-down attention does not override the disruption of performance associated with a salient color singleton.
fMRI activation
The fMRI activation varied significantly as a function of both age group and visual search performance. From two previous studies of visual search (de Fockert et al., 2004; Shulman et al., 2003), we selected eight frontoparietal ROIs (comprising MFG, FEF, SPL, and IPS, bilaterally). These ROIs were relevant for target detection, attentional capture by a salient distractor, and top-down attentional control, as well as for age-related differences in visual attention (Dennis and Cabeza, 2008; Grady et al., 2010; Madden et al., 2005; Nielson et al., 2002). Consistent with previous findings, these data yielded a significant age-related increase in frontoparietal activation, specifically MFG and FEF, and a corresponding trend was also evident in the parietal regions (Figures 5 and 6). We had expected, however, that both attentional capture from a salient distractor and top-down attention from target predictability would modulate this age-related difference in activation, which did not occur. Although both the presence of a salient distractor and the availability of a predictable target influenced the efficiency of feature search performance, the age-related increase in activation was independent of these variables. Thus, we isolated an age-related effect of attentional capture at the behavioral level but not at the neural level.
Two considerations are relevant to the interpretation of the age-related increase in activation. First, previous experiments linking older adults’ increased activation to attentional capture and top-down guidance (Madden et al., 2007b; Schmitz et al., 2010) used behavioral tasks with more complex perceptual discrimination than the feature search task in the present study. Although decreasing perceptual load appears to enhance the magnitude of older adults’ attentional capture at the behavioral level (Maylor and Lavie, 1998), increased complexity of the perceptual discrimination may be needed to detect the interaction of age with top-down attention and visual salience in activation data. Second, the age-related interaction in activation may depend on a task context in which sensory level or bottom-up processing is demonstrably lower for older adults, triggering a compensatory increase in activation (Davis et al., 2008; Schneider and Pichora-Fuller, 2000). Older adults in this study exhibited typical age-related decline in visual acuity and perceptual-motor speed (Table 1), but the visual displays were high contrast shapes presented well above threshold (250 ms), and whole-brain, voxelwise analyses (Supplementary Material) did not detect significant age-related decline in activation in visual sensory regions.
The increases in activation associated with both target presence (IPS and SPL, bilaterally) and distractor presence (right SPL) were independent of age group and target predictability (Figure 6). These parietal regions are frequently activated during visual search, particularly in response to abrupt sensory changes in the display, which in turn lead to attentional shifts within or across feature dimensions (Liu et al., 2003; Wei et al., 2011; Yantis et al., 2002). The IPS and SPL are also associated with various aspects of top-down attentional control (Egner et al., 2008; Pollmann et al., 2003; Shulman et al., 2003; Vossel et al., 2013), but the absence of a target predictability effect in these data indicate a greater contribution from the sensory-level aspects of parietal activation.
The location of the distractor-related SPL activation obtained here is consistent with that obtained by de Fockert et al. (2004), who found that color singletons were associated with bilateral SPL activation. The de Fockert et al. report also highlighted a correlation between left prefrontal cortex activation (at the location corresponding to our MFG ROI) and a RT measure of attentional capture by a color singleton distractor. The de Fockert et al. correlation was negative, with increasing MFG activation associated with decreased attentional capture, which these authors interpreted as representing top-down attentional control of task-irrelevant information. The present analyses also revealed an association between attentional capture and activation, in this case a correlation between scaled RT and three frontal ROIs, for both age groups combined, which was specific to the distractor-present trials (Table 5 and Figure 7). Our correlations, however, were positive, reflecting increased prefrontal activation as scaled RT increased on distractor-present trials, which likely represents the different type of search decision in this task relative to that of de Fockert et al.3 Our findings suggest that the distractor-related activation represents an increase in the number or complexity of information processing operations (task difficulty; Anderson et al., 2007; Donner et al., 2002), rather than the successful implementation of top-down attentional control (de Fockert et al., 2004). More generally, the independence of the age-related and distractor-related activation suggests that, at least in the context of low perceptual load, age-related increases in frontoparietal activation do not necessarily signal a specific compensatory attentional strategy.
The absence of any significant effects in the sustained activation (Table 4) was surprising. Madden et al. (2007b) reported an age-related increase in sustained, frontoparietal activation, during letter search with color singleton distractors, although the effect did not vary significantly across task conditions and was not entirely independent of the event-related activation. The perceptual load was lower in the present form of feature search than in the letter-search task used by Madden et al. (2007b), and this feature search task may not have required substantial allocation of sustained attention. In addition, the fMRI BOLD time series data did not exhibit the boxcar shape necessary to accurately model separate estimates of sustained and transient activity (Supplementary Material). It is possible that our off-task periods of 19.5 s were not of sufficient duration to yield stable, low-level BOLD activity throughout the off-task period. Thus, the event-related regressors may have captured the majority of the task-related variance.
The default assumption of neuroimaging studies is that brain-related variables are mediators of the relation between age and cognitive performance, but this assumption should be tested empirically (Salthouse, 2011). The mediation analyses enabled us to distinguish alternative models of the relations among age, search performance, and regional activation. The initial regression analyses of scaled RT on the distractor-present trials indicated that individual differences in activation, for each of three ROIs (left MFG, right MFG and left FEF), accounted for 38–52% of the age-related variance in scaled RT (Table 6), suggesting that frontal activation may have a causal or mediating influence on the age-related increase in attentional capture by the color singleton. When, however, we estimated the beta values for mediating effects with a resampling procedure (Mackinnon and Fairchild, 2009; Preacher and Hayes, 2008), and compared different arrangements of the mediating variables, a different conclusion emerged. For all three frontal ROIs, these analyses support a model in which age is a significant mediator of the relation between activation and performance on distractor-present trials (Table 7). The alternative model, with activation as a mediator of the age-RT relation, was not significant for any of the ROIs. This pattern is illustrated in Figure 7: Although the relation between activation and RT holds for both age groups combined, the relation is not significant within either age group, and the pattern relies on the age-related increase in both mean RT and mean activation. Thus, although activation shares age-related variance in RT, which is specific to the distractor-present trials, the relation between activation and RT is indirect and occurs because both frontal activation and RT increase with age. Years of age, in this context, represents not only the passage of time but also the combined influence of many unmeasured variables in the sensory/motor and central nervous systems, for which age is a proxy (Bishop et al., 2010; Heringa et al., 2014).
Limitations
The present form of top-down attentional guidance, target predictability, was associated with improved performance in visual feature search, but we did not detect any effects of target predictability in the activation data. Although increased activation was evident for target presence and distractor presence, and statistical power was sufficient to detect medium-sized effects, the analyses did not reveal significant effects in either event-related or sustained activation for top-down attention (constant vs. varied search). Larger sample sizes may be needed to detect the pattern of neural activity representing top-down attentional control in this form of feature search. Similarly, age-related differences in attentional capture were clearly evident in the behavioral data but no age-related interactions were significant in the activation data, and larger sample sizes with a more continuous distribution of age would provide additional power to detect smaller effects.
Conclusion
In a highly efficient version of feature search (detection of a shape singleton target), both younger and older adults were able to use top-down attention (target predictability) to improve performance. A salient but task-irrelevant distractor, a color singleton, disrupted performance for both age groups, but particularly older adults, and top-down attention did not protect either age group from this form of attentional capture. The occurrence of either a target shape or a color singleton distractor was associated with increased activation in parietal ROIs (IPS and SPL), for both age groups, whereas frontal ROIs (MFG and FEF) exhibited higher activation for older adults than for younger adults. On trials with a color singleton distractor, search performance declined as a function of increasing activation in three frontal ROIs (left MFG, right MFG and left FEF). This pattern suggests that the age-related increase in activation represents increased task difficulty, in the presence of a salient distractor, rather than a compensatory response. Mediational analyses, however, did not support a model in which the frontal activation was a mediator of the relation between age and distraction. Rather, age was a mediating variable: Both frontal activation and the time required to detect a target, when a salient distractor occurs, increase with adult age.
Supplementary Material
Acknowledgments
This research was supported by NIH research grant R01 AG039684 (DJM), R01 AG034138 (MTD), K23 MH087741 (GGP), R01 NS074045 (NKC), and R01 AG019731 (RC). We are grateful to Sally Cocjin, David Hoagey, Chris Petty, Matt Costello, Anne Shepler, and the members of the Brain Imaging and Analysis Center (Allen Song, Director), for their assistance and support. A preliminary version of these findings was presented at the Third International Conference on Visual Search and Selective Attention (VSSA III), Munich, Germany, July 2012.
Appendix A. Supplementary Data
Supplementary data to this article can be found online at http:// [to be added].
Footnotes
Note that although the mean of each participant’s standardized RT distribution is zero, there is no mathematical requirement that the mean of the final, scaled RT values would be exactly zero for either age group, or that the mean scaled RT, across the task conditions, would be equal for the two age groups. The shape of the RT distribution will differ across participants, as will the mapping between the individual trials for each of the eight task conditions and their respective locations on the distribution. The final, scaled RT values are also affected by the accuracy adjustment. Additional analyses of these data demonstrated that the overall pattern of results is very similar if just the standardized RTs are analyzed, without the accuracy adjustment, or if other methods for correcting for generalized slowing are used, such as the logarithmic transformation or proportional change scores (Faust et al., 1999). We find the scaled RT measure to be useful in this context because it corrects for generalized slowing while combining the influences of RT and accuracy into a single measure, facilitating the comparison between participant groups.
The activation effects were not entirely attributable to time on task (Grinband et al., 2011). Note that the main effect of target presence was not significant in the analysis of scaled RT (previous section, Visual search performance), although activation was reliably higher for target-present trials than for target-absent trials in both IPS and SPL bilaterally. The age group main effect in activation did correspond to the age group main effect in scaled RT, with older adults exhibiting both higher mean activation and higher scaled RT. In additional analyses, however, we found that within each age group, no correlation between mean scaled RT and mean activation was significant for any ROI, even at a more liberal threshold of p < .05, uncorrected for multiple comparisons.
Specifically, in the de Fockert et al. (2004) task, each display contained a shape singleton target (a circle among four diamonds), and participants made a two-choice response regarding the orientation of a line (horizontal/vertical) contained within the target shape. Thus, the de Fockert et al. task required an initial (covert) identification of the target shape and then the (overt) two-choice discrimination of the line located within the shape. This is an example of what Duncan (1985) referred to as compound search. The defining attribute of the target (shape) differs from the reported attribute (line orientation) defining the response. The present form of feature search, in contrast, is what Duncan referred to as simple search, in which the defining and reported attributes of the target are the same (presence vs. absence of a unique shape). Further, in the de Fockert et al. task, those trials with a color singleton (half of the trials overall) were divided equally between those on which one of the distractors was the color singleton and those on which the circle target was a color singleton. Thus, in the de Fockert et al. task, while color information was not needed for the line orientation response, color could facilitate the response when combined with the target. In the present task, the color singleton was always a distractor, never the target, and participants were instructed to ignore it. This design feature allows for the isolation of distractor and target related attentional effects.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ, McRobbie D, Kennard C. Involvement of prefrontal cortex in visual search. Exp Brain Res. 2007;180:289–302. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. Eur J Neurosci. 2006;23:591–596. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Bach M. The Freiburg Visual Acuity test automatic measurement of visual acuity. Optom Vis Sci. 1996;73:49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept Psychophys. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck depression inventory. Psychological Corporation; New York: 1978. [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo M, Nakayama JK. The role of attention in different visual-search tasks. Percept Psychophys. 1992;51:465–472. doi: 10.3758/bf03211642. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, Hasher L. Age differences in the frontoparietal cognitive control network: Implications for distractibility. Neuropsychologia. 2012;50:2212–2223. doi: 10.1016/j.neuropsychologia.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar N, Fukuda K, Bocklage A, Aurtenetxe S, Vogel EK, Gazzaley A. Prolonged disengagement from attentional capture in normal aging. Psychol Aging. 2013;28:77–86. doi: 10.1037/a0029899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci. 2006;10:131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert J, Rees G, Frith C, Lavie N. Neural correlates of attentional capture in visual search. J Cogn Neurosci. 2004;16:751–759. doi: 10.1162/089892904970762. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 1–54. [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15:16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Duncan J. Visual search and visual attention. In: Posner MI, Marin OSM, editors. Attention and performance XI. Erlbaum; Hillsdale, NJ: 1985. pp. 85–105. [Google Scholar]
- Dvorine I. Dvorine pseudo-isochromatic plates. 2. Harcourt; New York: 1963. [Google Scholar]
- Eckstein MP. Visual search: A retrospective. J Vis. 2011;11:1–36. doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- Egner T, Monti JM, Trittschuh EH, Wieneke CA, Hirsch J, Mesulam MM. Neural integration of top-down spatial and feature-based information in visual search. J Neurosci. 2008;28:6141–6151. doi: 10.1523/JNEUROSCI.1262-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychol Bull. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Geyer T, Zehetleitner M, Muller HJ. Contextual cueing of pop-out visual search: when context guides the deployment of attention. J Vis. 2010;10:20. doi: 10.1167/10.5.20. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57:303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new review. In: Bower GH, editor. The psychology of learning and motivation. Academic Press; Orlando: 1988. pp. 193–225. [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. Prog Brain Res. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, Teerlink T, Scheffer PG, van den Hurk K, Kappelle LJ, Dekker JM, Biessels GJ. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population - the Hoorn Study. Psychoneuroendocrinology. 2014;40:108–118. doi: 10.1016/j.psyneuen.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Hodsoll J, Mevorach C, Humphreys GW. Driven to less distraction: rTMS of the right parietal cortex reduces attentional capture in visual search. Cereb Cortex. 2009;19:106–114. doi: 10.1093/cercor/bhn070. [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZ, Li SC. Visual search across the life span. Dev Psychol. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Horowitz TS, Wolfe JM. Memory for rejected distractors in visual search? Vis Cogn. 2003;10:257–298. [Google Scholar]
- Humphrey DG, Kramer AF. Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychol Aging. 1997;12:704–717. doi: 10.1037//0882-7974.12.4.704. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, Theeuwes J. Age differences in the control of looking behavior: do you know where your eyes have been? Psychol Sci. 2000;11:210–217. doi: 10.1111/1467-9280.00243. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 189–249. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson A, Wang D, Nakayama K. The role of priming in conjunctive visual search. Cognition. 2002;85:37–52. doi: 10.1016/s0010-0277(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Gottlieb J, Goldberg ME. The lateral intraparietal area as a salience map: the representation of abrupt onset, stimulus motion, and task relevance. Vision Res. 2000;40:1459–1468. doi: 10.1016/s0042-6989(99)00212-6. [DOI] [PubMed] [Google Scholar]
- Leber AB, Egeth HE. It’s under control: top-down search strategies can override attentional capture. Psychon Bull Rev. 2006;13:132–138. doi: 10.3758/bf03193824. [DOI] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cereb Cortex. 2003;13:1334–1343. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Ludwig CJ, Gilchrist ID. Stimulus-driven and goal-driven control over visual selection. J Exp Psychol Hum Percept Perform. 2002;28:902–912. [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks R. Inhibitory deficit theory: Recent developments in a “new view”. In: Gorfein DS, MacLeod CM, editors. The place of inhibition in cognition. American Psychological Association; Washington, DC: 2007. pp. 145–162. [Google Scholar]
- Mackinnon DP, Fairchild AJ. Current directions in mediation analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ. Aging, attention, and the use of meaning during visual search. Cognitive Dev. 1987;2:201–216. [Google Scholar]
- Madden DJ. Aging and visual attention. Curr Dir Psychol Sci. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Allen PA. Adult age differences in visual search accuracy: attentional guidance and target detectability. Psychol Aging. 1999;14:683–694. doi: 10.1037//0882-7974.14.4.683. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Plude DJ. Selective preservation of selective attention. In: Cerella J, Rybash J, Hoyer W, Commons ML, editors. Adult information processing: Limits on loss. Academic Press; San Diego: 1993. pp. 273–300. [Google Scholar]
- Madden DJ, Spaniol J, Bucur B, Whiting WL. Age-related increase in top-down activation of visual features. Q J Exp Psychol (Colchester) 2007a;60:644–651. doi: 10.1080/17470210601154347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol Aging. 2007b;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL. Age-related changes in visual attention. In: Costa PT, Siegler IC, editors. Recent advances in psychology and aging. Elsevier; Amsterdam: 2004. pp. 41–88. [Google Scholar]
- Madden DJ, Whiting WL, Cabeza R, Huettel SA. Age-related preservation of top-down attentional guidance during visual search. Psychol Aging. 2004;19:304–309. doi: 10.1037/0882-7974.19.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA. Age-related changes in neural activity during visual perception and attention. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford University Press; Oxford: 2005. pp. 157–185. [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychol Aging. 1998;13:563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- McAvinue LP, Habekost T, Johnson KA, Kyllingsbaek S, Vangkilde S, Bundesen C, Robertson IH. Sustained attention, attentional selectivity, and attentional capacity across the lifespan. Atten Percept Psychophys. 2012;74:1570–1582. doi: 10.3758/s13414-012-0352-6. [DOI] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, Shalev L. Reflexive and preparatory selection and suppression of salient information in the right and left posterior parietal cortex. J Cogn Neurosci. 2009;21:1204–1214. doi: 10.1162/jocn.2009.21088. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychol Aging. 1989;4:98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Humphreys GW, Olivers CN, Muller K, Lohmann G, Wiggins CJ, Watson DG. Separating distractor rejection and target detection in posterior parietal cortex--an event-related fMRI study of visual marking. Neuroimage. 2003;18:310–323. doi: 10.1016/s1053-8119(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Porter G, Wright A, Tales A, Gilchrist ID. Stimulus onsets and distraction in younger and older adults. Psychol Aging. 2012;27:1111–1119. doi: 10.1037/a0028486. [DOI] [PubMed] [Google Scholar]
- Pratt J, Bellomo CN. Attentional capture in younger and older adults. Aging Neuropsychol C. 1999;6:19–31. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Some experiments and a model for changes in attentional selectivity with old age. In: Hoffmeister F, Muller C, editors. Brain function in old age: Evaluation of changes and disorders. Springer-Verlag; New York: 1979. pp. 82–94. [Google Scholar]
- Salthouse TA. Speed of behavior and its implications for cognition. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Van Nostrand Reinhold; New York: 1985. pp. 400–426. [Google Scholar]
- Salthouse TA. Mechanisms of age-cognition relations in adulthood. Erlbaum; Hillsdale: NJ: 1992a. [Google Scholar]
- Salthouse TA. What do adult age differences in the Digit Symbol Substitution Test reflect? J Gerontol. 1992b;47:121–128. doi: 10.1093/geronj/47.3.p121. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Madden DJ. Information processing speed and aging. In: Deluca J, Kalmar J, editors. Information processing speed in clinical populations. Psychology Press; New York: 2007. pp. 221–241. [Google Scholar]
- Schmitz TW, Cheng FH, De Rosa E. Failing to ignore: paradoxical neural effects of perceptual load on early attentional selection in normal aging. J Neurosci. 2010;30:14750–14758. doi: 10.1523/JNEUROSCI.2687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implication of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Erlbaum; Mahwah, NJ: 2000. pp. 155–219. [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Scialfa CT. The role of sensory factors in cognitive aging research. Can J Exp Psychol. 2002;56:153–163. doi: 10.1037/h0087393. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Corbetta M. Two cortical systems for the selection of visual stimuli. In: Posner MI, editor. Cognitive neuroscience of attention. The Guilford Press; New York, NY: 2004. pp. 114–126. [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d’Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- Theeuwes J. Top-down search strategies cannot override attentional capture. Psychon Bull Rev. 2004;11:65–70. doi: 10.3758/bf03206462. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Reimann B, Mortier K. Visual search for featural singletons: No top-down modulation, only bottom-up priming. Vis Cogn. 2006;14:466–489. [Google Scholar]
- Townsend JT, Ashby FG. The stochastic modeling of elementary psychological processes. Cambridge University Press; Cambridge, UK: 1983. [Google Scholar]
- Tsvetanov K, Mevorach C, Allen H, Humphreys G. Age-related differences in selection by visual saliency. Atten Percept Psychophys. 2013;75:1382–1394. doi: 10.3758/s13414-013-0499-9. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist. 2013 doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wecker NS, Kramer JH, Wisniewski A, Delis DC, Kaplan E. Age effects on executive ability. Neuropsychology. 2000;14:409–414. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- Wei P, Muller HJ, Pollmann S, Zhou X. Neural correlates of binding features within- or cross-dimensions in visual conjunction search: an fMRI study. Neuroimage. 2011;57:235–241. doi: 10.1016/j.neuroimage.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Whiting WL, Madden DJ, Babcock KJ. Overriding age differences in attentional capture with top-down processing. Psychol Aging. 2007;22:223–232. doi: 10.1037/0882-7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting WL, Madden DJ, Pierce TW, Allen PA. Searching from the top down: Ageing and attentional guidance during singleton detection. Q J Exp Psychol A. 2005;58:72–97. doi: 10.1080/02724980443000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting WL, Sample CH, Hagan SE. Top-down processing modulates older adults’ susceptibility to noise. Aging, Neuropsychology, and Cognition. 2014;21:370–385. doi: 10.1080/13825585.2013.826342. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. Psychology Press; East Sussex, UK: 1998. pp. 13–73. [Google Scholar]
- Wolfe JM. Guided search 4.0: Current progress with a model of visual search. In: Gray W, editor. Integrated models of visual systems. Oxford University Press; New York: 2007. pp. 99–119. [Google Scholar]
- Wolfe JM, Butcher SJ, Lee C, Hyle M. Changing your mind: on the contributions of top-down and bottom-up guidance in visual search for feature singletons. J Exp Psychol Hum Percept Perform. 2003;29:483–502. doi: 10.1037/0096-1523.29.2.483. [DOI] [PubMed] [Google Scholar]
- Yantis S. Control of visual attention. In: Pashler H, editor. Attention. Psychology Press; East Sussex, UK: 1998. pp. 223–256. [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.