Abstract
The human SLC52A1 gene encodes the riboflavin transporter-1 (RFVT-1), a plasma membrane protein that transports vitamin B2 (riboflavin, RF) into cells, and thus, plays a role in controlling cellular homeostasis of RF in those tissues that express the carrier protein (e.g. placenta and intestine). Currently, there is nothing known about transcriptional regulation of the SLC52A1 gene, therefore, we aimed to clone and characterize its 5′-flanking region. Using rapid amplification of the cDNA ends (5′-RACE), we identified one transcription start site (TSS). A 579 bp segment of the 5′-flanking region of this gene was cloned which exhibited robust promoter activity upon transfection in human intestinal epithelial cells. Deletion analysis revealed the core promoter activity to be embedded in a region between −234 and −23 that lacked TATA element, was GC-rich, and harbored several putative cis-regulatory sites including KLFs, AP-2, EGRF and Sp-1. Mutating each of these sites led to a significant decrease in promoter activity (which was highest for the Sp-1 site), suggesting their possible involvement in regulating SLC52A1 transcription. Focusing on the Sp-1 site, EMSA, super-shift and ChIP analysis was performed that established the interaction of the Sp-1 transcription factor with the SLC52A1 promoter; also, co-transfection of the minimal SLC52A1 promoter with an Sp-1 containing vector in Drosophila SL-2 cells led to significant promoter activation. These results are the first to reveal the identity of the minimal SLC52A1 promoter and to establish an important role for Sp-1 in its activity.
Keywords: SLC52A1, minimal promoter, riboflavin transporter, transcription regulation
1. Introduction
The water-soluble vitamin B2 (RF) in its co-enzymes forms i.e. flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), plays an important role in biochemical oxidation-reduction reactions. These reactions involve carbohydrate, lipid, and amino acid metabolism, conversion of other specific water-soluble vitamins (pyridoxine and folate) into their active forms (Cooperman and Lopez, 1984); as well as a role in proper protein folding (Tu et al., 2000). Recent studies have further shown that RF has anti-oxidant and anti-inflammatory properties (Sanches et al., 2014), can protect tissues against oxidative injury (Iwanaga et al., 2007, Mack et al., 1995, Seekamp et al., 1999), and plays a role in normal immune function (Toyosawa et al., 2004). Thus, it is not surprising that deficiency and sub-optimal levels of this essential micronutrient leads to clinical manifestations, including neurological disorders, anemia, and growth retardation (Cooperman and Lopez, 1984, Goldsmith et.al., 1975). Deficiency and sub-optimal levels of RF occurs in a variety of clinical conditions, including chronic alcoholism, diabetes mellitus, and inflammatory bowel diseases (Bonjour, 1980, Fennelly et al., 1964, Fernandez-Banares et al., 1989, Kodentsova et al., 1993, 1994, Majumdar et al., 1981, Rosenthal et al., 1973); they also occur in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency (Law et al., 2009, Liang et al., 2009), and those with Brown-Vialetto-Van Laere and Fazio Londe syndromes (BVVL/FL syndromes) (Anand et al., 2012, Bosch et al., 2011).
RF is obtained from exogenous sources, via intestinal absorption, as humans and all other mammals lack the ability to synthesize the vitamin endogenously. Two sources of RF are available to the mammalian gut: dietary sources and bacterial sources (the latter is in reference to the vitamin that is generated by the normal microbiota of the large intestine) (Wrong et al., 1981, Iinuma, 1955, Kasper, 1970, Said et al., 2000). Previous studies have shown that absorption of free RF in the small and large intestine is via specific carrier-mediated process (Said, 2004, Said and Arianas, 1991, Said et al., 1993). Recently, three specific human RFVTs have been identified: RFVT-1, RFVT-2 and RFVT-3; these transporters are encoded by the SLC52A1, SLC52A2 and SLC52A3 genes, respectively (Yamamoto et al., 2009, Yao et al., 2010, Yonezawa et al., 2008). The hRFVT-1, −2 & −3 are 448, 445, and 469 amino acid proteins, respectively, and are predicted to have 10-11 transmembrane domains. The hRFVT-1 & −2 share 87 percent identity at amino acid levels, whereas hRFVT-3 shares 41 and 44 percent identity with hRFVT-1 & −2, respectively (Yonezawa et al., 2008, Yao et al., 2010, Yonezawa and Inui, 2013). Expression of the RFVTs shows tissue specific distribution, but all are expressed in the intestine, although at different levels (Yonezawa et al., 2008, Subramanian et al., 2011). Also, while expression of RFVT-1 was found to be mainly localized at the basolateral membrane domain of the polarized intestinal epithelial cells, that of RFVT-3 was restricted only to the apical brush border membrane domain; expression of RFVT-2 is mostly intracellular with some expression at the basolateral membrane domain of these epithelial cells (Subramanian et al., 2011). Other studies have identified mutations in RFVT-3 and −2 as the cause of BVVL/FL syndromes (Green et al, 2010; Bosch et al., 2011, Foley et al., 2014). Also, a homozygous deletion of exons 2 and 3 in the SLC52A1 gene (RFVT-1) has been reported in a mother whose newborn developed clinical features of multiple acyl-CoA dehydrogenase deficiency that were corrected by RF supplementation (Ho et al., 2011).
Little is currently known about how the recently identified RFVTs are regulated at the transcriptional and post-transcriptional level in any cell type. We have been interested in understanding the mechanisms of RF transport and its regulation in intestine and other tissues for sometimes (see reviews, Said, 2004, Said and Ross, 2011, Said and Nexo, 2012, Said, 2011). With regards to the latter, we have for example shown that the intestinal RF uptake process is adaptively regulated by RF levels and during enterocyte differentiation (Said, 2011, Said and Nexo, 2012). In this study, we aimed to characterize the basal regulation of SLC52A1 transcription in a model of human intestinal epithelial cells (HuTu 80). Our results identify the SLC52A1 minimal promoter needed for basal activity, and establish an important role for the Sp-1 transcription factor in regulating activity of this promoter.
2. Materials and Methods
2.1. Materials
All molecular biology grade routine biochemical and cell culture reagents were obtained from Sigma (St. Louis, MO) and Fisher Scientific (Tustin, CA). Primers for promoter constructs, EMSA and ChIP analysis were obtained from Sigma Genosys (Woodlands, TX). PCR kit and DNA LA polymerase were bought from Clontech (Palo Alto, CA). Restriction enzymes were obtained from New England Biolabs (Beverly, MA). Anti-Sp-1 antibody and rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2. 5′-RACE
Transcription start site(s) for SLC52A1 in HuTu 80 cells was probed with the rapid amplification of the cDNA ends (RACE) utilizing 5′-RACE kit version 2.0 (Life Technologies) following the manufacturer’s protocols. For primer designing, the sequence of the human SLC52A1 mRNA was utilized from GenBank (accession no. NM_001104577.1). Two micrograms of total RNA from HuTu 80 cells was utilized for the initial RT-PCR. The following gene specific reverse primer was used 5′-ACCGGCTGTGCGTTTGGGT-3′. Next, the first-strand cDNAs were isolated and tailed. Then the tailed cDNAs was amplified using another gene-specific reverse primer 5′-CAAAGAGGGAGCTGATTTTCCTA-3′ and the manufacturer’s Abridged Anchor primer. Subsequently a nested PCR was done with the manufacturer’s Abridged Universal Amplification primer and the gene-specific primer 5′-CAGGAGGCTGAGGCAGGAGATT-3′. Finally PCR products were visualized on 2% agarose gel and also subcloned into the pGEM-T Easy vector (Promega, Madison, WI) for sequencing by Laragen Sequencing Facility (Los Angeles, CA).
2.3. Cloning of the 5′-regulatory region for the SLC52A1 gene
As an outline for designing primers to amplify SLC52A1 gene the 5′-flanking sequence of SLC52A1 was derived from GenBank (accession no. NG_033117.1; Homo sapiens solute carrier family 52, riboflavin transporter, member 1 (SLC52A1), RefSeqGene on chromosome 17). PCR was performed by using two different forward primers (forward-1: 5′-CGGGGTACCATGGTGATGCTCGCCTGT-3′ for 579-bp fragment, forward-2: 5′-CGGGGTACCGCCGGGGCAGGTGGATCACCT-3′ for 1000-bp fragment and one reverse primer 5′-CCGCTCGAGGCGACAGAACGAGATTCG-3′. Human genomic DNA (100 ng) from Promega, Madison, WI was used for templates. PCR conditions were following an initial denaturation at 95°C for 5 min, subsequent 34 cycles of denaturation at 95°C for 1 min, annealing at 54°C for 40 sec, extension for 1 min 35 sec at 72°C, and a final extension for 5 min at 72°C. The PCR products of 579-bp and 1000-bp length were purified from a 1.5% agarose gel, and cloned into the pGEM-T Easy vector (Promega, Madison, WI). The harvested PCR products were double digested with KpnI and XhoI (both the primers were underlined with this set of restriction sites), and subcloned into the KpnI/XhoI digested pGL3-Basic luciferase reporter vector (Promega) placed upstream of the luciferase gene. The DNA sequences were confirmed by sequencing (Laragen sequencing facility, Los Angeles, CA).
2.4. Construction of deleted and mutated fragments of the SLC52A1 promoter
5′-Deleted fragments of DNA were generated by PCR using specific primers (Table 1) followed by subsequent cloning of the PCR products into the KpnI/XhoI digested pGL3-Basic vector. Mutations were introduced using QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) with the primers containing mutated nucleotides (Table 2) following the manufacturer’s protocols in the consensus sites for transcriptional factors in the minimal promoter region. The sequence of all constructs (5′-deleted and mutated) was confirmed before using.
TABLE-1. Primer sequences used in PCR for deletion of 5′-regulatory region of SLC52A1 promoter.
| Sequence (5′-3′) | Position |
|---|---|
| CGGGGTACCGCTCTAGCCTGGGTGAC | −310 to −292 |
| CGGGGTACCAGCTGTGTGGGCTCTGG | −234 to −216 |
| CGGGGTACCGGATCCGGAGGTGCGG | −146 to −131 |
| CGGGGTACCAACTCGACCTGCCTCAGC | −23 to −7 |
| CGGGGTACCTGAGTGTCGCAAAAGCGC | +2 to +20 |
| CCGCTCGAGGCGACAGAACGAGATTCG | +134 to 150 |
KpnI and XhoI restriction sites are shown by bold and underline, respectively. The relative position of primers is based on numbering the start site of transcription of SLC52A1 as position+1.
TABLE-2. Sequence of primers used in PCR for generating mutant SLC52A1 minimal promoter.
| cis-elements | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| AP2 | CGGGATGCCCCCAGGAGGCAGGCGGG | CCCGCCTGCCTCCTGGGGGCATCCCG |
| EGR | CCCTGAGGCAGGCAAGAGGGCAGCACGG | CCGTGCTGCCCTCTTGCCTGCCTCAGGG |
| KLFs | CAGCACGGAGACGAACCTCCGGGCAGAG | CTCTGCCCGGAGGTTCGTCTCCGTGCTG |
| Sp-1 | CCTCCGGGCAGAGAACGCCCCAGCCACT | AGTGGCTGGGGCGTTCTCTGCCCGGAGG |
Location of point mutations are shown by bold and underlined text
2.5. Cell culture, transfection and Luciferase assay
The human-derived duodenal epithelial HuTu 80 cells (passages 5–20, American Type Culture Collection, Manassas, VA) were maintained in EMEM growth medium with 10% fetal bovine serum, sodium bicarbonate (3.6 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l). 60% confluent HuTu 80 cells grown in a 12-well plate were co-transfected with 1μg of test SLC52A1 promoter construct and 100 ng of control plasmid Renilla luciferase–thymidine kinase (pRL–TK) (Promega) along with Lipofectamine reagent (Life Technologies). The Renilla-normalized firefly luciferase activity was quantified after 48 h of transfection using the Dual Luciferase Assay system (Promega). Data are presented as fold increase over the expression of pGL3-basic set arbitrarily as one.
Drosophila SL-2 cells were seeded at approximately 5 × 105 cells per well in 12-well plates one day before transfection with Lipofectamine. Two micrograms of pGL3-Basic or SLC52A1 promoter construct were used along with the control plasmid pPac0 and 2 μg of the Drosophila Sp expression plasmid (pPacSp1 or pPacSp3 or pPacSp4), keeping the total amount of DNA constant at 4 μg (Courey and Tjian, 1988, Reidling and Said, 2003). At 48-h post-transfection, cells were harvested and firefly luciferase activity was assayed with the Luciferase Assay system (Promega). The activity was normalized to the Renilla luciferase activity from pRL-TK in the same extract. Data represented are means ± standard error of at least three independent experiments and given as fold expression over pGL3 basic expression.
2.6. Electrophoretic mobility shift assay (EMSA)
We prepared nuclear extract from HuTu 80 cells with the commercially available NE-PER Nuclear and Cytoplasmic extraction kit from Thermo Scientific Pierce, Rockford, IL following the manufacturer’s protocol. For EMSA, a 28-bp minimal promoter region (a sequence: 5′-CCTCCGGGCAGAGCCCGCCCCAGCCACT −3′), a sequence between −60 and −33 was used. The promoter was biotinylated using a Biotin 3′-end DNA labeling kit (Thermo Scientific Pierce). LightShift Chemiluminescent EMSA kit from Thermo Scientific Pierce was used for EMSA. Briefly, for binding reactions the reaction volume was 20 μl with 5 μg of nuclear extract, 20 fmol of biotin end-labeled DNA, and 50 ng/μl poly (dI.dC), and was incubated at room temperature for 20–30 min. Analysis of oligonucleotide competition was carried out with a 50-fold molar excess of unlabeled SLC52A1 DNA fragment. For super-shift assays pretreatment of the nuclear extract was carried out with 2 μg anti-Sp-1 antibody or 2 μg normal rabbit IgG. Subsequently DNA-protein complexes were separated on a 6% DNA retardation gel (Invitrogen Life Technologies) in 0.5× Tris borate-EDTA buffer at 100V, followed by electrophoretic transfer of binding reactions to nylon membrane and crosslinking of transferred DNA to membrane in UV. Biotinilated DNA was detected by chemiluminescence using Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific Pierce).
2.7. Chromatin immunoprecipitation (ChIP)
ChIP was performed with the ChIP assay kit (Millipore, Temecula, CA) following the provided manufacturer’s protocol. HuTu 80 cells (1 × 106 cells) were incubated with 1% formaldehyde (final concentration) for 10 min at 37°C to crosslink proteins to DNA, then lysed by SDS containing lysis buffer and sonicated to shear the DNA into 200–1000 bp fragments. The Sonicated cell supernatant was initially incubated with 10 μg of anti-Sp-1 or normal rabbit IgG antibody overnight at 4°C before precipitation of the immunocomplex with protein A agarose/salmon sperm DNA for 1 h at 4°C. Immunoprecipitates were finally eluted and incubated for 4 h at 65°C (to reverse protein/DNA crosslinks). Eluted samples were treated with proteinase K and the DNA was extracted by phenol/chloroform method followed by ethanol precipitation before using for PCR. Primers used for amplification of the SLC52A1 promoter region from −234 to +66 were 5′-AGCTGTGTGGGCTCTGGGC-3′ (forward) and 5′-ATGGACCCCGGGACAAGG-3′ (reverse). PCR conditions was as follows- denaturation at 95°C for 3 min, followed by 34 cycles of denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec, and extension at 72°C for 35 sec.
2.8. Data presentation and Statistical analysis
Data are presented as mean ± SE of at least three independent experiments. Statistical significance of each data was evaluated by Student’s t-test using Microsoft Excel software; p<0.05 and p<0.01 were considered as significant (*) and highly significant (**), respectively.
3. Results
3.1. Determination of the TSS of the human SLC52A1 gene in intestinal epithelial HuTu 80 cells using 5′-RACE
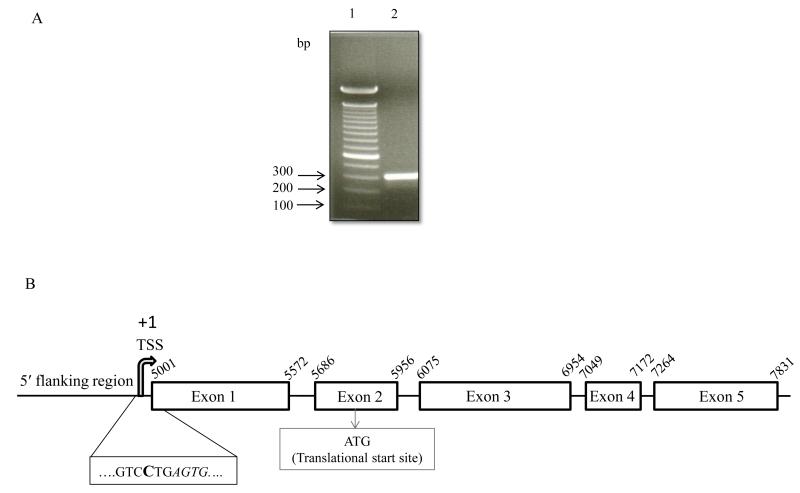
In this study, we used 5′-RACE techniques to identify the TSS of SLC52A1 in intestinal epithelial HuTu 80 cells as the first step in cloning of the putative promoter. RefSeqGene information for the SLC52A1 gene deposited in GenBank (accession number, NG_033117.1) was used for primer design. A number of clones for the single prominent PCR product of ~300 bp were obtained (Fig. 1A) and sequenced. The sequencing results from several randomly selected clones revealed the existence of one distinct TSS for SLC52A1 in HuTu 80 cells represented as cytosine (‘C’), located 2 bp before exon 1 and 828 bp upstream of the ATG codon of the SLC52A1 (Fig. 1B). We denoted the TSS as + 1 (Fig. 1B).
Fig. 1. Identification of the transcriptional start site of the SLC52A1 gene.
A. Results of 5′-RACE experiments on total RNA from HuTu 80 cells; DNA sequences were detected by gel electrophoresis using 2% agarose. Lane 1, 1Kb marker; Lane 2, A single DNA band of 300 bp was detected. B. Schematic diagram of SLC52A1 gene depicting the positions of the TSS. The TSS and the first four nucleotide of exon 1 are shown in bold type and italic, respectively. The start codon is shown in the box that is located inside exon 2.
3.2. Cloning of the 5′-regulatory region of the human SLC52A1 gene and determination of promoter activity
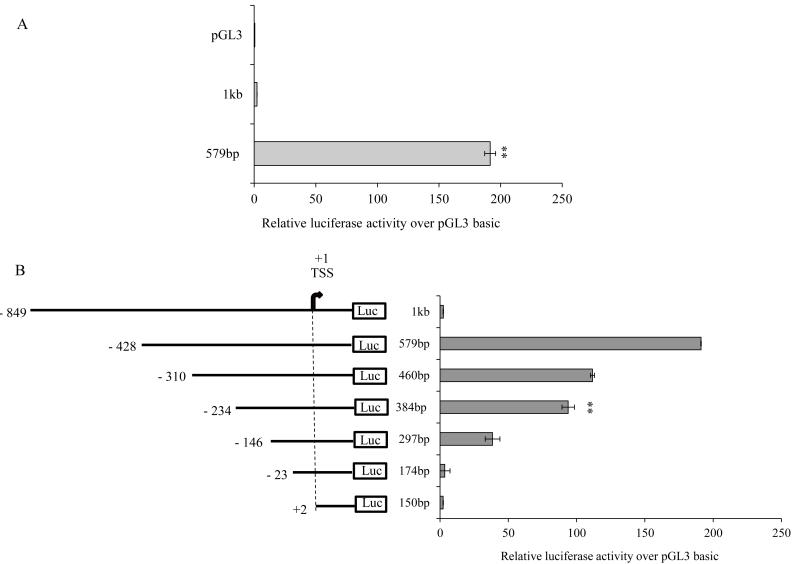
We cloned the 579-bp genomic fragment containing the 5′-regulatory region of the human SLC52A1 gene from human genomic DNA and verified its identity by sequencing. The cloned fragment starts within exon 1 of the SLC52A1 gene at position +150 (using TSS as +1) and extends to −428 upstream of the TSS. Transient transfection of human intestinal epithelial HuTu 80 cells with the cloned −428/+150 genomic fragment using the Firefly luciferase reporter construct resulted in a significant (P < 0.01) increase in promoter activity, which was over 190-fold above the activity observed with the pGL3-basic vector (Fig. 2A). In contrast, a larger 1000 bp genomic fragment (−849/+150) of the SLC52A1 5′-regulatory region cloned into pGL3-basic plasmid and transfected in HuTu 80 cells did not show any promoter (luciferase) activity (Fig. 2A). The latter observation suggests possible existence of strong suppressor element between - 849 nt and −429 nt of the SLC52A1 5′-regulatory region.
Fig. 2. SLC52A1 promoter activity in HuTu 80 cells and deletion analysis of the SLC52A1 promoter.
A. Promoter activity of 1000bp and 579bp promoter fragments of SLC52A1 in HuTu 80 cells. B. Schematic diagrams of the 5′-deleted promoter constructs are shown on the left. 5′-deletions were introduced into the 1000 bp promoter fragment as described in MATERIALS AND METHODS section. All construct are derivatives of the pGL3-basic vector. Promoter constructs in pGL3-basic vector were transiently expressed in HuTu 80 cells for luciferase assay. Firefly luciferase activity was normalized relative to Renilla luciferase activity and results were expressed as fold over pGL3-basic expression; data are presented as mean ± SE of at least 3 independent experiments.
3.3. Determination of the minimal SLC52A1 promoter
We performed 5′-deletion analysis to determine the minimal region required for basal activity of the SLC52A1 promoter. A series of 5′-deletion reporter plasmid constructs containing specific lengths of the SLC52A1 (ranging from −428 to +2) were generated by PCR amplification (and verified by sequencing) and cloned upstream of the Firefly luciferase gene. Promoter activity of each of the deletion constructs was assessed by measuring luciferase activities in transiently transfected HuTu 80 cells. The results showed luciferase activity of the shorter 384 bp promoter fragment (−234/+150) had ~50% less activity than the full-length 579 bp (−428/+150) promoter (Fig 2B). A further deletion of 88 bp (−234/−146) from 5′-end of the 384 bp fragment resulted in a drastic reduction in promoter activity, whereas deletion of an additional 123 bp (−146/−23) completely abolished the luciferase activity (Fig. 2B). These results suggest that the minimal promoter sequence of the SLC52A1 gene has encoded in the sequence between −234 and −23.
3.4. Identification of putative cis-regulatory elements in the SLC52A1 minimal promoter
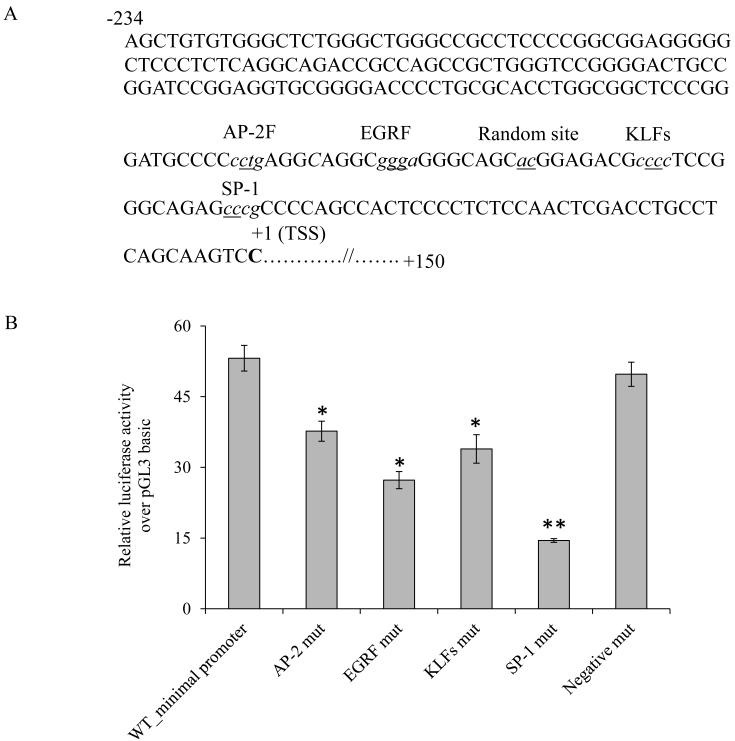
To obtain information on putative cis-regulatory binding sites in the minimal promoter region of the SLC52A1 gene, we subjected the DNA fragment (−234/−23) to MatInspector analysis (www.genomatix.de). The analysis revealed an absence of the typical TATA motif and the existence of multiple putative cis-regulatory elements including Stimulating protein 1 (Sp-1)/GC-box, Activator protein 2 (AP2), Krueppel like transcription factors (KLFs) and EGR/nerve growth factor induced protein C & related factors (EGRF) (Fig. 3A). To assess the involvement of these putative cis-regulatory sites in regulating the basal activity of the SLC52A1 promoter, we introduced specific point mutations in the core sequences of these sites and examined the effect of these mutations on promoter activity. As a negative control, the effect of mutating a random site (−71/−72) in the minimal promoter was examined. In this context we compared the minimal promoter (−234/+150) activity with mutant promoters after transfection into HuTu 80 cells. The results showed that mutating all the cis-elements identified by MatInspector, but not the randomly mutated sites, led to a significant (P < 0.01) reduction in promoter activity compared to the minimal promoter (Fig. 3B). The reduction, however, was most pronounced in the Sp-1 site located at −47/−41 position, where an approximate 72% reduction in promoter activity was observed. For this reason we focused on this site and its interacting transcription factor in subsequent experiments.
Fig. 3. Schematic of the SLC52A1 minimal promoter region and mutational analysi of cis-regulatory elements.
A. Schematic depicting the minimal promoter sequence (−234 to +150) with annotated putative cis-elements. Cis-elements on the complementary strand are shown in italicized letters. The nucleotides that were altered for mutational analysis are underlined. TSS is bold type and numbered as +1. B: The mutated minimal promoter constructs in pGL3-Basic were transiently expressed into HuTu 80 cells for luciferase assays. Firefly luciferase activity was normalized relative to Renilla luciferase activity and results were expressed as fold over pGL3-basic expression; data are presented as mean ± SE of at least 3 independent experiments.
3.5. Conformation of binding of Sp-1 in the SLC52A1 minimal promoter
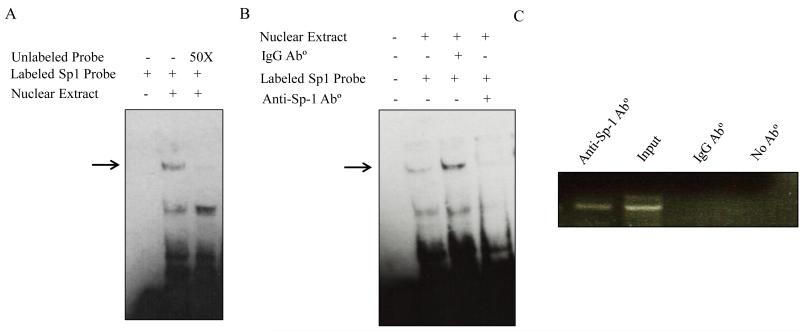
To further establish the importance of the Sp-1 cis-element in SLC52A1 promoter activity, we first examined the interaction of a 28-bp region (−60/−33) of the minimal promoter containing this site and nuclear extract from HuTu 80 cells, i.e., performed EMSA. The results showed the formation of two major DNA-protein complexes (two major bands, bands 1 and 2) with decreased gel mobility (Fig. 4A, lane 2). One of these two major DNA-protein complexes was specific and was competed away with a 50-fold molar excess of the unlabeled DNA probe (Fig. 4A, lane 3). To establish the involvement of the Sp-1 transcription factor in the formation of the protein - DNA complex that was detected in the EMSA, we then performed super-shift analysis in which we pre-incubated the HuTu 80 cells nuclear extract with specific anti-Sp-1 antibodies. The result showed clear disappearance of the specific protein-DNA complex (Fig. 4B, lane 4), indicating that the interacting protein is Sp-1. The nonspecific IgG antibodies (served as negative control) had no effect on mobility of the protein-DNA complex (Fig. 4B, lane 3). These results suggest that Sp-1 can specifically bind to its site in the SLC52A1 promoter fragment.
Fig. 4. Binding of Sp-1 transcription factor to the SLC52A1 promoter in vitro and in vivo.
A. DNA/protein profile from EMSA using the SLC52A1 minimal promoter region. Gel shift assays were conducted with HuTu 80 cells nuclear extract as described in MATERIALS AND METHODS section. The labeled fragment of the SLC52A1 promoter region (−60/−33) was used. DNA/protein complexes were resolved on 6% retardation acrylamide gel. Competition experiments were performed using a 50 fold molar excess of unlabeled promoter fragment. The specific complex is indicated with an arrow. B. Presence of Sp1 in DNA-protein complexes. Nuclear extracts were pre-incubated with anti-Sp-1 antibody or nonspecific IgG before the addition of the biotin labeled Sp-1 probe. Data are representative of three separate experiments. C. Chromatin immunoprecipitation analysis. Formaldehyde cross-linked chromatin from HuTu 80 cells was incubated with anti-Sp-1 antibody. As a negative control, the chromatin was incubated without antibodies or with nonspecific IgG antibody. Total input DNA at a 1:10 dilution was used as a positive control for the PCR reaction. Immunoprecipitated DNA was analyzed by PCR with primers specific for the SLC52A1 promoter.
To further confirm the existence of direct binding of Sp-1 to the SLC52A1 promoter and to do so in vivo, we carried out ChIP assay using cross-linked chromatin prepared from HuTu 80 cells. Chromatin was sheared by sonication into fragments of 200-1000 bp followed by immunoprecipitation using anti-Sp-1 antibodies or nonspecific rabbit IgG (as negative control). The purified DNA from the immune-precipitated chromatin was used as template for PCR amplification, using primers that specifically amplify a 300 bp region (−234/+66) harboring the Sp-1 site. As shown in Fig. 4C, the amplification of the region −234 to +66 was detected in immunoprecipitated obtained with anti-Sp-1 antibody, but not with the nonspecific rabbit IgG. These data further demonstrate that anti-Sp-1 antibody is able to specifically precipitate the minimal SLC52A1 promoter region, and provide a direct conformation of Sp-1 association with the minimal SLC52A1 promoter in vivo.
3.6. Role of Sp-1 in the activation of the SLC52A1 promoter: studies with Drosophila SL-2 cells
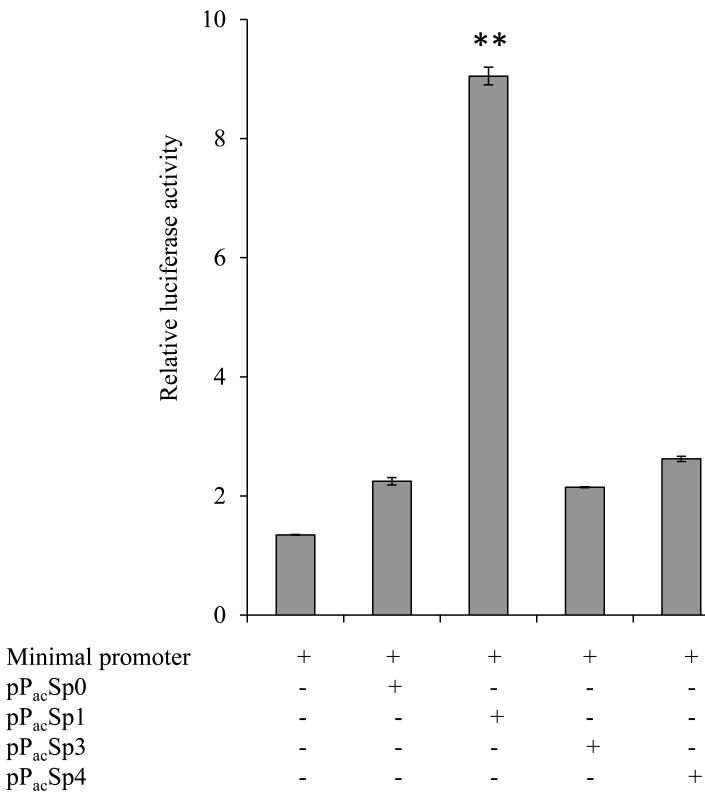
To further confirm the role of Sp-1 in the activation of the SLC52A1 promoter described earlier, we used Drosophila SL-2 cells since these cells lacks endogenous Sp factors (Courey and Tjian, 1988, Reidling and Said, 2003). For this, we co-transfected the minimal promoter construct (−234/+149) of SLC52A1 with Drosophila expression vectors pPacSp0, and pPacSp1 into Drosophila SL-2 cells and also tested the effect on promoter activity of pPacSp3 and pPacSp4. The level of reporter gene expression detected for the construct in the absence of co-expression of exogenous Sp protein was assigned the arbitrary value of 1. The results showed a significant (P < 0.01; 8-fold) induction in promoter activity upon co-expression with pPacSp1 but not with pPacSp3 or pPacSp4 (Fig. 5). These findings confirm the important role and specificity of Sp-1 in activating the SLC52A1minimal promoter.
Fig. 5. Co-transfection assays using both pPacSp1 or pPacSp3 or pPacSp4 vectors and SLC52A1 minimal promoter luciferase construct in Drosophila SL-2 cells.
SL-2 cells were co-transfected with 3μg of the minimal promoter construct in pGL3-basic and 2μg of either control plsmid pPac0 or Drosophila Sp expression plasmids (pPacSp1 or pPacSp3 or pPacSp4). At 48 h post-transfection, cells were harvested and firefly luciferase activity was assayed as described in MATERIALS AND METHODS. Firefly luciferase activity was normalized relative to Renilla luciferase activity and results were expressed as fold over pGL3-basic expression; data are presented as mean ± SE of at least 3 independent experiments.
4. Discussion
Our laboratory has been interested in delineating the mechanisms and regulation of RF transport in intestinal and other epithelial cells (Said, 2004, Said and Arianas, 1991, Said et al., 1993, Said and Ross, 2011, Said and Nexo, 2012, Said, 2011), but it has been difficult to characterize the regulatory aspects at the molecular level due to lack of knowledge of the identity of RF transporters. With the recent cloning and molecular identification of three specific RFVTs, this issue became feasible. Taking advantage of this advancement, we report here on the first characterization of the transcriptional regulation of the SLC52A1 gene (which encodes RFVT-1). First we identified the TSS of the SLC52A1 gene in human-derived intestinal HuTu 80 cells using 5′-RACE approach and found one TSS, located outside of predicted exon 1. Based on the location of TSS, we subsequently amplified and cloned a 579 bp and a 1000 bp genomic DNA fragments and tested their promoter activity after transfecting them into HuTu 80 cells. The 579 bp (−428/+150) fragment had a robust promoter activity in these cells while the 1000 bp (−849/+150) fragment had virtually no promoter activity. These findings suggest the presence of a possible suppressor in the upstream region (−849 to −429) of the SLC52A1 promoter; further studies, however, are needed to test this possibility. Our results showed the minimal SLC52A1 promoter region to be between −234 nt and −23 nt using a 5′-deletion approach. This region retained approximately 50% of the activity of the 579 bp promoter fragment. The minimal region was found to be TATA-less, GC-rich, and contained a number of putative cis-regulatory elements that include EGRF, AP-2, KLFs and Sp-1. The Role of these putative cis-elements in regulating the activity of the SLC52A1 minimal promoter was examined by mutating these sites and testing the effect of such mutations on promoter activity. Mutating all the putative cis-elements led to a significant reduction in promoter activity, with the degree of reduction being highest for the Sp-1 binding site. For that reason we focused our subsequent investigations on this site and on the nuclear factor that interacts with it.
To establish the interaction of Sp-1 with the SLC52A1 promoter, we first performed EMSA using a 28 bp region of the minimal promoter that contain this cis-regulatory site and observed two major bands representing DNA-protein complexes. One of these bands was found to be specific and was competed away by an excess of the unlabeled probe. We then performed super-shift analysis using anti Sp-1 antibody with the results showing a complete disappearance of the specific DNA-protein complex. Similar disappearance of the specific DNA-protein complex has been observed with other genes (Han et al., 2001; Zatyka et al., 2002; Omotehara et al., 2002; Chen et.al., 2007), which could be due to precipitation of the corresponding protein-DNA complex by Sp-1 antibodies, or due to interaction of the Sp-1 antibodies with the nuclear Sp-1 protein leading to inability of the latter to bind its specific site in the DNA. This suggests that the Sp-1 nuclear factor (which is ubiquitously expressed in mammalian cells) does indeed bind to the SLC52A1 promoter. The latter was further confirmed by the ChIP assay, which showed anti-Sp-1 antibody specifically precipitates the SLC52A1 promoter whereas nonspecific rabbit IgG antibody was not able to do so. Based on the above observations, it is concluded that the Sp-1 transcriptional factor does bind to the SLC52A1 promoter.
To further establish a role for Sp-1 in the activity of the SLC52A1 promoter, we also used the Drosophila SL-2 cellular system. These cells lack Sp nuclear factors and have been used as a model to verify role of Sp factors in activity of promoters of different genes (Courey and Tjian, 1988, Reidling and Said, 2003, Wang and Bannon, 2005, Wan et al., 2005). For this we co-transfected the minimal SLC52A1 promoter with a human Sp-1 containing vector and observed a significant induction to promoter activity. It is interesting that transfecting these cells with the transcription factors Sp-3 and Sp-4 (which can also interact with Sp cis-elements in gene promoters, Han et al., 2001, Wan et al., 2005, Nabokina and Said, 2004) did not affect the activity of the SLC52A1 minimal promoter demonstrating the specificity of the Sp-1 effect.
In conclusion, this study describes the first characterization of the SLC52A1promoter and reports on the identification of the minimal promoter and key transcription factors involved in its activity. Results of these investigations should assist us in understanding how expression of the SLC52A1 gene is affected in conditions like RF deficiency or over-supplementation, and how exposure to environmental/external factors like chronic alcohol use could affect this expression. The findings should serve as base for future investigations into the molecular regulation of this important human gene in health and disease.
Highlights.
SLC52A1 minimal promoter was identified
Roles of putative cis-regulatory elements were established by point mutations; Sp-1site appears to play a predominant role
Binding of Sp-1 to the minimal promoter was established by EMSA and ChIP assays
Expression of Sp-1 in Drosophila SL-2 cells activated SLC52A1 promoter
Sp-1 plays a key role in the transcriptional regulation of the SLC52A1 gene
Acknowledgements
This work was supported by grants from the DVA and the NIH (DK56061, DK58057).
Abbreviations
- RFVT-1
riboflavin transporter-1
- RF
riboflavin
- 5′-RACE
5′-rapid amplification of the cDNA ends
- TSS
Transcription start site
- FMN
flavin mononucleotide
- FAD
flavin adenine dinucleotide
- EMSA
Electrophoretic mobility shift assay
- ChIP
Chromatin immuneprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare.
Author contribution
SS, AG and HMS designed the research. SS and AG performed the experiments. SS, AG and HMS analyzed the data and wrote the paper.
References
- Anand G, Hasan N, Jayapal S, Huma Z, Ali T, Hull J, Blair E, McShane T, Jayawant S. Early use of high-dose riboflavin in a case of Brown-Vialetto-Van Laere syndrome. Dev. Med. Child. Neurol. 2012;54:187–189. doi: 10.1111/j.1469-8749.2011.04142.x. [DOI] [PubMed] [Google Scholar]
- Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int. J. Vitam. Nutr. Res. 1980;50:425–440. [PubMed] [Google Scholar]
- Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J. Inherit. Metab. Dis. 2011;34:159–164. doi: 10.1007/s10545-010-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang J, Baker SM, Chen G. Human constitutive androstane receptor mediated methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1) Toxicology. 2007;231:224–233. doi: 10.1016/j.tox.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperman JM, Lopez R, editors. Nutrional, Biochemical and Clinical Aspects. Dekker; New York: 1984. Handbook of Vitamins; pp. 299–327. [Google Scholar]
- Courey A, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988;55:887–888. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic. I. Aetiological role of aneurin and other B-complex vitamins. Br. Med. J. 1964;2:1290–1292. doi: 10.1136/bmj.2.5420.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1989;84:744–748. [PubMed] [Google Scholar]
- Foley AR, Menezes MP, Pandraud A, Gonzalez MA, Al-Odaib A, Abrams AJ, Sugano K, Yonezawa A, Manzur AY, Burns J, Hughes I, McCullagh BG, Jungbluth H, Lim MJ, Lin JP, Megarbane A, Urtizberea JA, Shah AH, Antony J, Webster R, Broomfield A, Ng J, Mathew AA, O’Byrne JJ, Forman E, Scoto M, Prasad M, O’Brien K, Olpin S, Oppenheim M, Hargreaves I, Land JM, Wang MX, Carpenter K, Horvath R, Straub V, Lek M, Gold W, Farrell MO, Brandner S, Phadke R, Matsubara K, McGarvey ML, Scherer SS, Baxter PS, King MD, Clayton P, Rahman S, Reilly MM, Ouvrier RA, Christodoulou J, Züchner S, Muntoni F, Houlden H. Treatable childhood neuronopathy caused by mutations in riboflavin transporter RFVT2. Brain. 2014;137:44–56. doi: 10.1093/brain/awt315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, et al. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in C20orf54. Am J Hum Genet. 2010;86:485–489. doi: 10.1016/j.ajhg.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith GA. In: Riboflavin deficiency. Rivlin R, editor. Ribroflavin, Plenum; New York: 1975. pp. 221–244. [Google Scholar]
- Han B, Liu N, Yang X, Sun HB, Yang YC. MRG1 expression in fibroblasts is regulated by Sp1/Sp3 and an Ets transcription factor. J. Biol. Chem. 2001;276:7937–7942. doi: 10.1074/jbc.M007470200. [DOI] [PubMed] [Google Scholar]
- Ho G, Yonezawa A, Masuda S, Inui K, Sim KG, Carpenter K, Olsen RK, Mitchell JJ, Rhead WJ, Peters G, Christodoulou J. Maternal riboflavin deficiency, resulting in transient neonatal-onset glutaric aciduria Type 2, is caused by a microdeletion in the riboflavin transporter gene GPR172B. Hum Mutat. 2011;32:E1976–1984. doi: 10.1002/humu.21399. [DOI] [PubMed] [Google Scholar]
- Iinuma S. Synthesis of riboflavin by intestinal bacteria. J. Vitaminol. (Kyoto) 1955;1:6–13. doi: 10.5925/jnsv1954.1.2_6. [DOI] [PubMed] [Google Scholar]
- Iwanaga K, Hasegawa T, Hultquist DE, Harada H, Yoshikawa Y, Yanamadala S, Liao H, Visovatti SH, Pinsky DJ. Riboflavin-mediated reduction of oxidant injury, rejection, and vasculopathy after cardiac allotransplantation. Transplantation. 2007;83:747–753. doi: 10.1097/01.tp.0000256283.06469.d4. [DOI] [PubMed] [Google Scholar]
- Kasper H. Vitamin absorption in the colon. Am. J. Proctol. 1970;21:341–345. [PubMed] [Google Scholar]
- Kodentsova VM, Vrzhesinskaia OA, Sokol’nikov AA, Alekseeva IA, Spirichev VB. Metabolism of riboflavin and B group vitamins functionally bound to it in insulin-dependent diabetes mellitus. Vopr. Med. Khim. 1993;39:33–36. [PubMed] [Google Scholar]
- Kodentsova VM, Vrzhesinskaia OA, Trofimenko EV, Sokol’nikov AA, Beketova NA, Blazheevich NV, Isaeva VA, Aleinik SI, Trofimenko LS, Dronova VI. Vitamin status of children with diabetes mellitus. Vopr. Med. Khim. 1994;40:45–48. [PubMed] [Google Scholar]
- Law LK, Tang NL, Hui J, Fung SL, Ruiter J, Wanders RJ, Fok TF, Lam CW. Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin. Chim. Acta. 2009;404:95–99. doi: 10.1016/j.cca.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Liang WC, Ohkuma A, Hayashi YK, Lopez LC, Hirano M, Nonaka I, Noguchi S, Chen LH, Jong YJ, Nishino I. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul. Disord. 2009;19:212–216. doi: 10.1016/j.nmd.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CP, Hultquist DE, Shlafer M. Myocardial flavin reductase and riboflavin: a potential role in decreasing reoxygenation injury. Biochem Biophys Res Commun. 1995;212:35–40. doi: 10.1006/bbrc.1995.1932. [DOI] [PubMed] [Google Scholar]
- Majumdar SK, Shaw GK, Thomson AD. Vitamin utilization status in chronic alcoholics. Int. J. Vitam. Nutr. Res. 1981;51:54–58. [PubMed] [Google Scholar]
- Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G822–9. doi: 10.1152/ajpgi.00234.2004. [DOI] [PubMed] [Google Scholar]
- Omotehara F, Kawamata H, Uchida D, Hino S, Nakashiro K, Fujimori T. Vesnarinone, a differentiation inducing drug, directly activates p21(waf1) gene promoter via Sp1 sites in a human salivary gland cancer cell line. Br J Cancer. 2002;87:1042–1046. doi: 10.1038/sj.bjc.6600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal WS, Adham NF, Lopez R, Cooperman JM. Riboflavin deficiency in complicated chronic alcoholism. Am. J. Clin. Nutr. 1973;26:858–860. doi: 10.1093/ajcn/26.8.858. [DOI] [PubMed] [Google Scholar]
- Reidling JC, Said HM. In vitro and in vivo characterization of the minimal promoter region of the human thiamin transporter SLC19A2. Am. J. Physiol. Cell. Physiol. 2003;285:633–41. doi: 10.1152/ajpcell.00076.2003. [DOI] [PubMed] [Google Scholar]
- Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am. J. Physiol. Cell. Physiol. 2000;278:C 270–276. doi: 10.1152/ajpcell.2000.278.2.C270. [DOI] [PubMed] [Google Scholar]
- Said HM. Recent advances in carrier-mediated intestinal absorption of water-soluble vitamins. Annu. Rev. Physiol. 2004;66:419–446. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- Said HM, Arianas P. Transport of riboflavin in human intestinal brush border membrane vesicles. Gastroenterology. 1991;100:82–88. doi: 10.1016/0016-5085(91)90586-a. [DOI] [PubMed] [Google Scholar]
- Said HM, Hollander D, Mohammadkhani R. Uptake of riboflavin by intestinal basolateral membrane vesicles: a specialized carrier-mediated process. Biochim Biophys Acta. 1993;1148:263–268. doi: 10.1016/0005-2736(93)90138-p. [DOI] [PubMed] [Google Scholar]
- Said HM, Ross C. In: Modern Nutrition in Health and Disease. ROSS C, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Lippincott Williams and Wilkins; New York: 2011. pp. 325–330. [Google Scholar]
- Said HM, Nexo E. Intestinal absorption of water-soluble vitamins. In: Johnson LR, Ghishan FK, Merchand JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. Elsevier Press; San Diego, CA: 2012. pp. 1711–1756. [Google Scholar]
- Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–72. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches SC, Ramalho LN, Mendes-Braz M, Terra VA, Cecchini R, Augusto MJ, Ramalho FS. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem. Toxicol. 2014;67:65–71. doi: 10.1016/j.fct.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Seekamp A, Hultquist DE, Till GO. Protection by vitamin B2 against oxidant-mediated acute lung injury. Inflammation. 1999;23:449–460. doi: 10.1023/a:1021965026580. [DOI] [PubMed] [Google Scholar]
- Subramanian VS, Subramanya SB, Rapp L, Marchant JS, Ma TY, Said HM. Differential expression of human riboflavin transporters-1, -2, and -3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta. 2011;1808:3016–3021. doi: 10.1016/j.bbamem.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyosawa T, Suzuki M, Kodama K, Araki S. Highly purified vitamin B2 presents a promising therapeutic strategy for sepsis and septic shock. Infect Immun. 2004;72:1820–1823. doi: 10.1128/IAI.72.3.1820-1823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000;290:1571–1574. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- Wrong OM, Edmonds CJ, Chadwich VS. Vitamins, In The Large Intestine; Its Role In mammalian Nutrition and Homeostasis. Wiley and Sons; New York: 1981. pp. 157–166. [Google Scholar]
- Wang J, Bannon MJ. Sp1 and Sp3 activate transcription of the human dopamine transporter gene. J. Neurochem. 2005;93:474–82. doi: 10.1111/j.1471-4159.2005.03051.x. [DOI] [PubMed] [Google Scholar]
- Wan J, Carr BA, Cutler NS, Lanza DL, Hines RN, Yost GS. SP1 AND SP3 regulate basal transcription of the human cyp2f1 gene. Drug. Metab. Dispos. 2005;33:1244–1253. doi: 10.1124/dmd.105.004069. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J. Biochem. 2009;145:437–443. doi: 10.1093/jb/mvn181. [DOI] [PubMed] [Google Scholar]
- Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J. Nutr. 2010;140:1220–1226. doi: 10.3945/jn.110.122911. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am. J. Physiol. Cell. Physiol. 2008;295:632–641. doi: 10.1152/ajpcell.00019.2008. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Inui K. Novel riboflavin transporter family RFVT/SLC52: Identification, functional characterization and genetic diseases of RFVT/SLC52. Mol Aspects Med. 2013;34:693–701. doi: 10.1016/j.mam.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Zatyka M, Morrissey C, Kuzmin I, Lerman MI, Latif F, Richards FM, Maher ER. Genetic and functional analysis of the von Hippel-Lindau (VHL) tumour suppressor gene promoter. J Med Genet. 2002;39:463–72. doi: 10.1136/jmg.39.7.463. [DOI] [PMC free article] [PubMed] [Google Scholar]