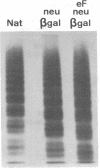
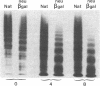
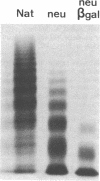
Abstract
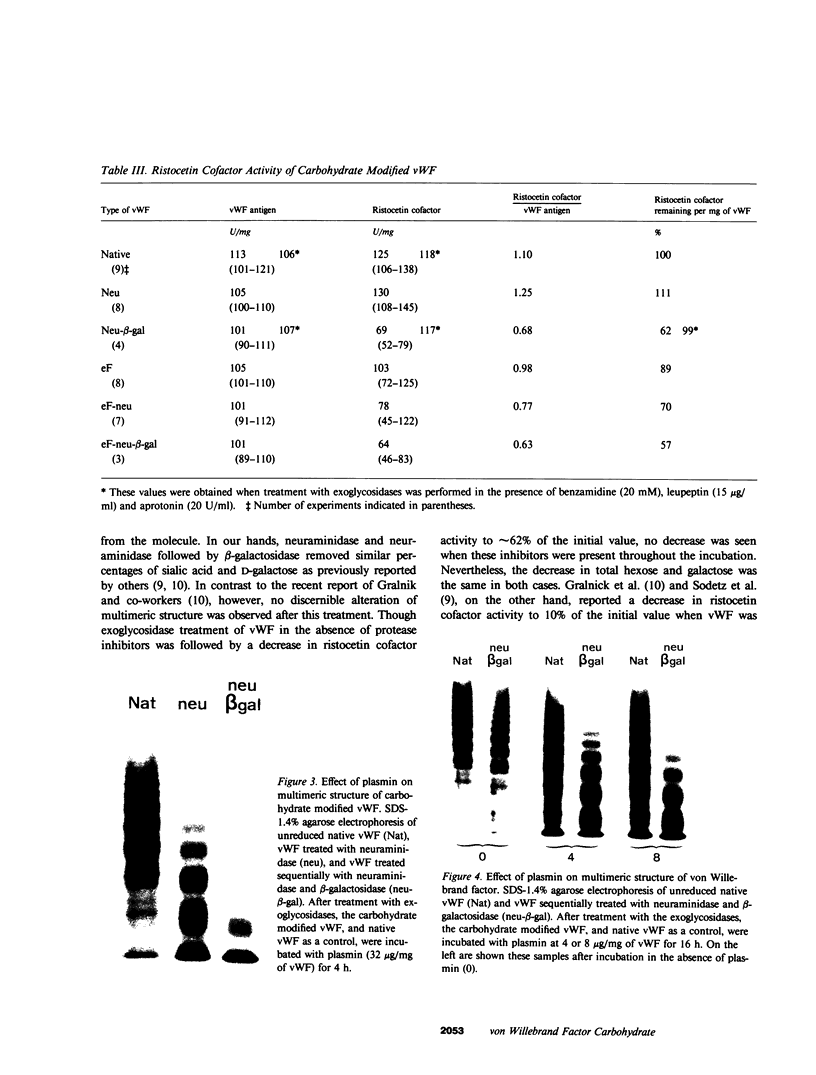
To better define the role of carbohydrate in the structure and ristocetin cofactor activity of von Willebrand factor, we have removed up to 83% of total hexose by sequential treatment of the molecule with endo-beta-N-acetyl-glucosaminidase F (endo F), neuraminidase, and beta-galactosidase. Endo F alone removed 69% of total hexose and D-galactose, and 71% of sialic acid. However, there was no discernible loss of large multimers and the ristocetin cofactor activity was decreased by only 11%. The reduced von Willebrand factor subunit migrated more rapidly in polyacrylamide gels containing SDS, consistent with a 10% decrease of molecular mass. All multimers of unreduced carbohydrate-modified von Willebrand factor migrated more rapidly in SDS-agarose, but the triplet pattern of individual multimers was unchanged. This alteration in multimer migration rate did not resemble alterations found so far in von Willebrand disease variants. Further treatment of von Willebrand factor with neuraminidase and beta-galactosidase reduced the D-galactose to 15% and ristocetin cofactor activity to 57%. A similar decrease in ristocetin cofactor activity was seen if von Willebrand factor was treated only with neuraminidase and beta-galactosidase. In contrast, treating von Willebrand factor with neuraminidase and beta-galactosidase in the presence of protease inhibitors (20 mM benzamidine, 20 U/ml aprotonin, 15 micrograms/ml leupeptin) resulted in a comparable removal of carbohydrate with no change in ristocetin cofactor activity. Moreover, the multimeric structure remained intact in spite of 80% removal of D-galactose. This suggested that carbohydrate was protecting von Willebrand factor against traces of one or more protease contaminants. Evidence in support of this hypothesis was obtained by exposing von Willebrand factor to plasmin after pretreatment with neuraminidase alone or with neuraminidase and beta-galactosidase. A loss of large multimers was observed from von Willebrand factor that had been pretreated with neuraminidase, but this was even greater if pretreatment was also with beta-galactosidase. In contrast, the multimeric structure of von Willebrand factor with intact carbohydrate was not affected by plasmin under similar conditions. These studies suggest that carbohydrate protects von Willebrand factor from disaggregation occurring secondarily to proteolytic attack but does not play a direct role in maintaining its multimeric structure or ristocetin cofactor activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard B. A., Yamada K. M., Olden K. Carbohydrates selectively protect a specific domain of fibronectin against proteases. J Biol Chem. 1982 Jul 25;257(14):8549–8554. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Ruggeri Z. M., Zimmerman T. S. Isoelectric focusing of human von Willebrand factor in urea-agarose gels. Blood. 1983 Feb;61(2):304–310. [PubMed] [Google Scholar]
- Gralnick H. R., Coller B. S., Sultan Y. Studies of the human factor VIII/von Willebrand factor protein. III. Qualitative defects in von Willebrand's disease. J Clin Invest. 1975 Oct;56(4):814–827. doi: 10.1172/JCI108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Cregger M. C., Williams S. B. Characterization of the defect of the factor VIII/von Willebrand factor protein in von Willebrand's disease. Blood. 1982 Mar;59(3):542–548. [PubMed] [Google Scholar]
- Gralnick H. R. Factor VIII/von Willebrand factor protein. Galactose a cryptic determinant of von Willebrand factor activity. J Clin Invest. 1978 Aug;62(2):496–499. doi: 10.1172/JCI109152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., Morisato D. K. Effect of multimeric structure of the factor VIII/von Willebrand factor protein on binding to platelets. Blood. 1981 Aug;58(2):387–397. [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., Rick M. E. Role of carbohydrate in multimeric structure of factor VIII/von Willebrand factor protein. Proc Natl Acad Sci U S A. 1983 May;80(9):2771–2774. doi: 10.1073/pnas.80.9.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. A., Perkin J., Koutts J., Firkin B. G. Quantitation of binding of factor VIII antigen to concanavalin A. Br J Haematol. 1981 Apr;47(4):607–615. doi: 10.1111/j.1365-2141.1981.tb02690.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Harrison J., Lazerson J., Abildgaard C. F. A new variant of dominant type II von Willebrand's disease with aberrant multimeric pattern of factor VIII-related antigen (type IID). Blood. 1984 Jun;63(6):1369–1371. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newman J., Johnson A. J., Karpatkin M. H., Puszkin S. Methods for the production of clinically effective intermediate- and high-purity factor-VIII concentrates. Br J Haematol. 1971 Jul;21(1):1–20. doi: 10.1111/j.1365-2141.1971.tb03413.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld L., Kirby E. P. The effects of neuraminidase treatment on the biological activities of factor VIII. Thromb Res. 1979;15(1-2):255–261. doi: 10.1016/0049-3848(79)90071-9. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Bader R., Barbui T. Factor VIII-related properties in platelets from patients with von Willebrand's disease. J Lab Clin Med. 1978 Jan;91(1):132–140. [PubMed] [Google Scholar]
- Ruggeri Z. M., Nilsson I. M., Lombardi R., Holmberg L., Zimmerman T. S. Aberrant multimeric structure of von Willebrand factor in a new variant of von Willebrand's disease (type IIC). J Clin Invest. 1982 Nov;70(5):1124–1127. doi: 10.1172/JCI110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodetz J. M., Paulson J. C., Pizzo S. V., McKee P. A. Carbohydrate on human factor VIII/von Willebrand factor. Impairment of function by removal of specific galactose residues. J Biol Chem. 1978 Oct 25;253(20):7202–7206. [PubMed] [Google Scholar]
- Sodetz J. M., Pizzo S. V., McKee P. A. Relationship of sialic acid to function and in vivo survival of human factor VIII/von Willebrand factor protein. J Biol Chem. 1977 Aug 10;252(15):5538–5546. [PubMed] [Google Scholar]
- Switzer M. E., McKee P. A. Studies on human antihemophilic factor. Evidence for a covalently linked subunit structure. J Clin Invest. 1976 Apr;57(4):925–937. doi: 10.1172/JCI108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Zimmerman T. S., Ruggeri Z. M. von Willebrand's Disease. Clin Haematol. 1983 Feb;12(1):175–200. [PubMed] [Google Scholar]
- Zimmerman T. S., Voss R., Edgington T. S. Carbohydrate of the factor VIII/von Willebrand factor in von Willebrand's disease. J Clin Invest. 1979 Nov;64(5):1298–1302. doi: 10.1172/JCI109585. [DOI] [PMC free article] [PubMed] [Google Scholar]