Abstract
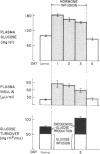
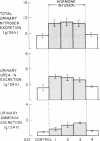
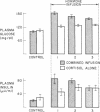
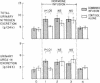
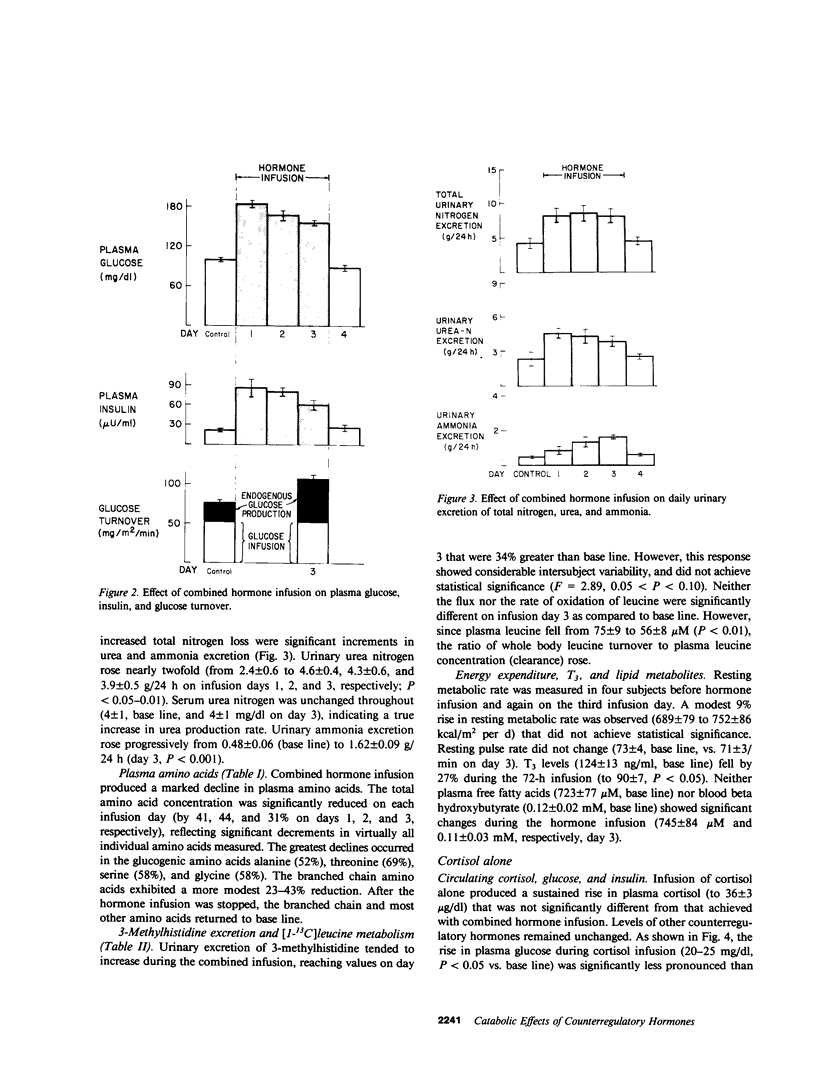
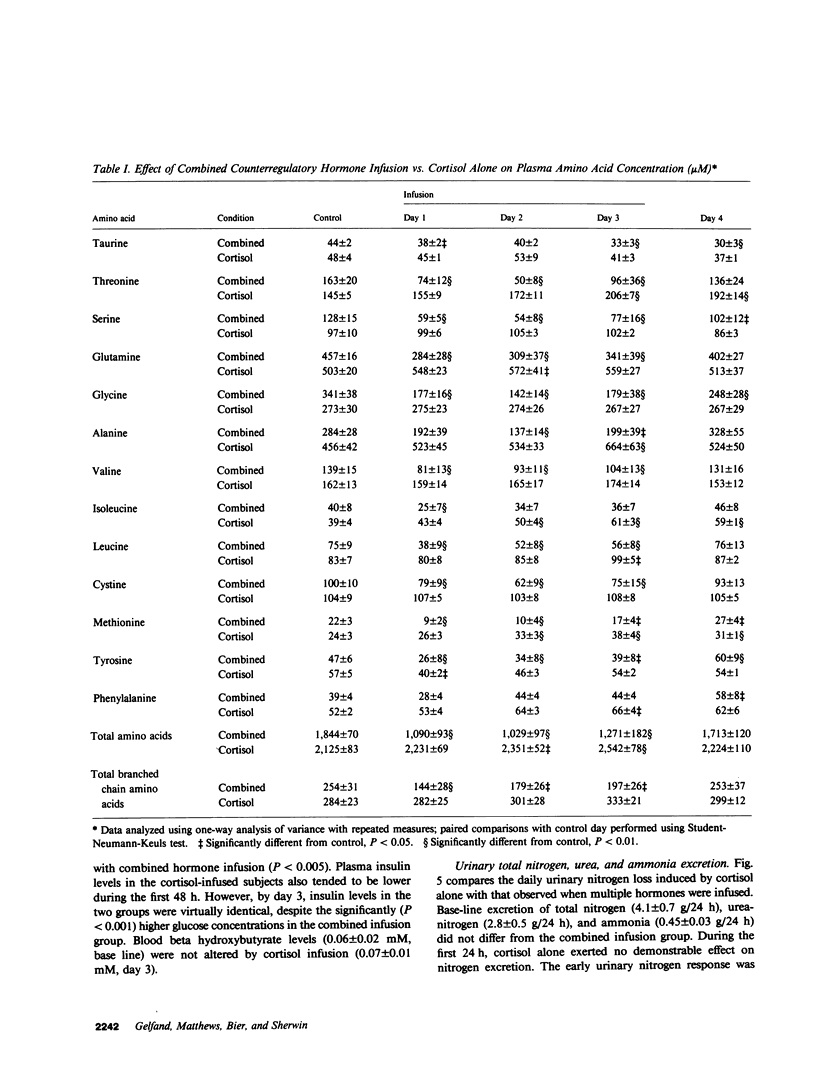
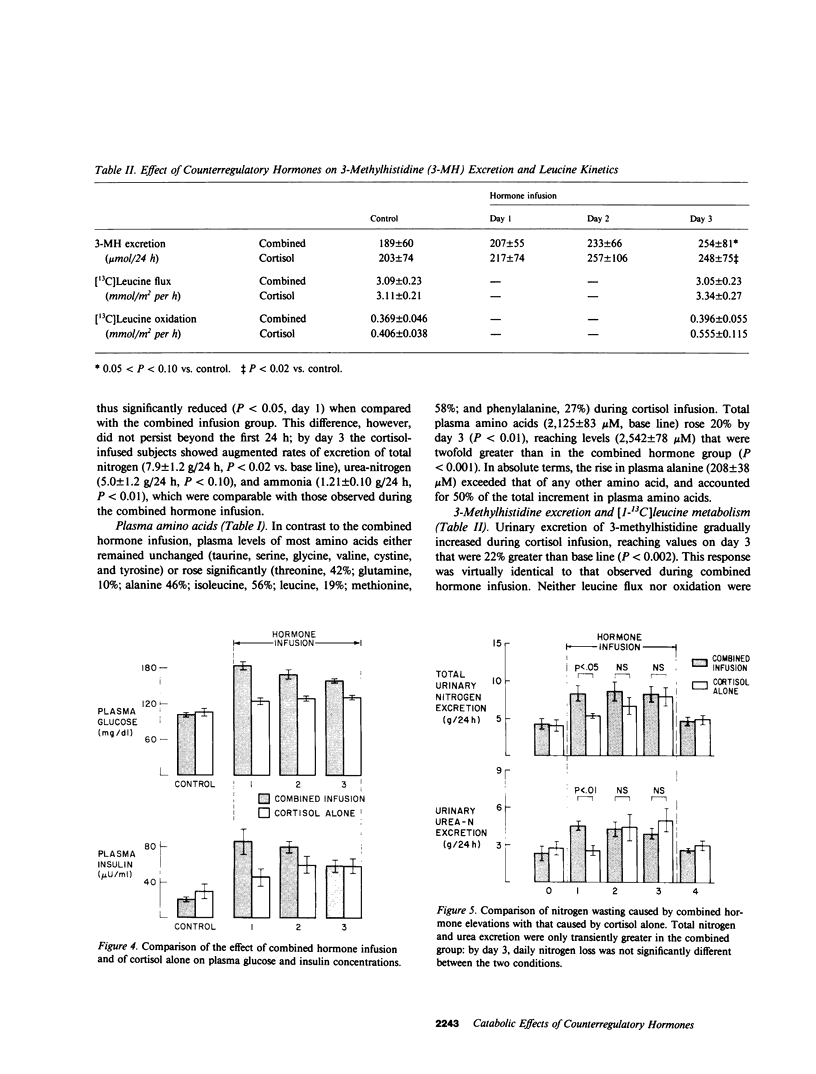
Patients with major injury or illness develop protein wasting, hypermetabolism, and hyperglycemia with increased glucose flux. To assess the role of elevated counterregulatory hormones in this response, we simultaneously infused cortisol (6 mg/m2 per h), glucagon (4 ng/kg per min), epinephrine (0.6 microgram/m2 per min), and norepinephrine (0.8 micrograms/m2 per min) for 72 h into five obese subjects receiving only intravenous glucose (150 g/d). Four obese subjects received cortisol alone under identical conditions. Combined infusion maintained plasma hormone elevations typical of severe stress for 3 d. This caused a sustained increase in plasma glucose (60-80%), glucose production (100%), and total glucose flux (40%), despite persistent hyperinsulinemia. In contrast, resting metabolic rate changed little (9% rise, P = NS). Urinary nitrogen excretion promptly doubled and remained increased by approximately 4 g/d, reflecting increased excretion of urea and ammonia. Virtually all plasma amino acids declined. The increment in nitrogen excretion was similar in three additional combined infusion studies performed in 3-d fasted subjects not receiving glucose. Cortisol alone produced a smaller glycemic response (20-25%), an initially smaller insulin response, and a delayed rise in nitrogen excretion. By day 3, however, daily nitrogen excretion was equal to the combined group as was the elevation in plasma insulin. Most plasma amino acids rose rather than fell. In both infusion protocols nitrogen wasting was accompanied by only modest increments in 3-methylhistidine excretion (approximately 20-30%) and no significant change in leucine flux. We conclude: (a) Prolonged elevations of multiple stress hormones cause persistent hyperglycemia, increased glucose turnover, and increased nitrogen loss; (b) The sustained nitrogen loss is no greater than that produced by cortisol alone; (c) Glucagon, epinephrine, and norepinephrine transiently augment cortisol-induced nitrogen loss and persistently accentuate hyperglycemia; (d) Counterregulatory hormones contribute to, but are probably not the sole mediators of the massive nitrogen loss, muscle proteolysis, and hypermetabolism seen in some clinical settings of severe stress.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Batstone G. F., Foster K. J., Johnston D. G. Relative role of various hormones in mediating the metabolic response to injury. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):141–146. doi: 10.1177/014860718000400214. [DOI] [PubMed] [Google Scholar]
- Beard J. C., Weinberg C., Pfeifer M. A., Best J. D., Halter J. B., Porte D., Jr Interaction of glucose and epinephrine in the regulation of insulin secretion. Diabetes. 1982 Sep;31(9):802–807. doi: 10.2337/diab.31.9.802. [DOI] [PubMed] [Google Scholar]
- Birkhahn R. H., Long C. L., Fitkin D., Geiger J. W., Blakemore W. S. Effects of major skeletal trauma on whole body protein turnover in man measured by L-[1,14C]-leucine. Surgery. 1980 Aug;88(2):294–300. [PubMed] [Google Scholar]
- Brandt M. R., Fernades A., Mordhorst R., Kehlet H. Epidural analgesia improves postoperative nitrogen balance. Br Med J. 1978 Apr 29;1(6120):1106–1108. doi: 10.1136/bmj.1.6120.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Shulman G. I., Lacy W. W. Differential time course of glucagon's effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes. 1981 Mar;30(3):180–187. doi: 10.2337/diab.30.3.180. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Martin H., Walji S., Hirsch E., Gazitua R., Goodfellow R. Blood insulin responses to blood glucose levels in high output sepsis and spetic shock. Am J Surg. 1978 Apr;135(4):577–583. doi: 10.1016/0002-9610(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Craig R. P., Tweedle D., Davidson H. A., Johnston I. D. Intravenous glucose, aminoacids, and fat in the postoperative period. A controlled evaluation of each substrate. Lancet. 1977 Jul 2;2(8027):8–11. doi: 10.1016/s0140-6736(77)90004-6. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D. P. Second annual Jonathan E. Rhoads Lecture. The metabolic response to injury and its nutritional implications: retrospect and prospect. JPEN J Parenter Enteral Nutr. 1979 May-Jun;3(3):108–129. doi: 10.1177/014860717900300302. [DOI] [PubMed] [Google Scholar]
- DE MOOR P., STEENO O., RASKIN M., HENDRIKX A. Fluorimetric determination of free plasma 11-hydroxycorticosteroids in man. Acta Endocrinol (Copenh) 1960 Feb;33:297–307. doi: 10.1530/acta.0.xxxiii0297. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Eigler N., Saccà L., Sherwin R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979 Jan;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Marliss E., Cahill G. F., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969 Oct 9;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani G., Vannini P., Marchesini G., Zoli M., Ciavarella A., Pisi E. Insulin-dependent metabolism of branched-chain amino acids in obesity. Metabolism. 1984 Feb;33(2):147–150. doi: 10.1016/0026-0495(84)90127-6. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. IV. beta-Adrenergic inhibition of amino acid release. J Biol Chem. 1976 Feb 10;251(3):851–857. [PubMed] [Google Scholar]
- Goldberg A. L., Goodman H. M. Relationship between cortisone and muscle work in determining muscle size. J Physiol. 1969 Feb;200(3):667–675. doi: 10.1113/jphysiol.1969.sp008715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem. 1969 Jun 25;244(12):3223–3229. [PubMed] [Google Scholar]
- Hoover H. C., Jr, Grant J. P., Gorschboth C., Ketcham A. S. Nitrogen-sparing intravenous fluids in postperative patients. N Engl J Med. 1975 Jul 24;293(4):172–175. doi: 10.1056/NEJM197507242930404. [DOI] [PubMed] [Google Scholar]
- Jättelä A., Alho A., Avikainen V., Karaharju E., Kataja J., Lahdensuu M., Lepistö P., Rokkanen P., Tervo T. Plasma catecholamines in severely injured patients: a prospective study on 45 patients with multiple injuries. Br J Surg. 1975 Mar;62(3):177–181. doi: 10.1002/bjs.1800620303. [DOI] [PubMed] [Google Scholar]
- Kien C. L., Young V. R., Rohrbaugh D. K., Burke J. F. Increased rates of whole body protein synthesis and breakdown in children recovering from burns. Ann Surg. 1978 Apr;187(4):383–391. doi: 10.1097/00000658-197804000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. B., Jefferson L. S. Effect of isoproterenol on amino acid levels and protein turnover in skeletal muscle. Am J Physiol. 1977 Feb;232(2):E243–E249. doi: 10.1152/ajpendo.1977.232.2.E243. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Lewis S. B., Cherrington A. D., Sinclair-Smith B. C., Lacy W. W. Effects of pharmacologic hyperglucagonemia on plasma amino acid concentrations in normal and diabetic man. Metabolism. 1981 Dec;30(12):1195–1199. doi: 10.1016/0026-0495(81)90041-x. [DOI] [PubMed] [Google Scholar]
- Long C. L., Birkhahn R. H., Geiger J. W., Betts J. E., Schiller W. R., Blakemore W. S. Urinary excretion of 3-methylhistidine: an assessment of muscle protein catabolism in adult normal subjects and during malnutrition, sepsis, and skeletal trauma. Metabolism. 1981 Aug;30(8):765–776. doi: 10.1016/0026-0495(81)90022-6. [DOI] [PubMed] [Google Scholar]
- Long C. L., Jeevanandam M., Kim B. M., Kinney J. M. Whole body protein synthesis and catabolism in septic man. Am J Clin Nutr. 1977 Aug;30(8):1340–1344. doi: 10.1093/ajcn/30.8.1340. [DOI] [PubMed] [Google Scholar]
- Long C. L., Spencer J. L., Kinney J. M., Geiger J. W. Carbohydrate metabolism in normal man and effect of glucose infusion. J Appl Physiol. 1971 Jul;31(1):102–109. doi: 10.1152/jappl.1971.31.1.102. [DOI] [PubMed] [Google Scholar]
- Marchuk J. B., Finley R. J., Groves A. C., Wolfe L. I., Holliday R. L., Duff J. H. Catabolic hormones and substrate patterns in septic patients. J Surg Res. 1977 Sep;23(3):177–182. doi: 10.1016/0022-4804(77)90018-x. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Unger R. H., Soeldner J. S., Cahill G. F., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970 Dec;49(12):2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. E., Motil K. J., Rohrbaugh D. K., Burke J. F., Young V. R., Bier D. M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980 May;238(5):E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- Meguid M. M., Aun F., Soeldner J. S. Temporal characteristics of insulin: glucose ratio after varying degrees of stress and trauma in man. J Surg Res. 1978 Nov;25(5):389–393. doi: 10.1016/s0022-4804(78)80002-x. [DOI] [PubMed] [Google Scholar]
- Meguid M. M., Brennan M. F., Aoki T. T., Muller W. A., Ball M. R., Moore F. D. Hormone-substrate interrelationships following trauma. Arch Surg. 1974 Dec;109(6):776–783. doi: 10.1001/archsurg.1974.01360060046013. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- O'Donnel T. F., Clowes G. H., Jr, Blackburn G. L., Ryan N. T., Benotti P. N., Miller J. D. Proteolysis associated with a deficit of peripheral energy fuel substrates in septic man. Surgery. 1976 Aug;80(2):192–200. [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Robertson R. P. Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc. 1973 Jul;32(7):1792–1796. [PubMed] [Google Scholar]
- Pozefsky T., Tancredi R. G., Moxley R. T., Dupre J., Tobin J. D. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes. 1976 Feb;25(2):128–135. doi: 10.2337/diab.25.2.128. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Hendler R., Felig P. Influence of continuous physiologic hyperinsulinemia on glucose kinetics and counterregulatory hormones in normal and diabetic humans. J Clin Invest. 1979 May;63(5):849–857. doi: 10.1172/JCI109384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà L., Vigorito C., Cicala M., Corso G., Sherwin R. S. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol. 1983 Sep;245(3):E294–E302. doi: 10.1152/ajpendo.1983.245.3.E294. [DOI] [PubMed] [Google Scholar]
- Sapir D. G., Pozefsky T., Knochel J. P., Walser M. The role of alanine and glutamine in steroid-induced nitrogen wasting in man. Clin Sci Mol Med. 1977 Sep;53(3):215–220. doi: 10.1042/cs0530215. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981 Jun;52(6):1235–1241. doi: 10.1210/jcem-52-6-1235. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Jacob R., Sherwin R. S. Epinephrine-induced hypoaminoacidemia in normal and diabetic human subjects: effect of beta blockade. Diabetes. 1980 Nov;29(11):875–881. doi: 10.2337/diab.29.11.875. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R., Felig P. Influence of physiologic hyperglucagonemia on urinary glucose, nitrogen, and electrolyte excretion in diabetes. Metabolism. 1977 Jan;26(1):53–58. doi: 10.1016/0026-0495(77)90127-5. [DOI] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOL I. G., WEINSHELBAUM E. I. Incorporation of C14-amino acids into protein of isolated diaphragms: role of the adrenal steroids. Am J Physiol. 1959 Nov;197:1089–1092. doi: 10.1152/ajplegacy.1959.197.5.1089. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W. Carbohydrate metabolism in trauma. Clin Endocrinol Metab. 1976 Nov;5(3):731–745. doi: 10.1016/s0300-595x(76)80048-5. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Long J. M., Mason A. D., Jr, Skreen R. W., Pruitt B. A., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180(4):653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. M., Culebras J. M., Aoki T. T., O'Connor N. E., Finley R. J., Kaczowka A., Moore F. D. The effects of glucagon on protein metabolism in normal man. Surgery. 1979 Aug;86(2):248–257. [PubMed] [Google Scholar]
- Wolfe R. R., Durkot M. J., Allsop J. R., Burke J. F. Glucose metabolism in severely burned patients. Metabolism. 1979 Oct;28(10):1031–1039. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]