Abstract
The vascular endothelial growth factor (VEGF)-C–induced down-regulation of VEGF receptor (VEGFR)-3 is important in lymphangiogenesis. Here, we demonstrate that VEGF-C, -D, and -C156S, but not VEGF-A, down-regulate VEGFR-3. VEGF-C stimulates VEGFR-3 tyrosyl phosphorylation and transient phosphorylation of ERK, p38, and c-Jun N-terminal kinases in lymphatic endothelial cells. VEGF-C–induced down-regulation of VEGFR-3 was blocked by a VEGF-C trap, tyrosine kinase inhibitor, and LPE (leupeptin, pepstatin, and E64), but was unaffected by Notch 1 activator and γ-secretase inhibitors. Our findings indicate that VEGF-C down-regulates VEGFR-3 in lymphatic endothelial cells through VEGFR-3 kinase activation and, in part, via lysosomal degradation.
Introduction
Vascular endothelial growth factors (VEGFs) are dimeric glycoproteins that are critical regulators of hemangiogenesis and lymphangiogenesis [1]. The mammalian VEGF family consists of five ligands, VEGF-A, -B, -C, -D, and placenta growth factor (PlGF), and three receptors, VEGFR-1, VEGFR-2, and VEGFR-3, which are members of the receptor tyrosine kinase superfamily. VEGF-C is a ligand for VEGFR-3, through which it is capable of inducing both lymphatic and blood vessel growth in many tissues including the mouse cornea [2–5]. VEGF-C, when applied to the cornea, induces corneal neovascularization and lymphangiogenesis by promoting lymphatic endothelial cell (LEC) proliferation, migration, and tube formation [6].
VEGF-C stimulation of VEGFR-3 leads to the autophosphorylation of VEGFR-3 intracellular tyrosine residues Y1230 and Y1231, resulting in binding of the SRC homology-containing signaling adaptor proteins and growth factor receptor binding protein 2, which activates ERK 1/2 and PI3K via phosphorylation [7,8]. Alternatively, autophosphorylation of the VEGFR-3 tyrosine residue Y1063 induces a survival signaling cascade via CRKI/II, leading to c-JUN expression [8]. The ERK and PI3K pathways have been implicated in endothelial cell (EC) proliferation, migration, and survival, whereas c-Jun is necessary for pro-survival signaling by VEGFR-3 [8–10].
VEGFR-3 activity is implicated in numerous pathologies, including chronic inflammation, corneal neovascularization and graft rejection, and tumor growth and metastasis [11,12]. Therefore, there is clear clinical value in exploring potential therapeutic approaches to blocking VEGFR-3 activity [13] [14] [15]. Experimentally demonstrated methods for blocking VEGFR-3 signaling include a recently discovered naturally occurring soluble VEGFR-3 [16], a synthetic soluble VEGFR-3 fusion protein [17], antibodies against VEGFR-3 [18,19], small interfering RNAs [20], tyrosine kinase inhibitors (TKIs) [21], which prevent phosphorylation of the tyrosine residues and further downstream signaling, and endostatin-containing fragments, which can be synthesized with receptor specificity [22].
However, little is known about the mechanisms that regulate VEGFR-3 in vivo. It has been shown that blockade of the Notch signaling pathway, a family of receptors that regulate cell fate determination through direct cell-cell interactions with the γ-secretase inhibitor DAPT (difluorophenylacetyl-alanyl-phenylglycine-t-butyl-ester) induces VEGFR-3 transcription in the retina [19,23]. Although some findings indicate that Notch may negatively regulate VEGFR-3, another group found that Notch induces VEGFR-3 expression through a complex with CBF-1/suppressor of hairless/Lag1 [23]. Moreover, in ECs, Notch can make VEGFR-3 more receptive to VEGF-C, promoting blood EC survival and morphological changes [23]. Thus, the relationship between Notch and VEGFR-3 appears to be important, but complicated.
Matrix metalloproteinases (MMPs) are generally considered to be responsible for extracellular matrix remodeling, but recent evidence shows they may also interact with members of the VEGF family of proteins [24] [25]. These relationships remain poorly understood, but there is evidence for MMP involvement in VEGFR-3 signaling. For example, MMP-9 may act through VEGFR-2 and -3 to promote cancer cell migration [26].
An important cellular mechanism used to inactivate proteins, including receptor tyrosine kinases (RTKs) such as VEGFR-3, is internalization and degradation. Activated RTKs undergo endocytosis and are, by the clathrin-mediated endosomal pathway, either recycled to the cell surface or transported to lysosomes for degradation, which represents a form of receptor down-regulation that can be crucial to the termination of cell proliferation signals produced by activated receptors [27–29]. Caveolin-1 has specifically is involved in the modulation of phosphorylated VEGFR-3 in ECs during angiogenesis [30] and tumor metastasis [31], strongly suggesting that endocytosis is a normal route for the regulation of VEGFR-3 activity.
Due to the roles of VEGF–VEGFR interactions in tumor growth and metastasis, methods for controlling or modulating the VEGF–VEGFR axis are continuously being studied. Here, we used a variety of in vitro methods to investigate the mechanisms of VEGFR-3 signaling in ECs.
Materials and Methods
Cell Culture
Simian virus 40 (SV40)-immortalized LECs from mesenchymal lymphangioblasts (SV-LECs; [32]), vECs [32], and primary human lung LECs (hLECs, Lonza, Walkersville, MD) were plated onto 100-mm dishes. SV-LEC and hLEC lines were plated at a density of 5.0×106 cells per 100-mm dish. Cells were starved in serum-free medium for 24 hours prior to experiments. Cells were stimulated with varying concentrations of VEGF-C (0–200 ng/mL; R&D Systems, Minneapolis, MN) for 0–24 hours, depending on the experiment. Some experiments also included incubations with VEGF-A, VEGF-D, VEGFR-3-Fc (all R&D Systems, Minneapolis, MN), GM6001 (Calbiochem, Billerica, MA), Jag1 (Anaspec Inc, Fremont, CA), DAPT (Sigma-Aldrich, St. Louis, MO), AZD2171 (Selleck Chemicals Co., Houston, TX), or LPE (Sigma-Aldrich) for 6 or 24 hours, depending on the experiment.
Western Blotting
Conditioned media were collected and concentrated for some experiments. For cell lysate experiments, cells were lysed in ice-cold radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% NP40; 0.25% Na-deoxycholate; 1 mM phenylmethylsulfonyl fluoride; and Roche complete protease inhibitor), and the supernatant was collected. Total protein concentration was measured using a protein assay (Bio-Rad, Hercules, CA) and adjusted to 1 mg/ml. Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon P membranes (Millipore, Bedford, MA). Membranes were blocked with 3% bovine serum albumin and non-fat milk for 60 min and then incubated for 1 hour with anti-VEGFR-3 (rat anti-mouse, 1:1,000, eBiosciences, San Diego, CA). rabbit anti-ERK (1:1000), mouse anti-phospho-ERK (1:1000), anti-JNK (1:200), mouse anti-phospho-JNK (1:200), anti-p38 (1:200), or rabbit anti-phospho p38 (1:1,000; all Cell Signaling Technology, Danvers, MA). The membranes were incubated for 30 min with horseradish peroxidase-conjugated donkey anti-rabbit, donkey anti-rat, or donkey anti-mouse IgG (1:20,000; Cell Signaling Technology or Amersham Biosciences) and washed with Tris-buffered saline. The target antigen was visualized using the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences). To obtain the data shown in Figure 3A, following 10 minutes of stimulation with 50 ng/mL VEGF-C, 500 μg cell lysate was incubated with 1 μg goat anti-human VEGFR-3 (R&D Systems) for immunoprecipitation before Western blotting with anti-VEGFR-3 (rat anti-mouse, 1:1,000, eBiosciences) or anti-phosphotyrosine 4G10 antibody (1:1000, Millipore, Danvers, MA).
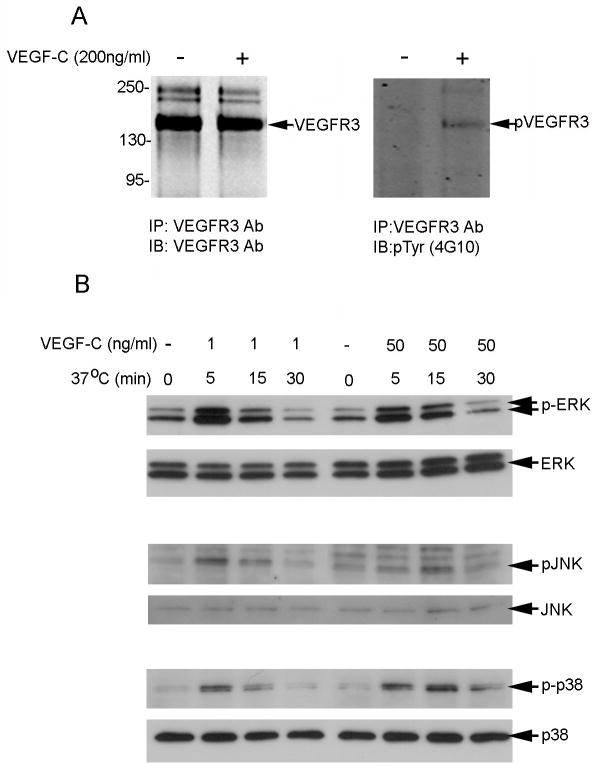
Figure 3.
VEGF-C induces tyrosyl phosphorylation of ERK, JNK, and p38 in serum-starved SV-LECs. Western blot analysis of: (A) total (left) and tyrosine phosphorylated (right) VEGFR-3 in SV-LECs left untreated or stimulated with VEGF-C for 10 min; and (B) total (lower rows) and phosphorylated (upper rows) ERK, JNK, and p38 in SV-LECs stimulated with VEGF-C (1 or 50 ng/mL as indicated) for 0, 5, 15, or 30 min.
Reverse Transcriptase-Polymerase Chain Reaction (PCR) and Real-Time PCR Analysis of VEGFR-2 and -3
RNA was purified from SV-LECs using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA was reverse transcribed using High-Capacity cDNA reverse transcription kit reagents (Applied Biosystems, Foster City, CA). The following primer sets were used for amplification: [33] VEGFR-2, sense 5′-AGGTTTGCGTGCTCTTCACAGT-3′ and antisense 5′-ACACGTAAGAGTCCGGAAGGAA-3′; VEGFR-3, sense 5′-CCCAGCCATGTACAGAAGGT-3′ and antisense 5′-GGCTGGAGTCAGAGGAGTTG-3′; and glyceraldehyde 3-phosphate dehydrogenase, sense 5′-TTGCCATCAATGACCCCTTCA-3′ and antisense 5′-ATGGGCTTCCTGTTGATGACA-3′. Real-time PCR was performed in triplicate using the ABI Prism 7900HT sequence detection system and SYBR Green PCR master mix (Applied Biosystems) and the following cycling conditions: activation of Taq enzyme at 50°C for 2 minutes, initial denaturation at 95°C for 10 minutes, 40 amplification cycles of 95°C for 15 seconds, and 60°C for 60 seconds. ABI PRISM SDS 2.3 software (Applied Biosystems) was used to convert the raw data. VEGFR-2 and -3 mRNA levels were normalized to GAPDH mRNA levels.
Results
VEGF-C Down-regulates VEGFR-3 Protein and mRNA Expression in LECs and vECs
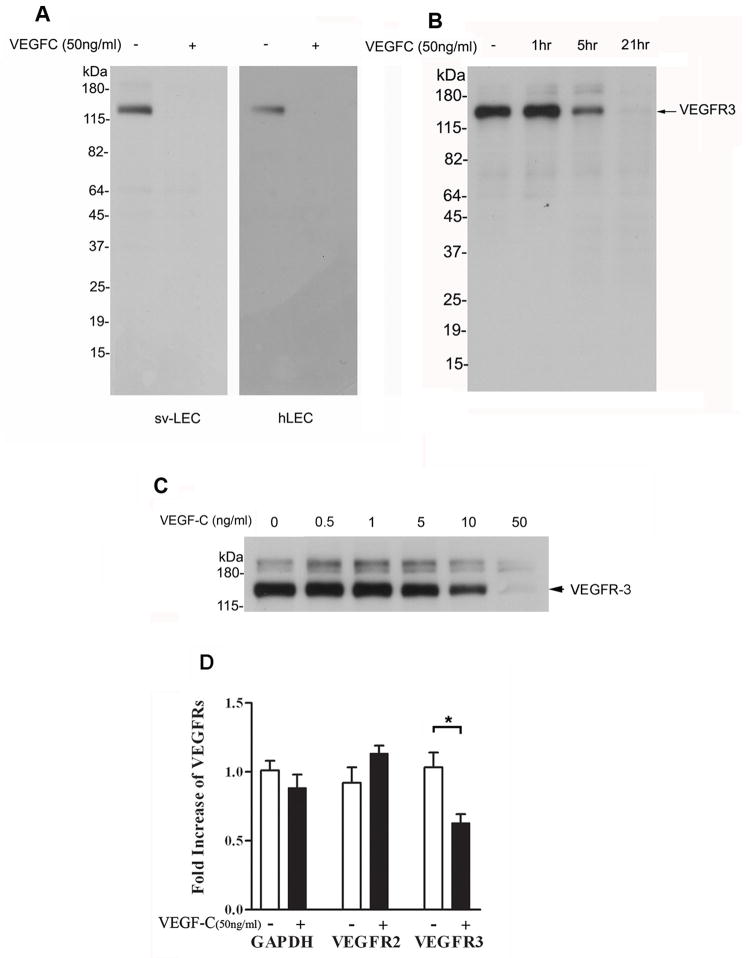
We first investigated the effect of VEGF-C stimulation on VEGFR-3 expression in immortalized SV-LECs, primary hLECs, and vECs. VEGFR-3 was detected by Western blotting in untreated SV-LECs and hLECs, but not in LECs stimulated with 50 ng/mL VEGF-C for 24 hours (Fig. 1A). To determine the circumstances under which this down-regulation occurs, time- and dose-dependent studies were performed. VEGFR-3 expression in vECs (Fig. 1B) was unchanged after 1 hour of VEGF-C stimulation, diminished after 5 hours of VEGF-C stimulation, and undetectable after 21 hours of VEGF-C stimulation. Dose-response experiments showed that VEGFR-3 expression in SV-LECs remained unaffected by VEGF-C doses of 0.5, 1, or 5 ng/mL, but was diminished by 10 ng/mL VEGF-C and undetectable after exposure to 50 ng/mL VEGF-C for 24 hours (Fig. 1C).
Figure 1.
VEGF-C treatment down-regulates VEGFR-3 protein and mRNA expression in SV-LECs and hLECs. Western blot analysis of VEGFR-3 in: (A) SV-LECs and hLECs left untreated or stimulated with VEGF-C for 24 hours; (B) vECs at the indicated times after VEGF-C (50 ng/mL) stimulation; and (C) serum-starved SV-LECs exposed to increasing concentrations of VEGF-C for 24 hours. (D) Quantitative real-time PCR analysis of VEGFR-2 and -3 as well as GAPDH mRNA levels in SV-LECs stimulated with VEGF-C for 24 hours.
To determine whether VEGFR-3 protein down-regulation occurs at the transcriptional level, we analyzed mRNA levels of VEGFR-2 and -3 in SV-LECs using quantitative real-time PCR. VEGFR-3 mRNA levels were reduced in VEGF-C–stimulated SV-LECs, but VEGFR-2 mRNA and control GAPDH mRNA levels were not altered by VEGF-C stimulation (Fig. 1D). Thus, VEGF-C does affect VEGFR-3 at both the transcriptional and protein levels, and although both VEGFR-2 and -3 are receptors for VEGF-C, VEGFR-2 is likely not down-regulated in the same way as VEGFR-3.
VEGFR-3–specific Ligand, VEGF-C (C156S), Down-regulates VEGFR-3 Expression in LECs
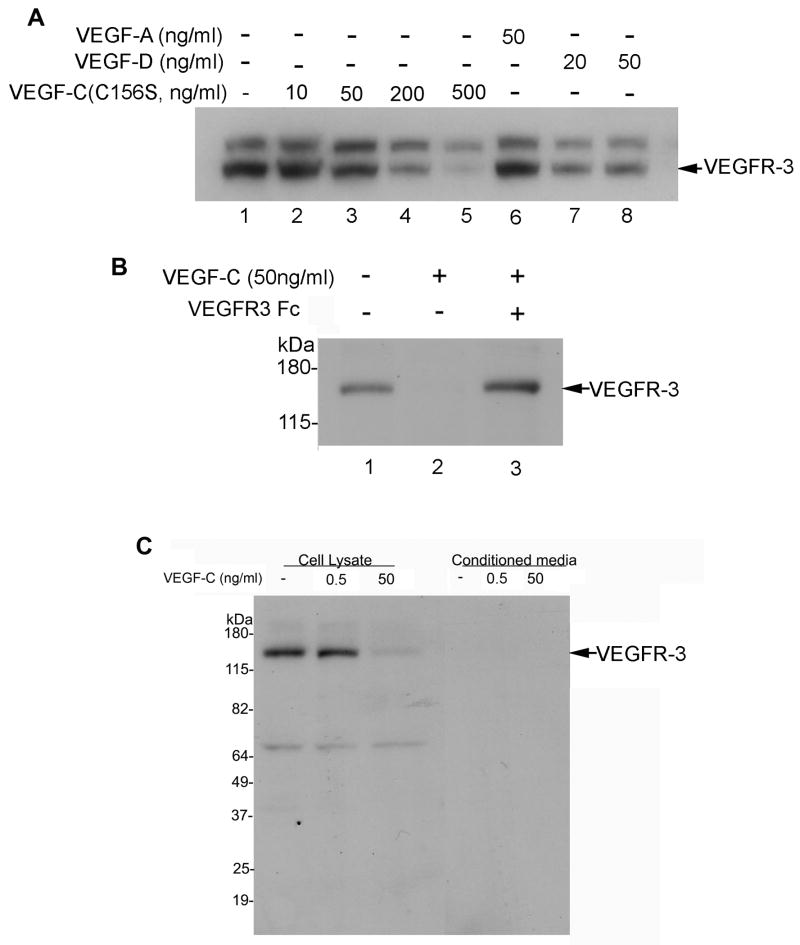
To assess the specificity of the VEGF-C/VEGFR-3 interaction, we examined whether other VEGFR ligands, such as VEGF-A, -C (C156S), and -D, exert effects similar to that of VEGF-C on VEGFR-3 expression. Stimulation of SV-LECs with VEGF-A for 24 hours did not change VEGFR-3 levels relative to those in untreated SV-LECs. High concentrations of the VEGFR-3–specific ligand VEGF-C156S down-regulated VEGFR-3 expression (200–500 ng/mL), as did low concentrations of the VEGFR-2 and -3 ligand VEGF-D (20–50 ng/mL, Fig. 2A). Addition of VEGFR-3-Fc (which sequesters VEGF-C, preventing it from binding to VEGFRs) to VEGF-C–stimulated LECs appeared to completely block VEGF-C–induced down-regulation of VEGFR-3 (Fig. 2B).
Figure 2.
VEGFR-3 protein expression is down-regulated by treatment with VEGF-C and -D, but not VEGF-A, and down-regulation by VEGF-C can be blocked by VEGFR-3-Fc. Western blot analysis of VEGFR-3 in: (A) serum-starved SV-LECs left untreated or stimulated with VEGF-C (C156S, 0–500 ng/mL), VEGF-A (50 ng/mL), or VEGF-D (20–50 ng/mL) for 24 hours; (B) serum-starved SV-LECs untreated or stimulated with VEGF-C for 24 hours with or without the the VEGF-C trap VEGFR-3-Fc (1 μg/mL); and (C) cell lysates and conditioned media collected from SV-LECs stimulated with VEGF-C for 24 hours.
Effect of VEGF-C on Distribution of VEGFR-3 in LECs
We then explored whether the distribution of VEGFR-3 is altered during VEGF-C–induced down-regulation. Figure 2C shows that VEGFR-3 was present after 24 hours in the lysates of untreated cells and cells stimulated with a low concentration of VEGF-C (0.5 ng/mL), whereas stimulation with a high concentration of VEGF-C (50 ng/mL) reduced VEGFR-3 levels in cell lysates. VEGFR-3 was undetectable by Western blotting in the conditioned media of both untreated and stimulated cells. Therefore, VEGF-C does not induce VEGFR-3 release via exosomes or the release of fragmented VEGFR-3 into the media. Its decrease in the cell lysate could be due to several potentially interacting mechanisms, including a reduction in VEGFR-3 transcription, as shown in Figure 1D, and a reduction in VEGFR-3 levels through proteolysis.
Effects of VEGF-C on VEGFR-3, ERK, p38, and JNK Phosphorylation in SV-LECs
VEGF-C binding induces autophosphorylation of VEGFR-3, which initiates lymphangiogenesis among LECs. Downstream targets of pVEGFR-3 include ERK, JNK, and p38. VEGFR-3 tyrosyl phosphorylation was examined following immunoprecipitation from VEGF-C–stimulated and unstimulated LEC lysates. VEGFR-3 tyrosyl phosphorylation was enhanced by VEGF-C stimulation (Fig. 3A, right panel). Next, the phosphorylation status of ERK, JNK, and p38 was examined in SV-LECs stimulated with VEGF-C (1 or 50 ng/mL). Maximal levels of phosphorylated ERK were present 5 min after stimulation, whereas JNK and p38 phosphorylation peaked 5 and 15 min after stimulation, respectively, with 1 or 50 ng/mL VEGF-C. JNK and p38 protein expression levels were equivalent at all time points, whereas ERK levels appeared to increase over time following stimulation with 50 ng/mL VEGF-C (Fig. 3B).
VEGF-C–induced Down-regulation of VEGFR-3 Does Not Require MMPs or Notch Signaling
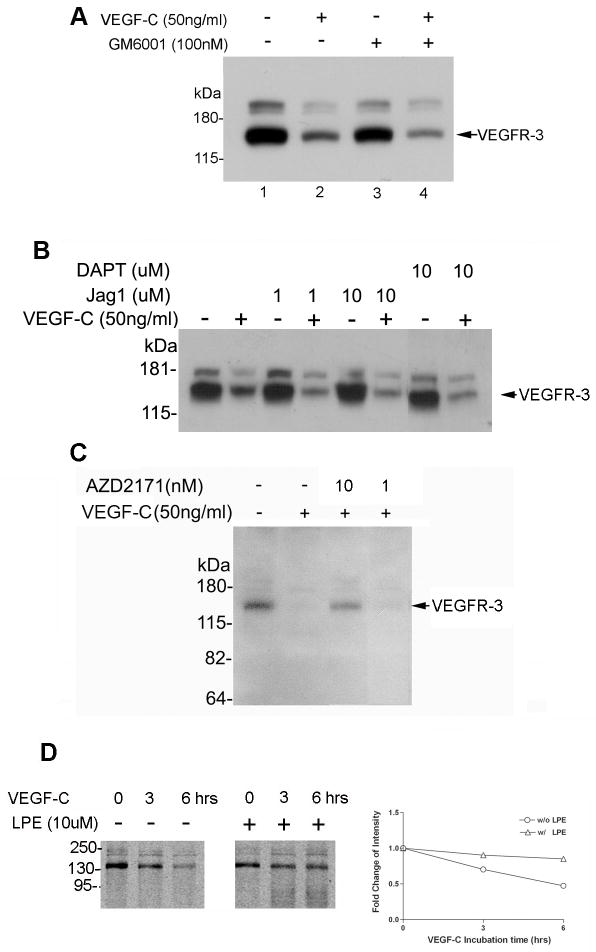
VEGFR-1 undergoes ectodomain shedding upon PlGF stimulation, and this shedding can be blocked by treatment with GM6001, a broad inhibitor of metalloproteinase family proteases [34], indicating that this shedding requires metalloprotease activity [34]. We tested whether an MMP participates in VEGFR-3 protein processing by treating SV-LECs with GM6001 and VEGF-C for 24 hours. Cells stimulated with VEGF-C displayed the expected down-regulation of VEGFR-3 relative to untreated cells (Fig. 4A, lane 2 vs. lane 1). Cells that received only GM6001 displayed VEGFR-3 expression similar to that in unstimulated control cells (Fig. 4A, lane 3), but cells receiving VEGF-C and GM6001 exhibited VEGFR-3 expression levels equivalent to those in VEGF-C–stimulated cells (Fig. 4A, lane 4). Therefore, GM6001 inhibition of MMP activity did not affect the VEGF-C–mediated down-regulation of VEGFR-3.
Figure 4.
VEGF-C–induced down-regulation of VEGFR-3 protein expression is not affected by a MMP inhibitor, Notch 1 activator, or γ-secretase inhibitor but is inhibited by AZD1217 and LPE. Western blot analysis of VEGFR-3 in: (A) serum-starved SV-LECs treated with either GM6001 (MMP inhibitor, 100 nM), VEGF-C (50 ng/mL), or both for 24 hours; (B) serum-starved SV-LECs left untreated or stimulated with VEGF-C (50 ng/ml) for 24 hours in the presence or absence of the Notch activator, Jag-1 (1 or 10 μM), or the γ-secretase inhibitor, DAPT (10 μM); (C) SV-LECs left untreated or stimulated with VEGF-C in the presence or absence of the TKI, AZD2171 (10 or 1 nM); and (D) inhibition of VEGF-C–induced VEGFR-3 down-regulation by lysosomal protease inhibitors (LPE) after 6 hours of incubation.
Because Tammela et al.[16] showed that Notch activation can block VEGFR-3 expression and that genetic or pharmacological disruption of Notch signaling enhances endothelial VEGFR-3 expression, we also analyzed the effect of inhibiting Notch signaling. We found that neither the Notch1 activator, Jag1 (Fig. 4B, lanes 3–6), nor the γ-secretase inhibitor of Notch, DAPT (Fig. 4B, lanes 7–8), substantially affected VEGF-C–induced down-regulation of VEGFR-3.
TKI and LPE Diminished VEGF-C–induced Down-regulation of VEGFR-3
To investigate whether tyrosine phosphorylation and lysosomal degradation are involved in VEGF-C–induced down-regulation of VEGFR-3, we analyzed the effects of a TKI (AZD2171) and lysosomal protease inhibitors (LPE) on VEGF-C–induced VEGFR-3 down-regulation. Exposure of hLECs to the TKI (Fig. 4C) or LPE (Fig. 4D) abolished or partially inhibited VEGF-C–induced VEGFR-3 down-regulation, respectively. These data confirm that VEGF-C induces VEGFR-3 down-regulation, at least in part, through activation of VEGFR-3 and lysosomal protease degradation.
Discussion
Activation of VEGFR-3 is sufficient for the growth, survival, and migration of cultured LECs as well as lymphangiogenesis in vivo [35]. Therefore, down-regulation of the VEGF-C/VEGFR-3 signaling pathway is a potentially viable method for inhibiting pathogenic lymphangiogenesis in addition to the growth and metastasis of tumors, which require access to the blood and lymphatic systems. We have shown that VEGF-C down-regulates VEGFR-3 protein and mRNA expression in LECs and vECs in vitro in a time- and concentration-dependent manner. To explore the mechanism by which this down-regulation occurs, we conducted experiments aimed at several known upstream effectors and downstream targets of VEGFR-3.
First we tested the timing and concentration-dependence of VEGF-C down-regulation of VEGFR-3. VEGFR-3 down-regulation was observed after 5 hours of incubation with a high dose of VEFG-C (50 ng/mL, Fig. 1B) and by 24 hours with a lower dose (10 ng/mL, Fig. 1C). Although 50 ng/mL is a high concentration, Weich et al (2004) found that transfected human 293 cells secrete up to 50 ng/mL VEGF-C over 6 days in culture [36].
We next explored the specificity of this interaction. VEGF-C is a known ligand for both VEGFR-2 and -3, and thus, the inhibitory effect of VEGF-C on VEGFR-2 was also studied. However, VEGF-C did not significantly down-regulate VEGFR-2 mRNA expression (Fig 1D). We also tested other ligands to VEGFR-3, as well as VEGF-A, which binds other VEGFRs but not VEGFR-3. Treatment with 50 ng/mL VEGF-A did not affect VEGFR-3 protein levels (Fig. 2A), whereas treatment with 200 ng/mL of the VEGFR-3–specific VEGF-C (C156S), which does not bind VEGFR-2, reduced VEGFR-3 protein levels (Fig. 2A). Treatment with 20 ng/mL VEGF-D, a separate ligand of both VEGFR-2 and -3, also reduced VEGFR-3 levels, but even at 50 ng/mL, VEGF-D could not reduce VEGFR-3 expression (Fig. 2A) to the degree that VEGF-C did (Fig. 1A–C). VEGF-D is structurally and functionally similar to VEGF-C (48% identical, [37]) and binds to the same receptors, but it appears to play a greater role in the pathology rather than the development of the lymphatic system[38]. The fact that VEGF-D binding leads to only partial degradation of VEGFR-3 is interesting, as it supports the idea that these two ligands have different downstream effects, even within the same cell and when interacting with the same receptors. VEGF-C156S, on the other hand, only binds to VEGFR-3. It induces lymphangiogenesis with less lymphatic sprouting compared to that induced by VEGF-C and does not exert its effects through VEGFR-2 as VEGF-C has been shown to [39] [21]. Interestingly, the observation that VEGF-C156S had a weaker effect on VEGFR-3 degradation (Fig. 2C) than VEGF-C (Fig. 1A–C) suggests that VEGF-C–induced degradation of VEGFR-3 may involve or be amplified by an interaction between VEGF-C and VEGFR-2.
We next determined whether the VEGF-C–induced down-regulation of VEGFR-3 could be inhibited by treatment with a soluble VEGFR-3 (VEGFR-3-Fc, Fig. 2B), which sequesters, or traps, VEGF-C and -D, preventing their interaction with membrane-bound VEGFR-3. Our results showed that VEGFR-3-Fc trap treatment abolished VEGF-C–induced VEGFR-3 protein down-regulation, confirming that the down-regulatory effect on VEGFR-3 occurs through the ligand binding to VEGFR-3 and not other extraneous factors. Our findings suggest that the ligands involved are VEGF-C and, to a lesser extent, VEGF-D.
A decrease in cellular VEGFR-3 could be accomplished either by expelling VEGFR-3 from the cell (e.g., via exosomes) or by absorbing and digesting VEGFR-3 within the cell (e.g., by lysosomal degradation). Most growth factors control cellular functions by activating specific RTKs. Deactivation of RTKs when they are no longer required is critical to maintaining normal cellular function [40], and one major route for cellular protein removal involves ligand-induced endocytosis of the RTKs and subsequent degradation of the ligand-activated RTK complex in lysosomes [41]. We found that VEGFR-3 was not present in the culture medium before or after VEGF-C stimulation (Fig. 2C, right lanes), indicating that VEGFR-3 is not expelled from the cell. Moreover, VEGFR-3 was present in the cell lysate prior to but not following stimulation with VEGF-C (Fig. 2C, left lanes), indicating that VEGF-C induces intracellular degradation of VEGFR-3.
Based on these observations, we analyzed the tyrosine phosphorylation and expression of pVEGFR-3, ERK/pERK, JNK/pJNK, and p38/p-p38, which have all been implicated in lymphangiogenesis. VEGF-C stimulation induced VEGFR-3 tyrosyl phosphorylation (Fig. 3A) and also transiently increased phosphorylation of ERK, JNK, and p38 (Fig. 3B). However, the level of tyrosyl phosphorylation of these downstream signaling molecules (ERK, JNK, and p38) remained relatively unchanged upon treatment with low concentrations of VEGF-C, indicating their high sensitivity to this ligand. The extremely transient nature of these increases (depleted by 30 minutes post-stimulation), compared to the long time scale over which VEGF-C induces VEGFR-3 degradation (6–24 hours) suggests that these two effects are likely unrelated.
We next aimed to determine whether known associates of the VEGF/VEGFR axis, MMPs and Notch, are involved in VEGF-C–induced degradation of VEGFR-3. An MMP inhibitor (GM6001) did not affect VEGFR-3 downregulation, indicating that degradation likely occurs via a separate proteolytic process. Notch proteins are cell surface proteins that regulate cell fate. Notch can directly target the VEGFR-3 gene by binding and transactivating the VEGFR-3 promoter. However, neither the Notch inhibitor (γ secretase inhibitor DAPT, Fig. 4B) nor the Notch activator Jag1 (Fig. 4B) showed an effect on VEGFR-3 downregulation in response to VEGF-C. However, directly blocking VEGFR-3 signal transduction with the TKI AZD2171 (Fig. 4C) did prevent VEGF-C–mediated down-regulation of VEGFR-3. Activation of VEGFR-3 autophosphorylation upon binding of VEGF-C is therefore essential to the subsequent down-regulation of activated VEGFR-3. Lysosomal protease inhibitors (LPE, Fig. 4D) also inhibited the down-regulatory effect of VEGF-C, supporting our conclusion that the lysosomal pathway is involved in this instance of ligand-induced receptor down-regulation.
Our understanding of the pathways involved in VEGFR inhibition and promotion is steadily increasing. This task is complicated by the complexity of the effects of VEGF-C and VEGFR-3 interactions during developmental, normal, pathological, and cancer processes and differences across cell types, time points, and malignancies. Further studies are needed to better define the precise mechanisms underlying VEGF-C–induced VEGFR-3 down-regulation. Understanding this and other methods of molecular modulation of VEGFR-3 protein levels will aid in the development of therapeutic interventions for many lymphangiogenesis-related disorders, including corneal transplant rejection, lymphedema, and tumor metastasis.
VEGF-C specifically down-regulates VEGFR-3 mRNA and protein in lymphatic endothelial cells
Down-regulation is mediated by VEGF-C binding to VEGFR-3 and VEGFR-3 autophosphorylation
Down-regulation does not require MMP activity or Notch signaling
Lysosomal protease inhibitors decrease the down-regulatory effect on VEGFR-3 protein levels
Results indicate a ligand-induced receptor down-regulation mechanism via lysosomal activity
Acknowledgments
This study was supported by grants from the National Institutes of Health EY10101 (D.T.A.), EY023691, EY021886, I01 BX002386 (J.H.C), EY01792, UL1TR000050, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Abbreviations
- BSA
bovine serum albumin
- DAPT
difluorophenylacetyl)-alanyl-phenylglycine-t-butyl-ester
- EC
endothelial cell
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- LEC
lymphatic endothelial cell
- LPE
leupeptin, pepstatin, and E64
- MMP
matrix metalloproteinase
- PCR
polymerase chain reaction
- PlGF
placenta growth factor
- RTK
receptor tyrosine kinase
- SV40
simian virus 40
- SV-LEC
simian virus 40-immortalized lymphatic endothelial cell
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–48. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joukov V, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–43. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 4.Albuquerque RJ, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–30. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coso S, Bovay E, Petrova TV. Pressing the right buttons: signaling in lymphangiogenesis. Blood. 2014;123:2614–24. doi: 10.1182/blood-2013-12-297317. [DOI] [PubMed] [Google Scholar]
- 6.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galvagni F, et al. Endothelial cell adhesion to the extracellular matrix induces c-Src-dependent VEGFR-3 phosphorylation without the activation of the receptor intrinsic kinase activity. Circ Res. 2010;106:1839–48. doi: 10.1161/CIRCRESAHA.109.206326. [DOI] [PubMed] [Google Scholar]
- 8.Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106:3423–31. doi: 10.1182/blood-2005-04-1388. [DOI] [PubMed] [Google Scholar]
- 9.Graupera M, Potente M. Regulation of angiogenesis by PI3K signaling networks. Exp Cell Res. 2013;319:1348–55. doi: 10.1016/j.yexcr.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–43. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Emami-Naeini P, Dohlman TH, Omoto M, Hattori T, Chen Y, Lee HS, Chauhan SK, Dana R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch Clin Exp Ophthalmol. 2014 doi: 10.1007/s00417-014-2749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omoto I, et al. Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol Lett. 2014;7:1027–1032. doi: 10.3892/ol.2014.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hos D, Bock F, Dietrich T, Onderka J, Kruse FE, Thierauch KH, Cursiefen C. Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Invest Ophthalmol Vis Sci. 2008;49:1836–42. doi: 10.1167/iovs.07-1314. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011;52:6529–35. doi: 10.1167/iovs.11-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YW, et al. Novel peptides suppress VEGFR-3 activity and antagonize VEGFR-3-mediated oncogenic effects. Oncotarget. 2014;5:3823–35. doi: 10.18632/oncotarget.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N, et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood. 2013;121:4242–9. doi: 10.1182/blood-2012-08-453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamada K, Oike Y, Takakura N, Ito Y, Jussila L, Dumont DJ, Alitalo K, Suda T. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood. 2000;96:3793–800. [PubMed] [Google Scholar]
- 18.Tammela T, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–13. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–60. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Wang GD, Tan YZ, Wang HJ. Inhibition of lymphangiogenesis of endothelial progenitor cells with VEGFR-3 siRNA delivered with PEI-alginate nanoparticles. Int J Biol Sci. 2014;10:160–70. doi: 10.7150/ijbs.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heckman CA, et al. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008;68:4754–62. doi: 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- 22.Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–20. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shawber CJ, et al. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest. 2007;117:3369–82. doi: 10.1172/JCI24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenach PA, Roghi C, Fogarasi M, Murphy G, English WR. MT1-MMP regulates VEGF-A expression through a complex with VEGFR-2 and Src. J Cell Sci. 2010;123:4182–93. doi: 10.1242/jcs.062711. [DOI] [PubMed] [Google Scholar]
- 25.Han KY, Fahd DC, Tshionyi M, Allemann N, Jain S, Chang JH, Azar DT. MT1-MMP modulates bFGF-induced VEGF-A expression in corneal fibroblasts. Protein Pept Lett. 2012;19:1334–9. doi: 10.2174/092986612803521639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karroum A, Mirshahi P, Faussat AM, Therwath A, Mirshahi M, Hatmi M. Tubular network formation by adriamycin-resistant MCF-7 breast cancer cells is closely linked to MMP-9 and VEGFR-2/VEGFR-3 over-expressions. Eur J Pharmacol. 2012;685:1–7. doi: 10.1016/j.ejphar.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189:871–83. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewan LC, et al. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–82. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 29.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galvagni F, Anselmi F, Salameh A, Orlandini M, Rocchigiani M, Oliviero S. Vascular endothelial growth factor receptor-3 activity is modulated by its association with caveolin-1 on endothelial membrane. Biochemistry. 2007;46:3998–4005. doi: 10.1021/bi061400n. [DOI] [PubMed] [Google Scholar]
- 31.Garouniatis A, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. Vascular endothelial growth factor receptors 1,3 and caveolin-1 are implicated in colorectal cancer aggressiveness and prognosis--correlations with epidermal growth factor receptor, CD44v6, focal adhesion kinase, and c-Met. Tumour Biol. 2013;34:2109–17. doi: 10.1007/s13277-013-0776-1. [DOI] [PubMed] [Google Scholar]
- 32.Ando T, Jordan P, Joh T, Wang Y, Jennings MH, Houghton J, Alexander JS. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol. 2005;3:105–15. doi: 10.1089/lrb.2005.3.105. [DOI] [PubMed] [Google Scholar]
- 33.Freitas-Andrade M, Carmeliet P, Stanimirovic DB, Moreno M. VEGFR-2-mediated increased proliferation and survival in response to oxygen and glucose deprivation in PlGF knockout astrocytes. J Neurochem. 2008;107:756–67. doi: 10.1111/j.1471-4159.2008.05660.x. [DOI] [PubMed] [Google Scholar]
- 34.Rahimi N, Golde TE, Meyer RD. Identification of ligand-induced proteolytic cleavage and ectodomain shedding of VEGFR-1/FLT1 in leukemic cancer cells. Cancer Res. 2009;69:2607–14. doi: 10.1158/0008-5472.CAN-08-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veikkola T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–31. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weich HA, et al. Quantification of vascular endothelial growth factor-C (VEGF-C) by a novel ELISA. J Immunol Methods. 2004;285:145–55. doi: 10.1016/j.jim.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Achen MG, Stacker SA. The vascular endothelial growth factor family; proteins which guide the development of the vasculature. Int J Exp Pathol. 1998;79:255–65. doi: 10.1046/j.1365-2613.1998.700404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch M, et al. VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. J Pathol. 2009;219:356–64. doi: 10.1002/path.2605. [DOI] [PubMed] [Google Scholar]
- 39.Chung ES, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–92. doi: 10.2353/ajpath.2009.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–74. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruns AF, Herbert SP, Odell AF, Jopling HM, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic. 2010;11:161–74. doi: 10.1111/j.1600-0854.2009.01001.x. [DOI] [PubMed] [Google Scholar]