Abstract
Objective
To examine the effect of Medicaid enrollment on the diagnosis, treatment, and survival of six surgically relevant cancers among poor and underserved Californians.
Data Sources
California Cancer Registry (CCR), California's Patient Discharge Database (PDD), and state Medicaid enrollment files between 2002 and 2008.
Study Design
We linked clinical and administrative records to differentiate patients continuously enrolled in Medicaid from those receiving coverage at the time of their cancer diagnosis. We developed multivariate logistic regression models to predict death within 1 year for each cancer after controlling for sociodemographic and clinical variables.
Data Collection/Extraction Methods
All incident cases of six cancers (colon, esophageal, lung, pancreas, stomach, and ovarian) were identified from CCR. CCR records were linked to hospitalizations (PDD) and monthly Medicaid enrollment.
Principal Findings
Continuous enrollment in Medicaid for at least 6 months prior to diagnosis improves survival in three surgically relevant cancers. Discontinuous Medicaid patients have higher stage tumors, undergo fewer definitive operations, and are more likely to die even after risk adjustment.
Conclusions
Expansion of continuous insurance coverage under the Affordable Care Act is likely to improve both access and clinical outcomes for cancer patients in California.
Keywords: Medicaid, cancer, surgery, access to care, survival
Cancer is the second leading cause of death in the United States (Hoyert and Xu 2012) and is predicted to be the number-one killer by 2030 (ASCO 2014). In California, an estimated 171,730 new cancer cases will be diagnosed, and nearly 58,000 lives claimed by cancer in 2014 (American Cancer Society 2014). Despite improvements in cancer screening and treatment, many services remain unavailable to the entire population.
Disparities in cancer care have been reported by geographic location, race, ethnicity, and socioeconomic status (SES). Low SES, in particular, is associated with increased cancer incidence, delayed or inadequate screening, advanced stage at diagnosis, poor adherence to treatment guidelines, and diminished survival (Siegel et al. 2011; Wu et al. 2012; Bristow et al. 2013; Vandergrift et al. 2013). SES appears to be important in navigating financial, structural, and personal barriers to accessing health care in the United States—barriers that may be more pronounced for individuals without adequate health insurance.
Due to the complexity of treatment regimens, lack of insurance or lack of effective insurance (“underinsurance”) may be particularly challenging in the treatment of cancer (Rojas et al. 1996; Morris et al. 2004; O'Connell, Maggard, and Ko 2004; Fiscella et al. 2005; Trivedi et al. 2005; Ell et al. 2007). The Institute of Medicine's report on cancer disparities found that “uninsured cancer patients generally have poorer outcomes and are more likely to die prematurely than persons with insurance” (Institute of Medicine Committee on the Consequences of Uninsurance 2002). While not sufficient to guarantee access, adequate insurance coverage remains an essential component to improving cancer screening, treatment, and clinical outcomes.
Medicaid and Health Status
Recent findings from Oregon's lottery-based Medicaid expansion generated new controversy regarding the program's impact on health. Medicaid enrollment resulted in limited health benefits for previously uninsured Oregonians over the 2-year study period, including no impact on a variety of common medical conditions (Baicker et al. 2013). Moreover, increases in emergency department visits among low-income Oregonians with Medicaid suggest that providing insurance alone may not improve access or increase the likelihood of having a stable source of care (Taubman et al. 2014). Combined with looming questions about the cost and quality of care, policy makers have begun to question whether Medicaid is the best option for insuring low-income Americans.
Medicaid's record on cancer, however, is more encouraging. Multiple studies show increased screening rates for Medicaid patients over the uninsured, particularly for cancers with evidence-based screening guidelines such as breast, cervical, and colon (Parker et al. 1998; Ioannou, Chapko, and Dominitz 2003; Ward et al. 2008). States with more generous Medicaid programs have smaller gaps in access between high- and low-income populations for Papanicolaou testing for cervical cancer (Weissman et al. 2008). Even in Oregon's controversial expansion, Medicaid increased cancer screening rates among the previously uninsured, including Pap smears, mammography, and PSA testing (Taubman et al. 2014). From the clinical perspective, Medicaid patients also present with less advanced disease in colorectal cancer (Halpern et al. 2009), cervical cancer (O'Malley et al. 2006), breast cancer (Perkins et al. 2001), head and neck cancers (Subramanian and Chen 2013), and melanoma (Pollitt et al. 2008) than similar populations without health insurance.
Expansion under the ACA
As of 2012, 47.9 million Americans remained uninsured—including over 7.1 million California residents (Kaiser Family Foundation 2013). Prior to the Affordable Care Act (ACA), California extended Medicaid coverage to eligible, but uninsured patients after a new cancer diagnosis. Compared to typical enrollees, patients who join Medicaid at the time of diagnosis may miss out on the opportunities of continuous coverage, such as screening, health maintenance, and nonemergent acute care visits. With its expansion under the ACA, millions of low-income adults who previously turned to Medicaid for emergency coverage—including many with cancer—will be allowed to use the program as a permanent source of health insurance (Lucia et al. 2013).
What effect more continuous insurance coverage will have on cancer outcomes, however, remains unknown. The objective of this study was to examine the continuity of Medicaid enrollment and its impact on the diagnosis, treatment, and survival in six surgically relevant cancers among poor and underserved Californians.
Conceptual Framework
Cancer mortality is strongly influenced by tumor stage and the timely receipt of therapy, including surgical resection. We hypothesized that the effect of continuous insurance coverage on mortality may be mediated through improved access to care, resulting in earlier diagnosis and an increased likelihood of surgical resection. Previous research has shown that health insurance decreases delays in seeking care (Sommers, Long, and Baicker 2014), and that cancer patients with more comprehensive plans are less likely to present with metastatic disease (Halpern et al. 2008). Implicit in this “cascade” from coverage to health outcomes is that patients who maintain enrollment have more timely access to care, visit their doctor more frequently, and have their cancers diagnosed earlier, either through screening or the evaluation of clinical symptoms (Eisenberg and Power 2000).
We also hypothesized that continuous enrollment among patients with surgical cancers may increase the likelihood of operation even after controlling for stage. This may occur for several reasons. Patients who access the health care system more frequently are more likely to be cared for in settings with established referral patterns, such as primary care offices, rather than in the emergency room where coordinating surgical follow-up can be problematic. Continuous participation in the health care system may also result in greater familiarity and trust, increasing the likelihood that patients follow through with surgical consultations. Previous work suggests that discontinuous insurance, even for a brief period, is associated with high rates of postponing or forgoing necessary medical care (Schoen and DesRoches 2000). In the setting of cancer, these delays can result in tumor progression, missed opportunities for intervention, and even death.
Based on this conceptual framework, we developed three testable hypotheses:
Continuous Medicaid is associated with decreased 1-year mortality when compared to discontinuous coverage after controlling for sociodemographic factors alone.
Any observed effect of continuous insurance on mortality will be attenuated after controlling for tumor stage and the receipt of a definitive cancer operation due to its mediation through these clinical pathways.
Compared to the discontinuously insured, continuous Medicaid patients will have less metastatic disease and undergo more surgical resections after controlling for both sociodemographic and clinical variables.
Methods
We assembled a retrospective cohort of all incident cases of six cancers (colon, esophageal, lung, pancreatic, stomach, and ovarian) in California between 2002 and 2008. Three datasets were used: (1) the California Cancer Registry (CCR), (2) California's Patient Discharge Database (PDD), and (3) the state's monthly Medicaid enrollment file. A confidential data file maintained by the CCR was used as the backbone for all linkages. Programmers at the CCR first used probabilistic data-matching techniques based on Social Security number (SSN), date of birth, and gender to link CCR records to hospitalizations in the PDD. Next, a finder file was provided to the California Department of Health Care Services (DHCS) for a deterministic linkage to enrollment data in the state's Medicaid file. Finally, deidentified data files were provided to the research team after both linkages had been performed.
California Cancer Registry
CCR is the largest statewide cancer surveillance system and has existed in its current form since 1985. By statute, all new diagnoses of cancer (except nonmelanoma skin cancers) must be reported to the CCR, after which a clinical registrar from one of eight regional centers is sent to collect demographic, diagnostic, and treatment data. While data are mostly observational, the CCR updates its files to capture cancer-related treatments (e.g., surgical, chemo-, or radiotherapy), and links to death certificates recorded by the California Department of Public Health. Despite its design as a longitudinal registry, CCR data are significantly more accurate for cancer-related hospitalizations than for ancillary treatments such as chemo- or radiotherapy (Malin et al. 2002). For that reason, a subset of CCR variables related to hospitalization data was chosen for our analysis including demographics, insurance status, cancer stage and site, operation, and census-level measurements of SES.
Patient Discharge Database
OSHPD collects data from all inpatients discharged from acute care hospitals licensed by the state. Data from over 4 million discharges are tabulated annually, including demographics, reason for admission (i.e., diagnosis-related group), and types and dates of procedures performed. Linkage to the PDD in our analysis provided additional information on insurance status, medical comorbidities, and operative care for patients hospitalized during their cancer treatment.
Monthly Medicaid Enrollment File
Medi-Cal, California's state Medicaid office, maintains a monthly enrollment list of its beneficiaries. While both CCR and PDD contain information on insurance status from the medical record, neither is able to establish the timing of coverage. Furthermore, abstracted insurance status is prone to error. Linkage to official Medicaid records allowed us to both confirm program participation and to differentiate continuous enrollees from those receiving coverage at the time of their diagnosis.
Case Identification
All patients diagnosed with one of six cancers between 2002 and 2008 were identified from the CCR by their corresponding International Classification of Diseases for Oncology (ICD-O) code. Several exclusion criteria were established to avoid expected sources of bias. First, patients with carcinoma in situ were excluded to focus on surgical candidates. Second, our cohort was restricted to patients under 65 years old to avoid interactions between age and insurance coverage as Medicare is nearly universal among age-eligible Americans. Finally, patients with unreliable follow-up data were excluded: patients whose diagnoses were made at federal hospitals, which do not report to the PDD, and non-California residents for whom the CCR does not accurately capture death statistics due to its use of the California Death Statistical Master File rather than the National Death Index.
Dependent Variables
The primary outcome of interest was death within 1 year of diagnosis. While additional follow-up data exist for some patients, 1-year mortality was chosen to analyze complete records for all patients. A fixed follow-up period was selected over a survival analysis to prevent up-weighting of earlier noncensored data, which may represent less current treatment strategies.
Two intermediate outcomes based on our conceptual model were also analyzed for cancers in which continuous Medicaid showed a survival benefit. Advanced stage was defined as a Surveillance, Epidemiology, and End Results (SEER) Summary Stage of “remote” compared to less extensive disease. Receipt of a definitive operation was also defined as a binary variable (Yes/No) based on either PDD data, if available, or CCR records consistent with an operation at the primary tumor site. Both variables were also included as independent covariates in regression models for mortality as described below.
Insurance
Insurance status was our primary regressor of interest, and we divided patients into one of five mutually exclusive categories: Privately Insured, Medicare, Continuous Medicaid, Discontinuous Medicaid, and Uninsured/Self-Pay.
We defined insurance status based on a hierarchical analysis of available data. First, patients were linked to Medicaid enrollment files for the 12 months prior to their diagnosis to determine their Medicaid status. Those who linked in their diagnosis month as well as continuously for at least 5 months leading up to their diagnosis (i.e., six total months) were classified as “continuous.” Patients who linked to enrollment records but did not meet these criteria were considered “discontinuous.” Six consecutive months was chosen for continuous coverage based on a review of enrollment data (Appendix SA2). Patients who linked to Medicaid and also reported Medicare in either CCR or PDD records were considered dual eligible (“Medi-Medi”) and reclassified as Medicare. Patients who did not link to Medicaid records were further classified based on CCR and PDD data.
Both the CCR and the PDD use similar insurance classifications: “Medicaid,” “Medicare,” “Private,” “Other public,” and “Uninsured.” “Medicaid” designations were disregarded among patients who did not link to Medicaid enrollment data, as official records were considered to be the gold standard. “Other public” and “Uninsured” were merged after a review of our data confirmed that these groups were functionally identical other than the type of hospital delivering their care: public versus private. For the remaining classifications, patients with congruent information (e.g., both CCR and PPD listed “Medicare”) were classified by their listed insurance status. Patients with incongruent information were coded as follows: patients coded as “Medicare” in either file received that designation, followed by “Private,” with the remaining being “Uninsured.”
Medicaid eligibility codes were also provided within the monthly Medi-Cal enrollment file. To promote comparability between continuous and discontinuous enrollees, we attempted to identify patients who may have been ineligible for Medicaid until the time of diagnosis. The Medi-Cal Aid Codes Master Chart (available at http://www.medi-cal.ca.gov) was used to identify patients with restricted benefit profiles in the month of diagnosis (Appendix SA3). Patients who linked to Medicaid enrollment files but were only eligible for emergency services were reclassified as “Uninsured.”
Covariates
Additional covariates were divided into two categories: sociodemographic and clinical. Sociodemographic variables included age at diagnosis, gender, race, distance to high-volume hospital, and census-tract measurements of SES. Age was treated as continuous, while gender and race were treated as both categorical and mutually exclusive based on CCR definitions: male versus female, and non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, and other.
Distance to high-volume hospital has previously been used as a measure of physician density (Liu et al. 2006; Scoggins et al. 2012). For our analysis, high-volume hospitals were identified as those in the top quintile of admissions for each cancer type. A linear distance between each patient and hospital zip code was calculated by cancer type, and the closest distance was used for all patients in that zip code. Three census-tract level measures of SES were included based on each patient's home address and U.S. Census data from 1999: percentage of the population living below 200 percent of the federal poverty level, percentage of the population without a high school diploma, and percentage of the population reporting poor English proficiency—a factor considered particularly relevant to California's Medicaid population.
Clinical variables included the SEER summary stage, cancer-specific anatomic location (e.g., cecum, ascending colon, etc.), receipt of a definitive cancer operation, and the presence of nononcologic comorbidities. SEER summary stages include localized, regional, and remote. Anatomic locations varied for each cancer type as defined by Collaborative Staging guidelines. As described above, receipt of a definitive operation was defined as binary variable based on a composite of available information. Comorbidity scores were generated for patients who linked to PDD records based on 27 items from the Elixhauser comorbidity index recorded during the surgical hospitalization; codes for tumor, weight loss, and metastatic disease were ignored due to their association with cancer staging.
Statistical Analysis
Descriptive statistics were performed for all available data. Bivariate tables were generated to compare baseline characteristics by insurance status for the full sample and within each cancer type. Given our conceptual model, we designed our regression models to capture both the overall effect of insurance on mortality (i.e., the total effect through any of the hypothesized mediating pathways), and the role of clinical processes—namely cancer stage and operation—in mediating any observed benefit. First, a separate multivariate logistic regression model was developed to predict death within 1 year for each cancer type after controlling only for sociodemographic variables. This was considered the simplest or “reduced form” model for baseline analyses.
Next, we developed additional regressions by first adding cancer stage, anatomic location, and comorbidity score; and then added these variables and operation to the baseline model. This created a nested series of models based on our conceptualization of stage and operation as potential mediators. For cancers in which continuous Medicaid was found to be beneficial, two additional logistic regression models were developed to predict advanced stage at diagnosis and receipt of a definitive cancer operation. For all regressions, predicted probabilities were calculated for each insurance subgroup, and the difference between the predicted values for continuous and discontinuous Medicaid enrollment—known as predictive margins—were used to summarize the influence of continuous enrollment on the dependent variable.
We conducted several sensitivity analyses to test the robustness of our estimates. Comorbidity data were missing for approximately 18 percent of the sample. As comorbid medical conditions may have had an impact on the likelihood of death within 1 year or of undergoing an operation, we attempted to control for their impact by imputing missing scores via a multivariate normal regression technique. Our multiple imputation models controlled for all variables in the full model for mortality after stratifying into age quartiles. Given the concern that comorbidity data were missing nonrandomly due to CCR including patients who were never hospitalized, we also performed a complete case analysis controlling for comorbidities first by composite score and then using binary comorbidity dummy codes. Neither of these strategies affected our results, and, consequently, we report regressions for the full sample with imputed scores for patients with missing data.
As our sample represents patients clustered within hospitals, cluster-robust Huber–White estimators were used as standard errors in all regression models. As an additional sensitivity analysis, we repeated our regressions using a hierarchical model with hospital-level random effects. The choice of model had no effect on our results, and we report results from our standard logistic regression models.
Data administration was performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA). Statistical analyses were performed using STATA/IC 13.0 (StataCorp, College Station, TX, USA). Chi-squared tests of independence for categorical variables and analysis of variance for continuous variables were used to compare unadjusted data by insurance status. Overall likelihood ratio chi-squared tests and Wald's z-tests were used to determine significance in logistic regression models; Taylor series linearization (“delta method”) was used to determine the significance of predictive margins. All reported p-values were two-sided, and values than.05 were considered significant. This study was approved by the Office of Human Research Protection Program at the University of California, Los Angeles, the California Committee for the Protection of Human Subjects, and California Department of Health Care Services.
Results
Descriptive and Bivariate Statistics
New cancer diagnoses of 291,565 were identified during the study period. Of these, 5,768 patients with carcinoma in situ, 188,847 patients aged 65 and older, 532 patients identified at federal hospitals, and 198 non-California residents were excluded (Figure1). The remaining patients (n = 96,220) were included in the analysis. Patients were treated at 469 separate hospitals throughout California. The number of patients per hospital ranged from 1 to over 1,700; 15 hospitals contributed over 1,000 patients, and the top 5 percent of hospitals by volume represented 25.5 percent of the sample.
Figure 1.
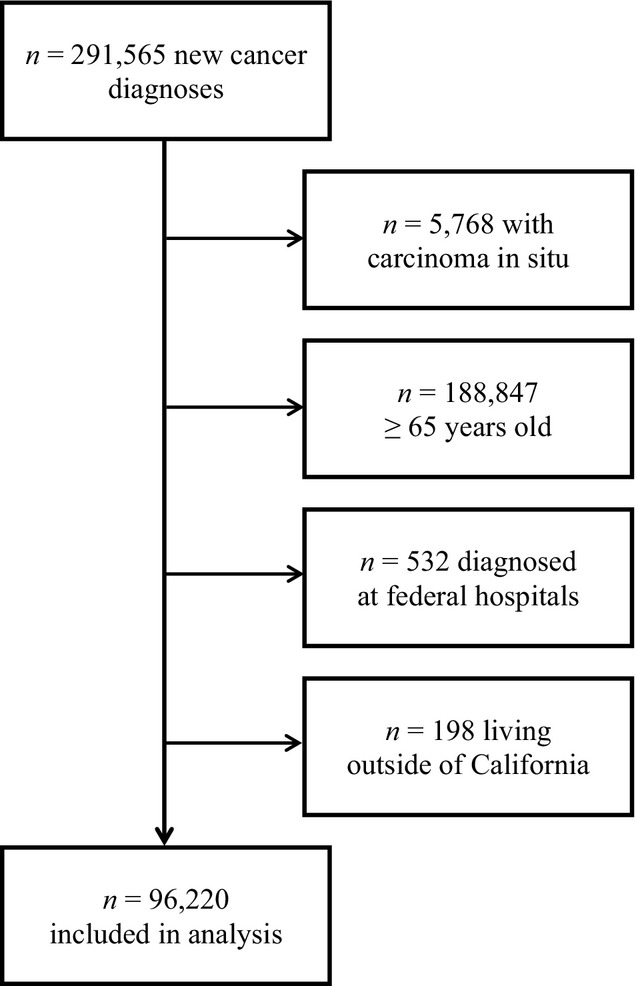
Inclusion/Exclusion Flow Diagram
Table1 shows descriptive statistics for the full cohort and compares independent variables by insurance status. Overall, 82.3 percent of patients linked to PDD records, and nearly half underwent a definitive cancer operation. All continuously enrolled and the majority of discontinuously enrolled Medicaid patients linked to PDD records. Colon cancer was the most common diagnosis overall (37.9 percent); however, lung cancer was predominant among both continuous (43.4 percent) and discontinuous Medicaid enrollees (41.4 percent). Among Medicaid patients for whom eligibility data were available (51.0 percent), 625 (6.7 percent) were identified as eligible for emergency services only and reclassified as uninsured.
Table 1.
Characteristics of Patients under Sixty-Five Years Old Diagnosed with Six Cancers in California, 2002–2008
|
Overall |
Private | Medicare | Continuous Medicaid | Discontinuous Medicaid | Uninsured | ||
|---|---|---|---|---|---|---|---|
| n | % | % | % | % | % | % | |
| Patients | 96,220 | 100.0 | 65.4 | 6.3 | 6.8 | 10.0 | 11.4 |
| Linked to PDD | 79,167 | 82.3 | 82.9 | 89.2 | 100.0 | 94.8 | 53.6 |
| Definitive operation | 45,270 | 47.1 | 53.1 | 38.3 | 38.5 | 32.6 | 34.9 |
| Cancer type | |||||||
| Colon | 36,437 | 37.9 | 41.6 | 29.8 | 28.6 | 28.5 | 35.0 |
| Esophagus | 3,009 | 3.1 | 3.0 | 3.3 | 3.3 | 3.6 | 3.1 |
| Lung | 33,187 | 34.5 | 31.1 | 48.2 | 43.4 | 41.4 | 34.8 |
| Ovary | 8,999 | 9.4 | 9.9 | 5.6 | 8.4 | 8.5 | 9.6 |
| Pancreas | 7,883 | 8.2 | 8.2 | 7.6 | 8.1 | 8.9 | 7.9 |
| Stomach | 6,705 | 7.0 | 6.2 | 5.6 | 8.2 | 9.1 | 9.6 |
| Sociodemographics | |||||||
| Age (mean ± SD) | 54.1 ± 8.3 | 54.1 ± 8.1 | 57.7 ± 6.2 | 53.3 ± 9.1 | 53.3 ± 8.9 | 53.1 ± 9.3 | |
| Female | 48,389 | 50.3 | 51.1 | 47.5 | 55.4 | 45.9 | 47.9 |
| Race | |||||||
| White | 58,470 | 60.8 | 66.7 | 65.3 | 44.2 | 48.8 | 44.6 |
| Black | 8,455 | 8.8 | 6.6 | 13.6 | 21.2 | 11.3 | 8.9 |
| Hispanic | 16,679 | 17.3 | 13.4 | 15.2 | 21.1 | 25.9 | 31.1 |
| Asian | 11,670 | 12.1 | 12.2 | 5.4 | 12.9 | 13.2 | 14.1 |
| Other | 946 | 1.0 | 1.0 | 0.5 | 0.7 | 0.8 | 1.3 |
| Distance to high-volume hospital (miles) | 8.9 ± 18.6 | 8.3 ± 17.5 | 10.7 ± 20.0 | 9.3 ± 19.9 | 9.6 ± 21.6 | 10.2 ± 20.3 | |
| % Below 200% of FPL | 30.1 ± 17.3 | 26.1 ± 15.5 | 35.8 ± 17.1 | 41.4 ± 17.6 | 36.9 ± 17.9 | 36.8 ± 18.3 | |
| % without a high school diploma | 21.7 ± 15.5 | 18.7 ± 13.8 | 25.2 ± 15.6 | 30.2 ± 16.6 | 27.4 ± 16.9 | 27.4 ± 17.4 | |
| % reporting poor English proficiency | 9.7 ± 10.0 | 8.1 ± 8.5 | 10.6 ± 10.3 | 14.1 ± 11.7 | 12.8 ± 11.7 | 13.3 ± 12.2 | |
| Clinical | |||||||
| Summary stage | |||||||
| Local | 22,863 | 23.8 | 27.3 | 21.2 | 19.3 | 11.7 | 18.2 |
| Regional | 26,781 | 27.8 | 29.4 | 26.6 | 26.3 | 24.1 | 23.8 |
| Remote | 42,301 | 44.0 | 40.7 | 45.6 | 48.2 | 60.1 | 45.3 |
| Unknown | 4,275 | 4.4 | 2.7 | 6.6 | 6.1 | 4.0 | 12.7 |
| Elixhauser Index* | 1.3 ± 0.01 | 1.2 ± 0.01 | 2.2 ± 0.02 | 1.9 ± 0.02 | 1.4 ± 0.02 | 1.3 ± 0.02 | |
Note. p < .001 for all comparisons based on chi-squared tests for categorical and ANOVA for continuous variables.
Means and standard deviations represent values after multiple imputation.
Fewer continuous Medicaid patients were diagnosed with metastatic disease, and more were diagnosed with local disease for each cancer type compared to discontinuous patients (Table2). Continuous Medicaid patients also had higher rates of operation for all cancers except pancreatic. One-year mortality was lowest among the privately insured for each cancer type, and lower among continuous Medicaid patients for colon, lung, and stomach cancer than for discontinuous patients.
Table 2.
One-Year Mortality, Advanced Stage at Diagnosis, and Definitive Operation by Insurance Status
| Private | Medicare | Continuous Medicaid | Discontinuous Medicaid | Uninsured | |
|---|---|---|---|---|---|
| N | 62,942 | 6,078 | 6,555 | 9,636 | 11,009 |
| Colon | |||||
| Advanced stage (%) | 21.5 | 28.5 | 29.8 | 41.7 | 35.5 |
| Definitive operation (%) | 70.9 | 69.6 | 69.2 | 59.4 | 56.8 |
| One-year mortality (%) | 8.4 | 20.8 | 19.7 | 23.1 | 16.4 |
| Esophagus | |||||
| Advanced stage (%) | 50.3 | 50.5 | 51.6 | 58.5 | 62.4 |
| Definitive operation (%) | 31.1 | 13.9 | 17.1 | 12.7 | 11.2 |
| One-year mortality (%) | 45.8 | 63.4 | 71.4 | 67.1 | 60.1 |
| Lung | |||||
| Advanced stage (%) | 62.1 | 63.9 | 67.8 | 78.5 | 75.1 |
| Definitive operation (%) | 26.8 | 17.6 | 14.9 | 9.2 | 9.0 |
| One-year mortality (%) | 45.6 | 57.3 | 63.3 | 67.4 | 56.8 |
| Ovary | |||||
| Advanced stage (%) | 55.9 | 67.0 | 59.8 | 69.4 | 62.1 |
| Definitive operation (%) | 86.5 | 68.7 | 67.8 | 63.5 | 68.2 |
| One-year mortality (%) | 10.5 | 23.6 | 23.9 | 22.5 | 18.3 |
| Pancreas | |||||
| Advanced stage (%) | 61.9 | 65.9 | 66.1 | 69.5 | 71.9 |
| Definitive operation (%) | 38.0 | 37.3 | 36.6 | 39.4 | 24.1 |
| One-year mortality (%) | 60.9 | 75.7 | 75.6 | 75.8 | 67.5 |
| Stomach | |||||
| Advanced stage (%) | 47.1 | 45.6 | 52.6 | 61.6 | 60.6 |
| Definitive operation (%) | 43.9 | 35.3 | 36.6 | 28.1 | 31.6 |
| One-year mortality (%) | 40.6 | 52.1 | 49.6 | 58.5 | 48.8 |
Note. p < .001 for all comparisons based on chi-squared tests.
Reduced Form Model
After controlling for sociodemographic factors, continuous Medicaid patients were less likely to die within 1 year for three of the six surgical cancers compared to discontinuous enrollees (Table3). Significant survival benefit was found in patients with colon (23.0 vs. 19.1 percent, predictive margin [PM] = −3.94, p = .001), lung (66.7 vs. 62.4 percent, PM = −4.32, p = .002), and stomach (57.8 vs. 49.0 percent, PM = −8.77 percent, p = .001) cancers. Esophageal, ovarian, and pancreatic cancer did not show a difference in mortality by Medicaid status.
Table 3.
Predicted Probability of Mortality and Predictive Margins at one Year for Continuous versus Discontinuous Medicaid Enrollment
|
Reduced Form* |
Clinical† |
Operation‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Discontinuous Medicaid (%) | Continuous Medicaid (%) | p-Value§ | Discontinuous Medicaid (%) | Continuous Medicaid (%) | p-Value§ | Discontinuous Medicaid (%) | Continuous Medicaid (%) | p-Value§ | |
| Colon | 23.0 | −3.94 | .001 | 15.6 | −0.15 | .855 | 14.9 | +0.48 | .548 |
| Esophagus | 66.6 | +3.79 | .329 | 64.0 | +5.56 | .137 | 61.1 | +6.66 | .073 |
| Lung | 66.7 | −4.32 | .002 | 60.5 | −0.56 | .595 | 59.2 | −0.12 | .905 |
| Ovary | 21.5 | +0.62 | .802 | 19.2 | −1.25 | .551 | 16.1 | −0.27 | .882 |
| Pancreas | 76.1 | −0.40 | .871 | 74.2 | −0.06 | .982 | 74.5 | −0.56 | .821 |
| Stomach | 57.8 | −8.77 | .001 | 51.7 | −4.12 | .083 | 50.3 | −3.08 | .198 |
Results from reduced form multivariate logistic model for mortality including insurance status, and sociodemographic variables (age, gender, race, distance to high-volume hospital, and percentage of population living in poverty, without a high school degree, and reporting poor English proficiency). Numbers in the discontinuous Medicaid column represent predicted probabilities of death at 1 year in percent, while numbers in the adjoining column represent predictive margins for continuous compared to discontinuous enrollment.
Results from model including reduced form and clinical variables (summary stage, anatomic location, comorbidity count).
Results from model including reduced form and clinical variables as well as receipt of a definitive cancer operation.
Based on standard error estimations using the delta method.
Demographic variables were associated with mortality in all six cancers (Appendix SA4). Census-level markers of education and language proficiency were associated with mortality in four of the six cancers (colon, lung, ovarian, and stomach); markers of poverty were only significant in colon cancer. Distance to high-volume hospital was not associated with mortality in any cancer after controlling for the remaining covariates (Appendix SA4).
Clinical
After adding clinical variables to the baseline model, the effect of continuous Medicaid on mortality disappeared entirely (Table3). Continuous enrollment demonstrated a trend toward improved survival in stomach cancer, which did not reach statistical significance (51.7 vs. 47.6 percent, PM = −4.12, p = .083). Esophageal, ovarian, and pancreatic cancer continued to show no difference in mortality by Medicaid status. Tumor stage and comorbidity count were associated with 1-year mortality in all six cancers; anatomic location was associated with mortality for all cancers except esophageal (Appendix SA4).
Operation
There continued to be no survival difference by Medicaid status after adding receipt of a definitive operation to our model controlling for tumor stage (Table3). Undergoing an operation was associated with decreased mortality in all six cancer types with predictive margins for operation versus no operation ranging from 7.4 percentage points in colon cancer to 29.7 percentage points in lung cancer (Appendix SA4; p < .001 for all).
Intermediate Outcomes
Based on our reduced form model, continuous Medicaid was associated with a survival benefit in three cancer types: colon, lung, and stomach. To further explore the mechanisms contributing to this effect, we performed separate regressions to predict advanced stage at diagnosis and receipt of a definitive operation. Continuous enrollment in Medicaid was associated with a lower likelihood of metastatic disease at diagnosis for all three cancers after controlling for sociodemographic variables (Table4). The largest effect was among colon cancer patients, where continuous enrollees had a 13.4 percentage point lower likelihood of metastatic disease than those with discontinuous coverage (p < .001).
Table 4.
Intermediate Outcomes for Cancers in Which Continuous Medicaid Demonstrated a Survival Benefit
|
Advanced Stage at Diagnosis* |
Definitive Cancer Operation† |
|||||
|---|---|---|---|---|---|---|
| Discontinuous Medicaid (%) | Continuous Medicaid (%) | p-Value‡ | Discontinuous Medicaid (%) | Continuous Medicaid (%) | p-Value‡ | |
| Colon | 40.2 | −13.4 | <.001 | 61.9 | +8.64 | <.001 |
| Lung | 77.0 | −12.1 | <.001 | 15.3 | +2.37 | .012 |
| Stomach | 58.2 | −9.78 | .003 | 31.0 | +6.17 | .015 |
Results from multivariate logistic model for summary stage of “remote” including insurance status and sociodemographic variables (age, gender, race, distance to high-volume hospital, and percentage of population living in poverty, without a high school degree, and reporting poor English proficiency). Numbers in the discontinuous Medicaid column represent predicted probabilities of advanced stage at diagnosis in percent, while numbers in the adjoining column represent changes in the predicted probability for continuous compared to discontinuous enrollment.
Results from multivariate logistic model for receipt of a definitive operation including sociodemographic and clinical variables (summary stage, anatomic location, comorbidity count).
Based on standard error estimations using the delta method.
Continuous Medicaid was similarly associated with increases in the likelihood of undergoing a definitive cancer operation for all three cancers after controlling for covariates including stage (Table4). Again, the largest effect was seen in colon cancer where patients continuously enrolled for at least 6 months had a 8.64 percentage point higher likelihood of having an operation compared to those with discontinuous enrollment periods (p < .001).
Discussion
Cancer remains a leading cause of mortality in the United States as well as a potential source of health care disparities when treatment is limited by access to care. Lack of continuous insurance among cancer patients in particular may contribute to access barriers due to the multidisciplinary nature of cancer treatment (Institute of Medicine 2013). Given its expansion under the ACA, we sought to determine the effect of Medicaid on all-cause mortality in six surgical cancers, and to explore the mechanisms contributing to differences in outcome by the continuity of enrollment.
We found that continuous enrollment in Medicaid for 6 months increased the likelihood of survival at 1 year in a variety of surgical cancers when compared to initiating coverage closer to the time of diagnosis. Our results align with two recent statewide analyses of Medicaid status on cancer survival in Michigan and Ohio, which found 1.1–1.5 fold increases in survival for continuous compared to discontinuous enrollees with colon and lung cancer (Bradley et al. 2005; Koroukian, Bakaki, and Raghavan 2012). While our description of the continuity of Medicaid enrollment in California also agrees with previous work—including the relative stability of coverage among patients enrolled for at least 6 months—our results suggest that only 40 percent of Medicaid beneficiaries with cancer are continuously enrolled compared to over 60 percent in previous studies (Schrag, Virnig, and Warren 2009). It is unclear whether this finding represents different patient populations or an actual shrinkage of continuous insurance over time. Either way, discontinuous enrollment appears substantial among Medicaid-eligible patients prior to the ACA with these individuals representing a disproportionate share of risk-adjusted cancer deaths.
Our results also suggest that the survival benefit observed for continuous Medicaid enrollment was strongly mediated by earlier diagnosis of tumors. This was demonstrated in two different ways. First, continuous Medicaid was associated with a decreased likelihood of advanced stage at presentation and an increased likelihood of undergoing an operation in the three cancers showing a survival benefit. Second, the effect of insurance on mortality in the reduced form model disappeared for these cancers when clinical variables—including stage—were added, suggesting that the insurance effect is partially, if not entirely, mediated by lower tumor stage among continuous Medicaid enrollees. Based on these results, observed disparities by Medicaid status may be due less to differences in how patients are treated after seeking care, and more to earlier diagnosis through repeated patient visits and screening protocols.
This conclusion is further borne out by the pattern of cancers in which we observed a survival benefit. Several studies have noted that inadequate insurance coverage reduces physician office visits in cancer (Friedman et al. 2002; Varghese et al. 2005). Compared to retroperitoneal cancers—such as ovarian, pancreatic, and esophageal—where clinical presentation is often cryptic, colon, lung, and stomach cancers are typically more symptomatic and easier to detect with common testing modalities. If continuous enrollment improves survival through more timely access to care, the largest impact would be expected in conditions that trigger the evaluation of clinical symptoms.
Moreover, as insurance is consistently linked to improved access to preventative care, continuous Medicaid enrollment may also increase the detection of tumors and precancerous lesions via screening protocols. Again, the pattern of our results appears to support this conclusion given the restriction of survival benefit to cancers with high screening potential. Colon cancer screening via routine lower endoscopy remains a mainstay of guidelines from multiple societies, including the United States Preventive Services Task Force (USPSTF) and the American Cancer Society (Levin et al. 2008; Whitlock et al. 2008). Although more recent guidelines for screening via low-dose computed tomography were not in effect during our study period, many physicians already screen for lung cancer using less sensitive methods, such as chest radiography and sputum cytology (Detterbeck et al. 2013; Humphrey et al. 2013). In contrast, USPSTF currently recommends against screening for ovarian and pancreatic cancers, and no widely accepted guidelines for stomach or esophageal cancer exist (USPSTF 2004; Moyer 2012). Strategies do exist for the detection of precancerous conditions, such as Barrett's esophagus and Helicobacter pylori infection, which may explain the survival benefit for continuous Medicaid in patients with stomach cancer. Further work is needed to explore these potential mechanisms, including an analysis of the differential usage of ambulatory services between continuous and discontinuous Medicaid enrollees.
While continuous enrollment appears to improve survival in at least a subset of surgical cancers, both Medicaid groups had lower risk-adjusted survival than the privately insured. Several factors may be involved in this relationship. First, privately insured patients typically have better access to care than even the best connected patients with public insurance, especially with respect to seeing specialists in a timely manner. Recognizing this problem, the American Society of Clinical Oncology listed improved access to cancer-related specialty care for Medicaid patients among the major opportunities for improving care under the ACA (Moy et al. 2011). Second, the privately insured are more likely to be of higher SES and less likely to exhibit negative health behaviors such as smoking. Therefore, while providing continuous Medicaid to low-income Californians with cancer appears to improve survival, additional work is needed to determine how to elevate the quality of care to the level of the privately insured.
Our results should be viewed in the context of other evidence on health benefits stemming from Medicaid expansion. Statewide programs to develop continuous eligibility periods have been linked to more stable Medicaid enrollment (Ku, Steinmetz, and Bruen 2013) as well as increased compliance with screening and treatment guidelines in certain cancers (Adams, Chien, and Gabram-Mendola 2012). Substantial expansions of Medicaid eligibility at the state level have also been linked to increased enrollment among the previously uninsured as well as reductions in rates of delayed care and all-cause mortality (Sommers, Baicker, and Epstein 2012; Sommers, Long, and Baicker 2014). Based on our results, we expect further expansion under the ACA to build on health improvements for Medicaid patients, both by stabilizing enrollment among the currently eligible and by extending coverage to individuals who previously would only have qualified for emergency services. Moreover, as the ACA is designed to increase coverage through a mixture of public and private insurance, we anticipate that our estimates may be conservative as the benefits of private insurance appear to be even more pronounced.
There are several limitations to our current work. First, our population represents Californians with six specific cancers, and our results may not generalize to other states or types of cancer. As Medicaid is administered at the state level, the impact of the program may differ based on the specific services offered. Second, SES and distance to high-volume hospital were measured at the census tract rather than the individual level. To the extent that individuals differed from the average person in their census tract, this was not captured by our analysis.
Third, while similar probabilistic linkage techniques have been validated previously, both random and nonrandom errors may have occurred in the data matching process. Most notably, while a valid SSN is not required to receive Medicaid, it was used—along with date of birth and gender—in the linkage algorithm. Patients who receive Medicaid but do not have a valid SSN may not link to the enrollment file, making them falsely appear uninsured. Other statewide analyses suggest that linkages including SSN, but not first and last name may fail to capture up to 10 percent of Medicaid enrollees due to invalid or missing data (Koroukian, Bakaki, and Raghavan 2012). Additional data-matching efforts may improve results, especially if the health or follow-up period differs for cancer patients with and without a valid SSN.
Fourth, as our analysis is based on retrospective data, we were unable to control for certain unmeasured differences between continuous and discontinuous Medicaid patients. Although we attempted to recategorize patients who may have been ineligible for certain services both directly via aid codes and indirectly by including SSN in our linkage algorithm, it is possible that some Medicaid patients with restricted coverage remain unidentified. In addition, without reliable data on patient motivation or health beliefs, we cannot control for complex issues that may have prevented patients from seeking both continuous coverage and other necessary health services, such as screening, surgery, or postoperative chemotherapy.
Finally, our results are limited to the health effects of Medicaid among cancer patients. Participating in the Medicaid program may impact patients in meaningful ways, such as financial security or quality of life, which are overlooked by our current analysis.
Conclusion
Continuous Medicaid coverage for at least 6 months appears to provide a survival benefit over enrollment at the time of diagnosis in a subset of surgical cancers. The observed benefit of continuous coverage is primarily mediated through earlier stage at diagnosis with the strongest effects seen in tumors that typically lead to earlier symptoms and cancers where routine screening is feasible and recommended. As both Medicaid and private insurance are set to expand under the ACA, low-income Californians with cancer are likely to experience both better access to care and better clinical outcomes than ever before.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This manuscript reflects work that was a presented at the 2014 AcademyHealth Annual Research Meeting. Dr. Dawes was supported by the VA Office of Academic Affiliations through the VA/Robert Wood Johnson Clinical Scholars Program. Dr. Zingmond was supported by a grant from the American Cancer Society (RSGI-11-005-01-CPHPS).
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Author Matrix.
Appendix SA2: Length of Continuous Enrollment among Patients Who Link to Monthly Medicaid Enrollment Files.
Appendix SA3: Listing and Description of Medi-Cal Aid Codes for Restricted Eligibility.
Appendix SA4: Full Regression Results from Nested Models in All Six Cancers.
References
- Adams EK, Chien LN, Gabram-Mendola SG. Treatment Patterns among Medicaid-Eligible Women with Breast Cancer in Georgia: Are Patterns Different under the Breast and Cervical Cancer Prevention and Treatment Act? Journal of Oncology Practice. 2012;8(1):46–52. doi: 10.1200/JOP.2011.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- ASCO. The State of Cancer Care in America, 2014: A Report by the American Society of Clinical Oncology. Journal of Oncology Practice. 2014;10(2):119–42. doi: 10.1200/JOP.2014.001386. [DOI] [PubMed] [Google Scholar]
- Baicker K, Taubman SL, Allen HL, Bernstein M, Gruber JH, Newhouse JP, Schneider EC, Wright BJ, Zaslavsky AM, Finkelstein AN, Carlson M, Edlund T, Gallia C, Smith J. The Oregon Experiment–Effects of Medicaid on Clinical Outcomes. New England Journal of Medicine. 2013;368(18):1713–22. doi: 10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CJ, Gardiner J, Given CW, Roberts C. Cancer, Medicaid Enrollment, and Survival Disparities. Cancer. 2005;103(8):1712–8. doi: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, Mutch DG, Cliby WA. Disparities in Ovarian Cancer Care Quality and Survival According to Race and Socioeconomic Status. Journal of the National Cancer Institute. 2013;105(11):823–32. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143(5 Suppl):e78S–92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg JM, Power EJ. Transforming Insurance Coverage Into Quality Health Care – Voltage Drops from Potential to Delivered Quality. Journal of the American Medical Association. 2000;284(16):2100–7. doi: 10.1001/jama.284.16.2100. [DOI] [PubMed] [Google Scholar]
- Ell K, Vourlekis B, Lee PJ, Xie B. Patient Navigation and Case Management Following an Abnormal Mammogram: A Randomized Clinical Trial. Preventive Medicine. 2007;2007(44):1. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Franks P, Medrum S, Barnett S. Racial Disparity in Surgical Complications in New York State. Annals of Surgery. 2005;242(2):151–5. doi: 10.1097/01.sla.0000171031.08435.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman C, Ahmed F, Franks A, Weatherup T, Manning M, Vance A, Thompson BL. Association between Health Insurance Coverage of Office Visit and Cancer Screening among Women. Medical Care. 2002;40(11):1060–7. doi: 10.1097/00005650-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bion B, Chan AY. Association of Insurances Status and Ethnicity with Cancer Stage at Diagnosis for 12 Cancer Sites: A Retrospective Analysis. Lancet Oncology. 2008;9:222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Pavluck AL, Ko CY, Ward EM. Factors Associated with Colon Cancer Stage at Diagnosis. Digestive Diseases and Sciences. 2009;54(12):2680–93. doi: 10.1007/s10620-008-0669-0. [DOI] [PubMed] [Google Scholar]
- Hoyert D, Xu JQ. Deaths: Preliminary Data for 2011. Hyattsville, MD: National Center for Health Statistics; 2012. and. National vital statistics reports. [PubMed] [Google Scholar]
- Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for Lung Cancer with Low-Dose Computed Tomography: A Systematic Review to Update the US Preventive Services Task Force Recommendation. Annals of Internal Medicine. 2013;159(6):411–20. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- Institute of Medicine Committee on the Consequences of Uninsurance. Coverage Matters: Insurance and Health Care. Washington, DC: National Academy Press; 2002. [Google Scholar]
- Ioannou GN, Chapko MK, Dominitz JA. Predictors of Colorectal Cancer Screening Participation in the United States. American Journal of Gastroenterology. 2003;98:2082–91. doi: 10.1111/j.1572-0241.2003.07574.x. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. 2013. “ Health Insurance Coverage of the Total Population ” [accessed on April 17, 2014, 2013]. Available at http://kff.org/other/state-indicator/total-population/
- Koroukian SM, Bakaki PM, Raghavan D. Survival Disparities by Medicaid Status: An Analysis of 8 Cancers. Cancer. 2012;118(17):4271–9. doi: 10.1002/cncr.27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku L, Steinmetz E, Bruen BK. Continuous-Eligibility Policies Stabilize Medicaid Coverage for Children and Could Be Extended to Adults with Similar Results. Health Affairs. 2013;32(9):1576–82. doi: 10.1377/hlthaff.2013.0362. [DOI] [PubMed] [Google Scholar]
- Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA: A Cancer Journal for Clinicians. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the Utilization of High-Volume Hospitals for Complex Surgery. Journal of the American Medical Association. 2006;296(16):1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- Lucia L, Jacobs K, Watson G, Dietz M, Roby D. Medi-Cal Expansion under the Affordable Care Act: Significant Increase in Coverage with Minimal Cost to the State. Berkeley, CA: UC Berkeley Institute for Research on Labor and Employment and UCLA Center on Health Policy Research; 2013. and. [Google Scholar]
- Malin JL, Kahn KL, Adams J, Kwan L, Laouri M, Ganz PA. Validity of Cancer Registry Data for Measuring the Quality of Breast Cancer Care. Journal of the National Cancer Institute. 2002;94(11):835–44. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]
- Morris AM, Billingsley KG, Master NN, Baldwin L-M. Racial Disparities in Rectal Cancer Treatment. Archives of Surgery. 2004;139:151–5. doi: 10.1001/archsurg.139.2.151. [DOI] [PubMed] [Google Scholar]
- Moy B, Polite BN, Halpern MT, Stranne SK, Winer EP, Wollins DS, Newman LA. American Society of Clinical Oncology Policy Statement: Opportunities in the Patient Protection and Affordable Care Act to Reduce Cancer Care Disparities. Journal of Clinical Oncology. 2011;29(28):3816–24. doi: 10.1200/JCO.2011.35.8903. [DOI] [PubMed] [Google Scholar]
- Moyer VA. Screening for Ovarian Cancer: U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. Annals of Internal Medicine. 2012;157(12):900–4. doi: 10.7326/0003-4819-157-11-201212040-00539. [DOI] [PubMed] [Google Scholar]
- O'Connell J, Maggard M, Ko CY. Cancer-Directed Surgery for Localized Disease: Underuse in the Elderly. Annals of Surgical Oncology. 2004;11:962–9. doi: 10.1245/ASO.2004.03.052. [DOI] [PubMed] [Google Scholar]
- O'Malley CD, Shema SJ, Clarke LS, Clarke CA, Perkins CI. Medicaid Status and Stage at Diagnosis of Cervical Cancer. American Journal of Public Health. 2006;96(12):2179–85. doi: 10.2105/AJPH.2005.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J, Gebretsadik T, Sabogal F, Newman J, Lawson HW. Mammography Screening among California Medicare Beneficiaries: 1993-1994. American Journal of Preventive Medicine. 1998;15(3):198–205. doi: 10.1016/s0749-3797(98)00045-2. [DOI] [PubMed] [Google Scholar]
- Perkins CI, Wright WE, Allen M, Samuels SJ, Romano PS. Breast Cancer Stage at Diagnosis in Relation to Duration of Medicaid Enrollment. Medical Care. 2001;39(11):1224–33. doi: 10.1097/00005650-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Pollitt R, Clarke C, Shema S, Swetter S. California Medicaid Enrollment and Melanoma Stage at Diagnosis A Population-Based Study. American Journal of Preventive Medicine. 2008;35(1):7–13. doi: 10.1016/j.amepre.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Mandelblatt J, Cagney K, Kerner J, Freeman H. Barriers to Follow-Up of Abnormal Screening Mammograms among Low-Income Minority Women. Cancer Control Center of Harlem. Ethnicity and Health. 1996;1(3):221–8. doi: 10.1080/13557858.1996.9961790. [DOI] [PubMed] [Google Scholar]
- Schoen C, DesRoches C. Uninsured and Unstably Insured: The Importance of Continuous Insurance Coverage. Health Services Research. 2000;35(1 Pt 2):187–206. [PMC free article] [PubMed] [Google Scholar]
- Schrag D, Virnig BA, Warren JL. Linking Tumor Registry and Medicaid Claims to Evaluate Cancer Care Delivery. Health Care Financing Review. 2009;30(4):61–73. [PMC free article] [PubMed] [Google Scholar]
- Scoggins JF, Fedorenko CR, Donahue SM, Buchwald D, Blough DK, Ramsey SD. Is Distance to Provider a Barrier to Care for Medicaid Patients with Breast, Colorectal, or Lung Cancer? Journal of Rural Health. 2012;28(1):54–62. doi: 10.1111/j.1748-0361.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics, 2011: The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA: A Cancer Journal for Clinicians. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Sommers BD, Baicker K, Epstein AM. Mortality and Access to Care among Adults after State Medicaid Expansions. New England Journal of Medicine. 2012;367(11):1025–34. doi: 10.1056/NEJMsa1202099. [DOI] [PubMed] [Google Scholar]
- Sommers BD, Long SK, Baicker K. Changes in Mortality after Massachusetts Health Care Reform a Quasi-experimental Study. Annals of Internal Medicine. 2014;160(9):585–93. doi: 10.7326/M13-2275. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Chen A. Treatment Patterns and Survival among Low-Income Medicaid Patients with Head and Neck Cancer. JAMA Otolaryngology–Head and Neck Surgery. 2013;139(5):489–95. doi: 10.1001/jamaoto.2013.2549. [DOI] [PubMed] [Google Scholar]
- Taubman SL, Allen HL, Wright BJ, Baicker K, Finkelstein AN. Medicaid Increases Emergency-Department Use: Evidence from Oregon's Health Insurance Experiment. Science. 2014;343(6168):263–8. doi: 10.1126/science.1246183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the Quality of Care and Racial Disparities in Medicare Managed Care. New England Journal of Medicine. 2005;353(7):692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- USPSTF. 2004. “ Screening for Pancreatic Cancer Recommendation Statement ” [accessed on April 18, 2014, 2004]. Available at http://www.uspreventiveservicestaskforce.org/3rduspstf/pancreatic/pancrers.pdf.
- Vandergrift JL, Niland JC, Theriault RL, Edge SB, Wong YN, Loftus LS, Breslin TM, Hudis CA, Javid SH, Rugo HS, Silver SM, Lepisto EM, Weeks JC. Time to Adjuvant Chemotherapy for Breast Cancer in National Comprehensive Cancer Network Institutions. Journal of the National Cancer Institute. 2013;105(2):104–12. doi: 10.1093/jnci/djs506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese RK, Friedman C, Ahmed F, Franks AL, Manning M, Seeff LC. Does Health Insurance Coverage of Office Visits Influence Colorectal Cancer Testing? Cancer Epidemiology, Biomarkers and Prevention. 2005;14(3):744–7. doi: 10.1158/1055-9965.EPI-04-0477. [DOI] [PubMed] [Google Scholar]
- Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis S, Bandi P, Siegel R, Stewart A, Jemal A. Association of Insurance with Cancer Care Utilization and Outcomes. CA: A Cancer Journal for Clinicians. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- Weissman JS, Zaslavsky AM, Wolf RE, Ayanian JZ. State Medicaid Coverage and Access to Care for Low-Income Adults. Journal of Health Care for the Poor and Underserved. 2008;19(1):307–19. doi: 10.1353/hpu.2008.0021. [DOI] [PubMed] [Google Scholar]
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for Colorectal Cancer: A Targeted, Updated Systematic Review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2008;149(9):638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of Race, Insurance, Socioeconomic Status, and Hospital Type on Receipt of Guideline-Concordant Adjuvant Systemic Therapy for Locoregional Breast Cancers. Journal of Clinical Oncology. 2012;30(2):142–50. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Matrix.
Appendix SA2: Length of Continuous Enrollment among Patients Who Link to Monthly Medicaid Enrollment Files.
Appendix SA3: Listing and Description of Medi-Cal Aid Codes for Restricted Eligibility.
Appendix SA4: Full Regression Results from Nested Models in All Six Cancers.


