Abstract
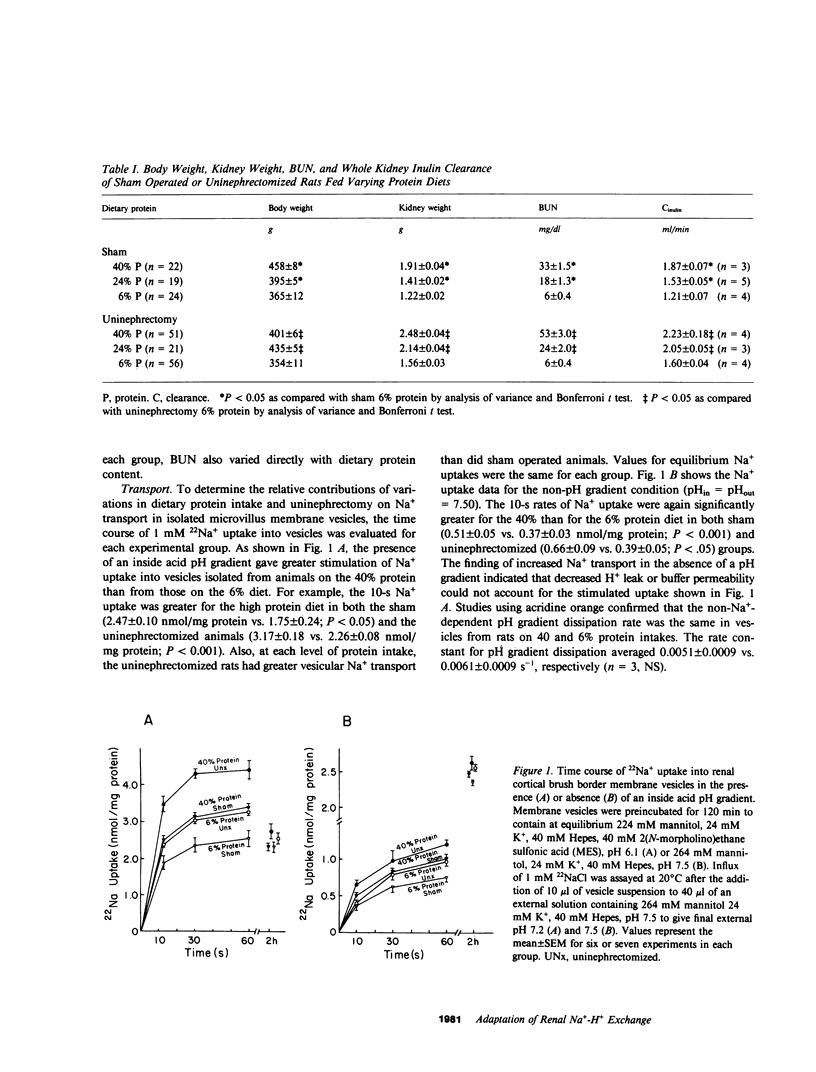
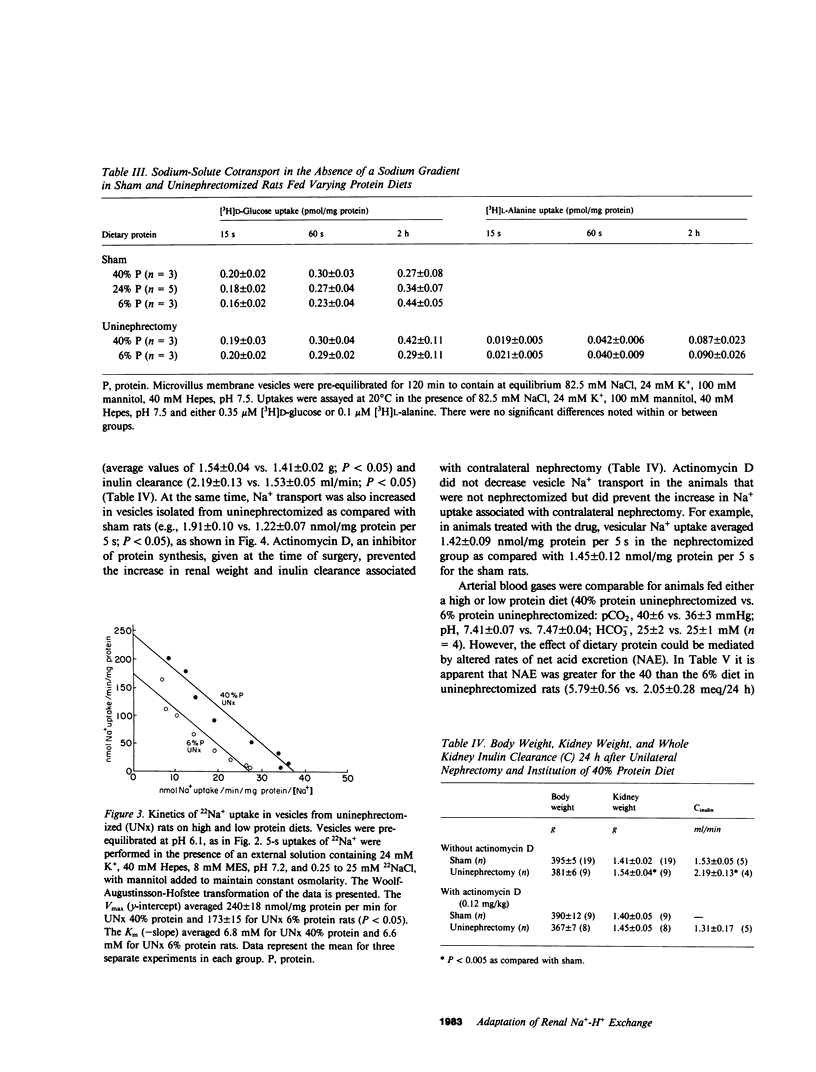
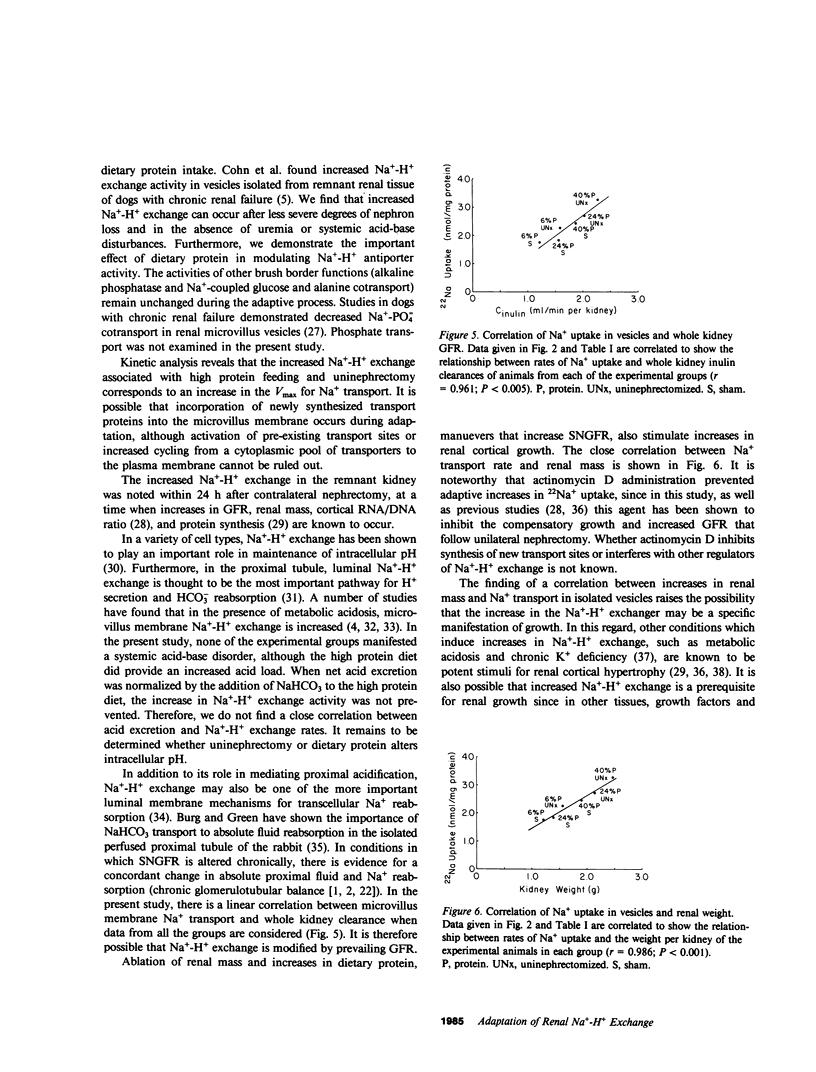
The ablation of renal mass and institution of a high protein diet both lead to renal cortical hypertrophy and increased glomerular filtration rate (GFR). We studied Na+ transport in rat microvillus membrane vesicles isolated from uninephrectomized or sham operated rats fed 6% (low), 24% (standard), or 40% (high) protein diets. The feeding of high protein, as compared with low protein, was associated with a 50% increase in rates of pH-stimulated 22Na+ transport in isolated vesicles from sham and uninephrectomized animals. Values for the standard protein diet were intermediate to values for high and low protein. At each level of dietary protein intake, vesicular Na+ transport was greater in the uninephrectomized than in sham rats. The high protein diet was also associated with increased vesicular 22Na+ flux inhibitable by 1 mM amiloride. Increases in total and amiloride sensitive flux were also noted in the absence of a pH gradient. Conductive Na+ and H+ transport were not altered, nor were sodium-glucose and sodium-alanine cotransport. Kinetic studies revealed evidence for an increased Vmax of Na+-H+ exchange in uninephrectomized animals fed a 40 vs. a 6% protein diet whereas Km was unchanged. Supplements of NaHCO3 in the 40% protein diet, to adjust for an increased rate of net acid excretion, did not prevent the increased rates of Na+-H+ exchange. However, treatment with actinomycin D (0.12 mg/kg) prevented the increased Na+-H+ activity as well as the increased renal mass and GFR noted 24 h after unilateral nephrectomy. Na+-H+ exchange rate was closely correlated with GFR (r = 0.961; P less than 0.005) and renal mass (r = .986; P less than 0.001). These observations provide evidence for modification of the luminal membrane Na+-H+ exchanger in response to changes in dietary protein content and nephron number.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol. 1978 Jul 21;42(1):81–98. doi: 10.1007/BF01870395. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- Arrud J. A., Carrasquillo T., Cubria A., Rademacher D. R., Kurtzman N. A. Bicarbonate reabsorption in chronic renal failure. Kidney Int. 1976 Jun;9(6):481–488. doi: 10.1038/ki.1976.62. [DOI] [PubMed] [Google Scholar]
- Bank N., Su W. S., Aynedjian H. S. A micropuncture study of HCO3 reabsorption by the hypertrophied proximal tubule. Yale J Biol Med. 1978 May-Jun;51(3):275–282. [PMC free article] [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Bennett C. M., Springberg P. D., Falkinburg N. R. Glomerular-tubular balance for bicarbonate in the dog. Am J Physiol. 1975 Jan;228(1):98–106. doi: 10.1152/ajplegacy.1975.228.1.98. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Sapirstein V. S. Characteristics of an amiloride-sensitive sodium entry pathway in cultured rodent glial and neuroblastoma cells. J Cell Physiol. 1983 Aug;116(2):213–220. doi: 10.1002/jcp.1041160213. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Galla J. H. Influence of postglomerular hematocrit and protein concentration on rat nephron fluid transfer. Am J Physiol. 1971 Jan;220(1):148–161. doi: 10.1152/ajplegacy.1971.220.1.148. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D., Simon E. Effect of reduced renal mass on ammonium handling and net acid formation by the superficial and juxtamedullary nephron of the rat. Evidence for impaired reentrapment rather than decreased production of ammonium in the acidosis of uremia. J Clin Invest. 1983 Jun;71(6):1661–1675. doi: 10.1172/JCI110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Green N. Role of monovalent ions in the reabsorption of fluid by isolated perfused proximal renal tubules of the rabbit. Kidney Int. 1976 Sep;10(3):221–228. doi: 10.1038/ki.1976.101. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Hruska K. A., Klahr S., Hammerman M. R. Increased Na+-H+ exchange in brush border vesicles from dogs with renal failure. Am J Physiol. 1982 Sep;243(3):F293–F299. doi: 10.1152/ajprenal.1982.243.3.F293. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Klahr S., Hammerman M. R. Metabolic acidosis and parathyroidectomy increase Na+-H+ exchange in brush border vesicles. Am J Physiol. 1983 Aug;245(2):F217–F222. doi: 10.1152/ajprenal.1983.245.2.F217. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Maddox D. A., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VII. Response to reduced renal mass. Am J Physiol. 1974 Sep;227(3):556–562. doi: 10.1152/ajplegacy.1974.227.3.556. [DOI] [PubMed] [Google Scholar]
- Freiberg J. M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson A. B., Shear L., Gabuzda G. J. Protein metabolism in vivo in kidney, liver, muscle, and heart of potassium-deficient rats. J Lab Clin Med. 1973 Aug;82(2):287–296. [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968 Apr;47(4):774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter T. H., Olson J. L., Rennke H. G., Venkatachalam M. A., Brenner B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981 Jul;241(1):F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Klahr S., Hammerman M. R. Decreased luminal membrane transport of phosphate in chronic renal failure. Am J Physiol. 1982 Jan;242(1):F17–F22. doi: 10.1152/ajprenal.1982.242.1.F17. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lotspeich W. D. Metabolic aspects of acid-base change. Science. 1967 Mar 3;155(3766):1066–1075. doi: 10.1126/science.155.3766.1066. [DOI] [PubMed] [Google Scholar]
- Malt R. A. Compensatory growth of the kidney. N Engl J Med. 1969 Jun 26;280(26):1446–1459. doi: 10.1056/NEJM196906262802606. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Northrup T. E., Malvin R. L. Cellular hypertrophy and renal function during compensatory renal growth. Am J Physiol. 1976 Oct;231(4):1191–1195. doi: 10.1152/ajplegacy.1976.231.4.1191. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra W. W., Warnock D. G., Yee V. J., Forte J. G. Proton gradients in renal cortex brush-border membrane vesicles. Demonstration of a rheogenic proton flux with acridine orange. J Biol Chem. 1981 Nov 25;256(22):11663–11666. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Schafer J. A. Salt and water absorption in the proximal tubule. Physiologist. 1982 Apr;25(2):95–103. [PubMed] [Google Scholar]
- Schmidt R. W., Bricker N. S., Gavellas G. Bicarbonate reabsorption in the dog with experimental renal disease. Kidney Int. 1976 Oct;10(4):287–294. doi: 10.1038/ki.1976.111. [DOI] [PubMed] [Google Scholar]
- Schoolwerth A. C., Sandler R. S., Hoffman P. M., Klahr S. Effects of nephron reduction and dietary protein content on renal ammoniagenesis in the rat. Kidney Int. 1975 Jun;7(6):397–404. doi: 10.1038/ki.1975.57. [DOI] [PubMed] [Google Scholar]
- Tabei K., Levenson D. J., Brenner B. M. Early enhancement of fluid transport in rabbit proximal straight tubules after loss of contralateral renal excretory function. J Clin Invest. 1983 Sep;72(3):871–881. doi: 10.1172/JCI111058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trizna W., Yanagawa N., Bar-Khayim Y., Houston B., Fine L. G. Functional profile of the isolated uremic nephron. Evidence of proximal tubular "memory" in experimental renal disease. J Clin Invest. 1981 Sep;68(3):760–767. doi: 10.1172/JCI110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]
- Wilcox C. S., Cemerikic D. A., Giebisch G. Differential effects of acute mineralo- and glucocorticosteroid administration on renal acid elimination. Kidney Int. 1982 Apr;21(4):546–556. doi: 10.1038/ki.1982.61. [DOI] [PubMed] [Google Scholar]


