Abstract
The relationship between alterations in chromatin structure and changes in gene expression during cell differentiation has served as a paradigm to understand the link between genome organization and function. Yet the factors involved and the mechanisms by which the three-dimensional organization of the nucleus is established remain poorly understood. The use of Chromosome Conformation-Capture (3C) based approaches has resulted in a new appreciation of the role of architectural proteins in the establishment of 3D genome organization. Architectural proteins orchestrate higher-order chromatin organization through the establishment of interactions between regulatory elements across multiple spatial scales. The regulation of these proteins, their interaction with DNA, and their co occurrence in the genome, may be responsible for the plasticity of 3D-chromatin architecture that dictates cell and time-specific blueprints of gene expression.
Keywords: CTCF, Transcription, Chromatin, Development, Differentiation
Nuclear organization
Chromosomes are tightly packed in the nucleus within chromosome territories [1-4]. The three-dimensional arrangement of the chromatin fiber in these territories during interphase is not random and, in principle, could be a consequence of genome function or it could be a pre-established effector of nuclear activity [5]. Nuclear processes such as transcription and replication require the assembly of large multi-protein complexes at promoters, enhancers and replication origins [5-7]. These proteins often contain multiple interacting domains and, therefore, they may drive the formation of intra- and inter-chromosomal contacts that contribute to the establishment of a specific three-dimensional arrangement of the chromatin fiber. Because this arrangement may be a consequence of genome function, it should be, at least in part, cell-type specific, correlating with the transcriptional state of the cell. In addition to this transcription-driven organization, the cell appears to also employ specific protein complexes whose main role is to establish contacts between distant sites in the genome to facilitate its three-dimensional organization and allow the execution of specific functional outcomes. These proteins, generally referred to as insulator proteins, were originally characterized for their ability to interfere with enhancer-promoter interactions and to shield the expression of transgenes from the effects of adjacent sequences [8]. More recent results suggest that insulator sequences and their associated proteins may not only inhibit but also facilitate enhancer-promoter interactions, as well as regulate other aspects of transcription, in addition to more general roles in chromosome organization [9]. Given the varied, and sometimes contradictory, functions mediated by these proteins, we will refer to them as architectural instead of insulator proteins.
Starting with the premise that architectural proteins can mediate interactions between different sequences in order to regulate genome function, here we will discuss mechanisms by which the interaction of these proteins with DNA or other proteins can be regulated to create specific patterns of nuclear 3D organization in order to elicit distinct functional outcomes that may contribute to the establishment of specific cell lineages during development.
Architectural proteins: structure and organization
Architectural proteins have been described in organisms ranging from yeast to humans [10]. In S. cerevisiae and S. pombe the main architectural protein characterized to date is the RNA polymerase III-associated factor TFIIIC, which is present at genes transcribed by this polymerase, such as tRNA genes, as well as at many non-transcribed regions of the genome known as extra TFIIIC (ETC) loci [11, 12]. TFIIIC co-localizes with cohesin and condensin, which have been shown to be required for its function in protecting against the spreading of histone covalent modifications associated with transcription silencing [13]. The best characterized architectural protein in vertebrates is CTCF, which also requires association with cohesin for its enhancer blocking function [10]. Recent experiments showing that tRNA genes can block enhancer function and that TFIIIC co-localizes with CTCF at many ETC loci through the mouse and human genomes suggest a conservation in the function of TFIIIC as an architectural protein from yeast to humans [14]. Other proteins shown to co-localize or directly interact with CTCF in vertebrates include YY1, Kaiso, chromodomain helicase DNA-binding protein 8 (CHD8), PARP1, MYC-associated zinc-finger protein (MAZ), JUND, zinc-finger protein 143 (ZNF143), PR domain zinc-finger protein 5 (PRDM5) and nucleophosmin [15-17]. Drosophila has also been a rich source of information aimed at understanding the structure and organization of this class of proteins. Several DNA-binding architectural proteins, including CTCF, Su(Hw), BEAF-32, DREF and TFIIIC, interact directly with the DNA [18, 19]. These DNA-binding proteins recruit other accessory architectural proteins that do not bind to DNA directly, including cohesin (Rad21), condensins (Cap-H2 and Barren), Mod(mdg4), CP190, L(3)mbt, Fs(1)h-L, Chromator, Zw5, and GAF [18, 19]. These proteins are present in the genome in different combinations at what are termed Architectural Protein Binding Sites (APBSs) [20]. Some sites in the genome contain one DNA-binding architectural protein and several accessory proteins and are called low occupancy APBSs. Others contain several DNA binding proteins bound within a short genomic region that recruit all or most accessory proteins and are called high occupancy APBSs. These two types of sites play different roles in genome organization and function [20]. Architectural proteins in Drosophila and mammals have been shown to interact with RNAs [21-25], perhaps as a means of stabilizing these large multi-protein complexes, but the mechanistic role of these transcripts in their function has not been studied in detail.
The role of architectural proteins in 3D genome organization
The recent use of Cromosome Conformation Capture-derived approaches such as 5C and Hi-C to measure interaction frequencies has allowed the establishment of comprehensive interaction maps over large regions or whole genomes [3, 19, 26-29]. Results from these experiments suggest that the Drosophila and mammalian genomes are compartmentalized into discrete regions termed Topologically Associating Domains (TADs) [27, 30]. TADs are regions of the genome that show a high frequency of intra-domain interactions, whereas the frequency of interactions with other TADs is very low. Therefore, the interaction-based division of the genome into TADs is caused by the presence of sequences and associated proteins within TADs that frequently interact with their neighbors while, concurrently, other sequences and associated proteins form TAD borders that preclude interactions between adjacent TADs. In both Drosophila and mammals TAD borders contain highly transcribed genes, including housekeeping genes, and architectural proteins [19, 30, 31]. Overall, only 15% of CTCF sites are present at TAD borders in mouse and human cells. Instead, most CTCF binding sites (85%) localize within topological domains, where they mediate interactions aimed at regulating various steps of the transcription process [27, 32]. These findings together suggest the existence of various functional subclasses, border-associated versus non-border, architecthural protein binding sites (APBS). It appears that the functional difference between the two types of APBSs rests on the number of other architectural proteins present. In mammals, there is a strong association between TAD borders and the presence of CTCF, TFIIIC, cohesin and PRDM5. Similarly, in Drosophila CTCF clusters at TAD borders with condensins, cohesin, TFIIIC, BEAF-32, Su(Hw), CP190, Mod(mdg4), DREF, Chromator, L(3)mbt [20] (Fig. 1 and Fig 2).
Figure 1.
Structure and organization of architectural protein binding sites (APBS) in yeast, Drosophila and mammals. Each DNA-binding architectural protein interacts with a particular sequence motif in the genome. For example the TFIIIC protein interacts with the B-box sequence in tRNA genes or ETC sites. DNA-binding architectural proteins require interaction with accessory proteins to accomplish their function. For example, CTCF often interacts with Cohesin, whereas in Drosophila dCTCF, BEAF and Su(Hw) interact with Mod(mdg4) and CP190. High occupancy binding sites are dense clusters of architectural proteins present at specific genomic regions and have been found in both Drosophila and mammals.
Figure 2.
Model for how architectural proteins influence genome organization at various length scales (TADs and sub-TADs) via long- range interactions. TADs are defined as regions of the genome undergoing high frequency of local interactions. TADs are separated by borders that preclude interactions between adjacent TADs. Highly occupied APBSs, containing multiple architectural proteins, are enriched at TAD borders whereas low occupancy APBSs are enriched inside TADs. Dynamic changes in the number and co-localization of architectural proteins may modulate TAD border strength across different cell types, allowing or restricting inter-TAD interactions to establish new patterns of gene expression during cell-type specification.
Work in mouse and human cells suggests that 60-70% of TADs are conserved between embryonic stem and differentiated cells, and even between mouse and human cells [27]. One interpretation of these observations is that TADs are static domains of genome organization that allow interactions among genes and regulatory sequences located in the same TAD but preclude interactions between sequences located in different TADs [1, 2, 33]. However, it is important to consider that the concept of the TAD border is relative. Borders are determined computationally using algorithms that, either implicitly or explicitly, set thresholds for the relative frequency of interactions within and between TADs flanking the border. Based on the difference in frequency between inter- and intra-TAD interactions, it is possible to establish the concept of border strength [20, 34, 35]. Strong TAD borders are those for which Hi-C interaction matrices do not show interactions between sequences in the two adjacent TADs whereas weak borders separate TADs with a high frequency of inter-TAD interactions. If one considers TAD borders as relative structures whose strength can be modulated, for example during cell differentiation, then it is possible to speculate that the apparent conservation of TADs between different cell types does not preclude the existence of interactions between genes and regulatory sequences present in different TADs. This has been observed for some enhancers involved in controlling the expression of genes during the differentiation of the mesoderm in Drosophila. In this case, some enhancers present in on TAD are able to contact promoters present in a different TAD [32]. What is responsible for the differences in the strength of borders separating different TADs? Recent results suggest that both in Drosophila and mammals the strength of TAD borders directly correlates with the number of architectural proteins present at the border [20, 34]. In Drosophila, high occupancy APBSs containing 8-12 architectural proteins form strong TAD borders and show enhancer blocking activity in functional reporter assays, whereas APBSs with 5-8 proteins have weak border strength and weak enhancer blocking activity. Interestingly, APBSs with 2-5 architectural proteins are enriched inside TADs and do not interfere with enhancer-promoter interactions in functional reporter assays [20]. These results agree with the view that architectural proteins located inside TADs may facilitate interactions between gene promoters and their regulatory sequences whereas those present at TAD borders may preclude interactions between genes and regulatory sequences located in different TADs. The role of architectural proteins in controlling TAD organization and TAD border strength has been clearly demonstrated in several recent studies that analyzed the consequence of depletion of CTCF and cohesin on 3D genome organization [35-37]. Embryonic kidney cells depleted of cohesin show a general loss of intrachromosomal interactions without affecting the TAD organization, whereas depletion of CTCF causes a similar decrease in the frequency of intra-TAD interactions concomitant with an increase in the frequency of interactions between adjacent TADs. Cohesin-deficient mouse astrocytes also show a reduced number of CTCF- and cohesin-mediated long-range interactions together with a relaxation of TAD organization. This TAD relaxation could be a consequence of a decrease in TAD border strength due to the lack of cohesin binding or to an increase in the frequency of inter-TAD interactions as observed in CTCF-depleted cells. It is therefore possible that cells may be able to regulate border strength by controlling the number of architectural proteins present at specific borders, thus allowing or constraining inter-TAD interactions to elicit novel patterns of gene expression during cell differentiation (Fig. 2).
Regulation of architectural protein localization
CTCF is located at 55,000-65,000 sites in the genome of mammalian cells [38]. Of these, approximately ∼50% reside within intergenic regions, ∼15% are located near promoters and ∼40% are present in introns and exons [38, 39]. In Drosophila, CTCF and other architectural proteins are present in the genome at ∼20-fold fewer sites, in agreement with the difference in genome size, and their distribution with respect to genome features such as promoters, introns and exons is similar to that of CTCF in mammals [30]. This conserved distribution at intergenic regions, 5′ UTRs and introns suggests that, in addition to their role at TAD borders, architectural proteins may also play roles in the regulation of enhancer-promoter interactions, transcription pausing or elongation, and splicing. Although 60-70% of the TADs are conserved among stem and differentiated cells corresponding to various lineages [27], the rest are not, suggesting that cells may have the ability to regulate the localization of architectural proteins during cell differentiation in order to regulate TAD border strength as well as various aspects of the transcription process. Thus, an important question in the field is how the distribution of architectural proteins is regulated in order to effect different functional outcomes during cell fate specification. Recent results suggest that the location of various architectural proteins can be modulated by controlling their interaction with DNA or with other proteins via post-translational modification [40]. Covalent modifications of mammalian CTCF by Poly(ADP-ribosyl)ation affects its ability to bind DNA [41], whereas the same modification of Drosophila CTCF affects its ability to interact with CP190 [40]. The interaction of CTCF with DNA can be also modulated by changes in the methylation status at its binding site [42]. Recent studies combining ChIP-seq and bisulphite sequencing in multiple human cell types revealed that 41% of the cell-specific CTCF binding is linked to differential DNA methylation [43]. Other studies have also reported a negative correlation between CTCF DNA binding and the DNA methylation status of CpGs within the CTCF binding sites [44], although the picture seems to be more complex. CTCF sites bound by this protein show the same methylation level as all the sites in the genome, and the binding affinity of CTCF correlates with the level of unmethylation, suggesting that CTCF can bind with low affinity to sites in the genome that are partially methylated [45]. Equally intriguing is the fact that CTCF can actively inhibit DNA methylation at CTCF binding sites by interacting with PARylated poly[-ADP-ribose] polymerase 1 (PARP1), which in turns inhibits DNA methyl-transferase 1 (DNMT1) activity [46]. Thus, it is not clear from these data whether DNA methylation has a causal role in CTCF binding or is a consequence of this process. Taken together, these studies underscore the complexity and possible importance of DNA methylation and protein covalent modifications in modulating the occupancy and interactions of architectural proteins [47]. It is possible that, by controlling the interactions of architectural proteins with DNA and other proteins, the cell can regulate their location in the genome during cell fate specification and, therefore, control different steps of the transcription process to establish or maintain patterns of gene expression during cell differentiation.
Architectural proteins mediate functional chromatin interactions during cell fate specification
The mechanisms by which cell-to-cell differences in chromatin architecture arise and how these various topologies can result in diverse functional outcomes remains a major gap in our understanding of cell fate specification processes. Below we review recent examples that illustrate how architectural proteins are responsible for the establishment of cell-specific 3D chromatin structures that may contribute to the spatio-temporal regulation of transcription during pluripotency and along various differentiation pathways.
Architectural proteins, types and co-occurrence, drive the transcriptional plasticity of ESCs
Data gathered from independent studies using 5C, Hi-C, and ChIA-PET, comparing 3D chromatin organization in human and mouse embryonic stem cells (ESCs) indicate that the pluripotent genome displays unique topological and functional features. These include global low-levels of transcription, a lack of long-range contacts at a global scale, and disorganization of the heterochromatin in the nuclei [48-50]. As ES cells differentiate there is a dynamic reorganization of the network of interactions genome wide. This involves compartimentalization of the genome into high-frequency interaction domains coupled with very tight spatiotemporal regulation of transcription [51-55] (Fig 2). Interestingly, ESC-specific TADs are mainly shaped around the pluripotency factors Oct4, Sox2 and Nanog, and interactions occur between genome regions that are rich in superenhancers and genes that control the pluripotent state [49, 56]. An important question arising from these studies is how these long-range interaction maps are reconfigured in the transition from ESCs to differentiated cells, and whether architectural proteins play a key role in pluripotency. Previous studies using ChIA-PET and ChIP-sequencing have suggested a role for CTCF, mediator and cohesin as chromatin organizers in ESCs, showing that they engage in functional interactions with pluripotent genes and transcription factors [57, 58]. This is supported by results obtained during the differentiation of ESCs into the endodermal lineage, where CTCF has been shown to directly recruit TAF3, a TBP-associated core promoter factor, to distal regulatory sequences. TAF3 present at CTCF/cohesin sites cooperates with these two proteins in mediating interactions between these enhancers and promoters during the differentiation of ESCs into endoderm [59]. Changes in CTCF occupancy during differentiation of ESCs are associated with alterations in nucleosome positioning and DNA demethylation [62].
A comprehensive analysis of APBS occupancy patterns in the context of ESC differentiation was obtained by comparing 5C interaction maps in ESCs and NPCs. The study revealed two classes of interactions: ES-cell specific enhancer-promoter short-range contacts involving cohesin and Mediator but not CTCF and larger loops coinciding with CTCF and cohesin binding. Loops at the sub-megabase scale show clear reorganization during differentiation, whereas CTCF-mediated megabase loops remain invariant and were proposed to play a role in chromosome folding [63]. These observations can be interpreted in the context of a model in which the regulation in the occupancy of various subclasses of architectural proteins results in changes in chromatin organization that allow the cell to switch between various transcription programs. Consistent with this hypothesis, two independent studies in ES-cells using conditional knockdowns in cohesin and Mediator, found either an artificial induction of differentiation of ESCs or impairing reprogramming in iPSCs [51, 64]. Whether architectural protein binding alone defines pluripotency, or whether pluripotency is instead driven by state-specific transcription factors and enhancers remains unanswered.
Together these findings support a key role of architectural proteins in the dynamic folding of the genome during cell fate specification. Yet several important issues remain. For example, how general is the relationship between architectural proteins, pluripotent transcription factors and/or enhancers? Are architectural proteins causal to changes in the pluripotent state, or a consequence of the binding of pluripotent transcription factors? Do TADs and TAD borders play a regulatory role in the transition between pluripotent and differentiated chromatin states?
CTCF and cohesin regulate lymphocyte differentiation
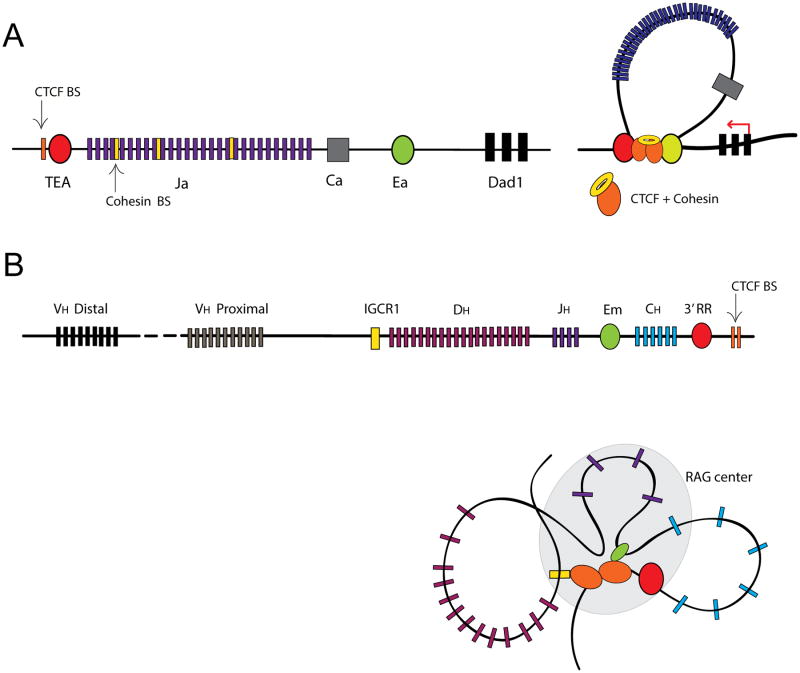
Lymphocyte differentiation provides a compelling example of the role of architectural proteins and chromatin 3D-architecture in generating cell diversity. B and T lymphocytes have a unique antigen receptor that is highly variable and cell-specific, and the basis of adaptive immunity. The variable portion of the B cell immunoglobulin (Ig) and T cell receptor (Tcr) loci is encoded by multiple copies of variable (V), diversity (D) and joining (J) gene segments that span across large genomic regions. Antigenic diversity in B and T lymphocytes is generated by gene rearrangements of these V, D and J gene segments catalyzed by the RAG1/2 recombinase. Growing evidence suggests that changes in 3D-chromatin architecture are key to the generation of B and T lymphoctyte receptor diversity [65]. The antigen receptor loci are particularly enriched in binding sites for CTCF and cohesin [66-69], leading to the proposal that these two proteins function together in modulating lymphocyte differentiation by at least two mechanisms. First, by forming rosette-like structures that facilitate lineage-specific enhancer-promoter communication and differentially activate transcription. In the Tcrα/δ locus of CD4+ CD8+ double positive thymocytes, binding of CTCF and cohesin at sites flanking the TEA promoter and the Eα enhancer is required for the long-range promoter-enhancer interactions that control Tcra transcription (Fig 3A) [66, 67]. This is supported by functional studies in mice where Rad21 deficient thymocytes show reduced interactions between the Tcrα enhancer Eα and the TEA promoter, and reduced TEA transcription, while provision of pre-rearranged TCR transgenes largely rescues thymocyte differentiation [66]. A second mechanism by which CTCF contributes to B and T cell development is by alternately facilitating and repressing V(D)J rearrangements via modulation of chromatin accessibility at the antigen receptor locus [67, 70, 71]. This has been shown using 3C-based analyses in pre-pro-B cells that reveal long-range interactions between CTCF binding sites near SIS (Silencer in Intervening Sequence), Vκ gene segments, and the boundaries of the Igκ locus. These interactions physically restrict the communication between the Jκ-Cκ-enhancer and the proximal Vκ promoter, thereby promoting rearrangement with distal Vκ segments, whereas the conditional knockout of CTCF results in more interations between the intronic Igk enhancer and the proximal Vκ segments and a bias toward proximal Vκ recombination [72]. Likewise, in the IgH locus, ChIP sequencing and 3C data show that colocalization of CTCF and Rad21 at ∼60 sites throughout the VH region and two CTCF binding sites within the Intergenic Control Region 1 (IGCR1) form the bases of the multiloop rossette structures that mediate ordered and lineage-specific VH-to-DJH recombination by biasing distal over proximal VH rearrangements [69, 73]. That is, IGCR1, which is positioned between the VH and DH clusters, suppresses the rearrangement of proximal VH segments by forming a CTCF-mediated loop that presumably isolates the proximal VH promoter from the influence of the downstream Eμ enhancer (Fig. 3B). Similarly, in CD4+ CD8+ double-positive thymocytes, the Tcrα enhancer Eα activates 3′ Vα promoters and the TEA promoter at the 5′ end of the Jα array to initiate Vα-to-Jα rearrangement. It has been shown that cohesin depletion in CD4+ CD8+ double-positive mouse thymocytes impaired the functional separation between Tcrα and the neighbouring housekeeping gene Dad1 [66]. More recent 3C data revealed the role of CTCF as an important regulator of Tcrα locus recombination. Here, Vα-to-Jα recombination occurs within a chromatin hub that is dependent on long-range interations between CTCF-binding sites and the Tcrα enhancer. The loss of CTCF in DP thymocytes dysregulates chromatin looping and locus contraction impairing Vα-to-Jα rearrangement [67].
Figure 3.
CTCF and cohesin regulate antigen receptor diversity in T and B lymphocytes. Antigen receptor diversity of B and T cells is generated by the rearrangement of different variable (V), diversity (D), and joining (J) gene segments in individual lymphocytes. CTCF influences the outcome of V(D)J recombination by regulating enhancer-promoter interactions and locus compaction. The general organization of the TCRα and IgH loci are shown. (A) In the TCRα locus of thymocytes, co-binding of the CTCF/cohesin complex at the TEA promoter and the Eα enhancer results in a DNA loop that is required to activate transcription of the nearby housekeeping gene Dad1. (B) In the Igh locus of pre-pro-B cells, CTCF-mediated looping between the Eμ enhancer and 3′ regulatory region (3′ RR) with distinct DH-JH-CH gene segments is required for ordered (DH–JH) recombination. CTCF-binding at intergenic control region 1 (IGCR1) blocks the influence of the Eμ enhancer on proximal variable (VH) regions.
CTCF/cohesin mediate monoallelic gene expression in neuronal differentiation
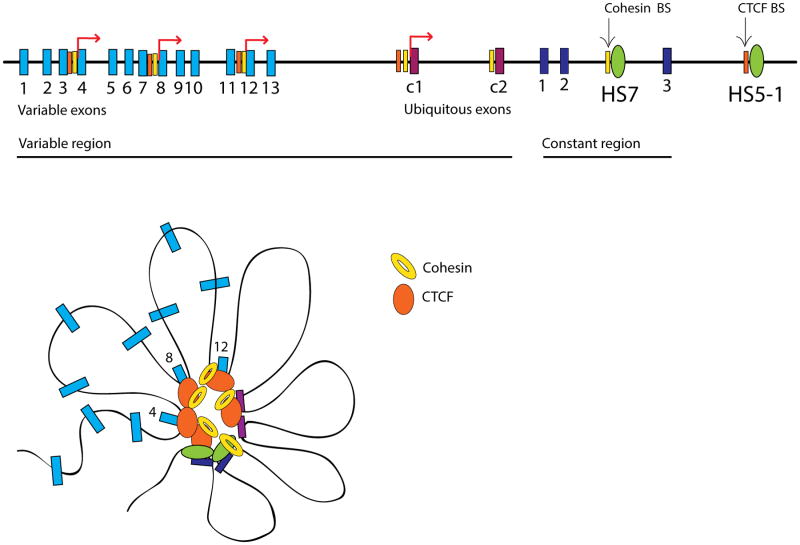
The differentiation of the hundreds of specialized neuronal types present in the brain requires the establishement of specific patterns of gene expression. Among the many genes that are transcribed in a neuron-specific manner, the mechanisms underlying the expression of Protocadherins have been studied in great detail. Protocadherins are part of the larger family of calcium-dependent cell adhesion molecules in the central nervous system. In mammals there are more than 50 protocadherin isoforms grouped into three gene clusters named α, β, and γ. Interestingly, the genomic organization of the Pcd gene clusters resembles that of the immunoglobulin and T-cell receptor genes, albeit the mechanism of regulation differ slightly in that it does not involve somatic rearrangements. In neurons, single-cell diversity results from the monoallelic gene expression of a protocadherin gene cluster, so only one isoform is transcribed at a time. This is achieved by stochastic promoter choice from the 15-variable first exons, followed by alternative pre-mRNA cis-splicing of the chosen alternative exons to three downstream constant exons [74]. Two independent studies in human and mouse cell lines provide evidence that CTCF and cohesin-mediated interactions are ultimately responsible of the monoallelic expression at the protocadherin α cluster [75, 76]. In the first of these studies, Maniatis and colleagues used a human diploid neuroblastoma cell line SK-N-SH expressing a select number of alternative Pcdh isoforms. In the case of Pcdhα, the cluster is composed of a set of 14-15 variable exons, each containing its own promoter and two cis-regulatory elements with enhancer activity (HS7 and HS5-1). CTCF/cohesin co-bound sites interact with the TSS and first exon of α4, α8, and α12 isoforms, and activate specific transcription of these isoforms. In the second study, the same authors showed that DNA looping at Pcdhα requires specific co-binding of the CTCF/cohesin complex to two symmetrically aligned binding sites in both the transcriptionally active promoters and the HS5-1 enhancer. In addition, this study identified a unique regulatory role for cohesin, which binds to another enhancer (HS7) independently of CTCF. Functional analyses demonstrated that CTCF or cohesin deletion and/or deletion of the CTCF-bound HS5-1 enhancer dysregulates chromatin architecthure at this locus and results in non-specific expression of Pcdhα isoforms [77, 78]. The findings suggest a primary role for CTCF/cohesin in establishing interactions between the two downstream enhancers and individual exon promoters that drive Pcdhα specific enhancer-promoter communication (Fig. 4). A question that arises from these studies is whether CTCF/cohesin may function by additional mechanisms, for example by regulating chromatin accessibility and compactation at this locus. In addition, the mechanisms underlying the exclusion of homologous alleles remain unclear and it will be an important issue for future work.
Figure 4.
CTCF and cohesin mediate monoallelic gene expression in neurons. The human protocadherin A (PCDH α) gene cluster contains 13 variable exons (1–13) and two c-type first exons (c1 and c2) which are expressed ubiquitously in neurons. Monoallelic gene expression of alternative isoforms occurs stocastically via a promoter choice mechanism that determines the splice site and as such, which variable exon is included in a Pcdh mRNA. Promoter choice requires the formation of a chromatin hub that is mediated by the co-binding of the CTCF/cohesin complex to the distal HS5-1 enhancer and two symmetrically aligned binding sites (yellow: cohesin, orange: CTCF) in the active promoters (α4, α8, and α12). An additional binding site for cohesin exist in the HS-7 enhancer.
Architectural proteins, 3D organization and Hox gene regulation during limb development
In vertebrates, Hox genes, present in four clusters named A to D, are activated sequentially relative to their positions within their genomic loci, leading to an anterior-posterior patterning of gene expression along the body axis. Recent studies employing various 3C techniques suggest that dynamic changes in chromatin architecture are key to transcriptional regulation of Hox gene clusters and underlie the collinearity in transcription during limb and trunk development [79-81]. In mouse limbs, the HoxD locus is located at the cusp of adjacent TADs [82]. The early HoxD1-9 genes are expressed in the proximal limb and regulated by enhancers located at the 3′ telomeric gene desert, whereas the late HoxD12-10 genes are expressed in the distal limb and regulated by enhancers located in a 5′ centromeric gene desert [82]. The transition from early to late limb development involves topological and functional switches between the regulatory archipelagos located at either side of the Hox gene cluster. Following this switch new sets of interactions are progressively established and collinearity progresses with two subsequent waves of transcription [80]. Thus far, however, the regulatory sequences and the mechanisms underlying the conformational and functional switches between domains remain obscure. It has been hypothesized that CTCF binding sites located at the TAD borders act as enhancer-blocking barriers that insulate early and late HoxD genes. Consistent with an involvement of CTCF in HoxD regulation, ChIP-chip analyses revealed CTCF binding sites flanking seven of the nine HoxD genes, as well as CTCF sites in the centromeric and telomeric gene deserts. The conditional inactivation of CTCF in mice results in massive apoptosis leading to a nearly complete loss of limb structure [83]. The situation is more complex in the case of the HoxA cluster, where studies in human cell lines and mouse embryos have reported different HoxA architectures [81, 84]. A common theme in these studies, however, is the selective gene activation through chromatin looping, which seems to depend on CTCF. Supporting evidence for this conclusion comes from a recent study using 5C in a human leukemia cell line, showing that HoxA gene activation coincides with a progressive loss of contacts throughout the region and the re-configuration of CTCF-mediated interactions between the two TAD boundaries [84, 85]. However, CTCF-dependent chromatin looping at the HoxA/D gene clusters is still inssuficient to explain the topological and functional changes that preclude the transition between early and late regulation during limb development. Further, the fact that Hox gene clusters display different topologies and apparently different transcription regulatory mechanisms across cell types and developmental processes [79, 81, 82, 85] questions the role of CTCF as the sole player in this process. In fact, Polycomb complexes have been shown to be directly involved in regulating changes in the topology of Hox loci in different developmental settings [86]. It is therefore likely that the presence of both Pc-G and architectural proteins at TAD borders and within TADs maybe responsible for shaping the three-dimensional organization of Hox gene clusters, resulting in distinct functional outcomes during cell differentiation.
Concluding remarks
By mediating communication between distant DNA sequences, architectural proteins contribute to the organization of the genome into topological and functional domains. However, the particulars of the different classes of architectural proteins associated with these domains, and how they facilitate or preclude interactions, remain obscure. In the context of cell-differentiation, an emerging theme from recent studies is that the dynamic regulation of the localization of architectural proteins, and their interactions with DNA and other proteins, modulate the network of interactions that result in cell-specific chromatin configurations. This provides a novel mechanism for cell-state-specific regulation of transcription in pluripotency and cell-fate specification. During the transition from ES to differentiated cells, genome-wide interaction maps are reshaped around cell-type specific enhancers and master transcription factors, at the same time that the binding landscapes of various architectural proteins are disrupted. However, whether architectural proteins are directly responsible for these changes is unclear. Filling this gap will require understanding the dynamics of architectural protein co-occupancy and their integration with TFs throughout the genome. Meanwhile, locus-specific studies, such as those in lymphocytes and neurons, have provided compelling and direct evidence of the importance of chromatin looping mediated by architetural proteins (CTCF/cohesin) in regulating differentiation. Much of our current knowledge is based on data obtained in different cell lines or tissue types that primarily lack functional validation. Thus, whether architectural protein binding is sufficient and necessary to engage in functional chromatin loops, remains unclear. Future research should investigate the mechanisms regulating architectural protein localization and cooperative binding, as well as the dynamics of three-dimensional landscapes across various cell-types and differentiation stages. Answers to these questions are key to our understanding of the regulation of differentiation and developmental processes.
Highlights.
The eukaryotic genome is organized into highly interactive domains called TADs
Architectural proteins control TAD border strength to regulate inter- and intra-TAD interactions
Architectural proteins control the transcription process at different steps
The effects of architectural proteins on transcription determine cell differentiation outcomes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Nora EP, et al. Segmental folding of chromosomes: a basis for structural and regulatory chromosomal neighborhoods? Bioessays. 2013;35:818–828. doi: 10.1002/bies.201300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickmore WA. The spatial organization of the human genome. Annu Rev Genomics Hum Genet. 2013;14:67–84. doi: 10.1146/annurev-genom-091212-153515. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman-Aiden E, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer CR, et al. Condensin II promotes the formation of chromosome territories by inducing axial compaction of polyploid interphase chromosomes. PLoS Genet. 2012;8:e1002873. doi: 10.1371/journal.pgen.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Bortle K, Corces VG. Nuclear organization and genome function. Annu Rev Cell Dev Biol. 2012;28:163–187. doi: 10.1146/annurev-cellbio-101011-155824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert D, et al. Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press; 2010. Space and Time in the Nucleus Developmental Control of Replication Timing and Chromosome Architecture; pp. 143–153. [DOI] [PubMed] [Google Scholar]
- 7.Li B, et al. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- 9.Phillips-Cremins JE, Corces VG. Chromatin insulators: linking genome organization to cellular function. Mol Cell. 2013;50:461–474. doi: 10.1016/j.molcel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong CT, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet. 2014;15:234–246. doi: 10.1038/nrg3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraga S, et al. TFIIIC localizes budding yeast ETC sites to the nuclear periphery. Mol Biol Cell. 2012;23:2741–2754. doi: 10.1091/mbc.E11-04-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moqtaderi Z, et al. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nat Struct Mol Biol. 2010;17:635–640. doi: 10.1038/nsmb.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Ambrosio C, et al. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008;22:2215–2227. doi: 10.1101/gad.1675708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Bortle K, Corces VG. tDNA insulators and the emerging role of TFIIIC in genome organization. Transcription. 2012;3:277–284. doi: 10.4161/trns.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli GG, et al. Genomic and proteomic analyses of Prdm5 reveal interactions with insulator binding proteins in embryonic stem cells. Mol Cell Biol. 2013;33:4504–4516. doi: 10.1128/MCB.00545-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlatanova J, Caiafa P. CTCF and its protein partners: divide and rule? J Cell Sci. 2009;122:1275–1284. doi: 10.1242/jcs.039990. [DOI] [PubMed] [Google Scholar]
- 17.Xie W, et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013;153:1134–1148. doi: 10.1016/j.cell.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz YB, et al. Nature and function of insulator protein binding sites in the Drosophila genome. Genome Res. 2012;22:2188–2198. doi: 10.1101/gr.138156.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sexton T, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148:458–472. doi: 10.1016/j.cell.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Van Bortle K, et al. Insulator function and topological domain border strength scale with architectural protein occupancy. Genome Biol. 2014;15:R82. doi: 10.1186/gb-2014-15-5-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei EP, Corces VG. RNA interference machinery influences the nuclear organization of a chromatin insulator. Nat Genet. 2006;38:936–941. doi: 10.1038/ng1850. [DOI] [PubMed] [Google Scholar]
- 22.Moshkovich N, et al. RNAi-independent role for Argonaute2 in CTCF/CP190 chromatin insulator function. Genes Dev. 2011;25:1686–1701. doi: 10.1101/gad.16651211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saldaña-Meyer R, et al. CTCF regulates the human p53 gene through direct interaction with its natural antisense transcript, Wrap53. Genes Dev. 2014;28:723–734. doi: 10.1101/gad.236869.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S, et al. Jpx RNA Activates Xist by Evicting CTCF. Cell. 2013;153:1537–1551. doi: 10.1016/j.cell.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao H, et al. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 2010;24:2543–2555. doi: 10.1101/gad.1967810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belton JM, et al. Hi-C: a comprehensive technique to capture the conformation of genomes. Methods. 2012;58:268–276. doi: 10.1016/j.ymeth.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon JR, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonis M, et al. An evaluation of 3C-based methods to capture DNA interactions. Nat Methods. 2007;4:895–901. doi: 10.1038/nmeth1114. [DOI] [PubMed] [Google Scholar]
- 29.de Wit E, de Laat W. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 2012;26:11–24. doi: 10.1101/gad.179804.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou C, et al. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol Cell. 2012;48:471–484. doi: 10.1016/j.molcel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nora EP, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghavi-Helm Y, et al. Enhancer loops appear stable during development and are associated with paused polymerase. Nature. 2014 doi: 10.1038/nature13417. [DOI] [PubMed] [Google Scholar]
- 33.Gibcus JH, Dekker J. The hierarchy of the 3D genome. Mol Cell. 2013;49:773–782. doi: 10.1016/j.molcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Bortle K, et al. Drosophila CTCF tandemly aligns with other insulator proteins at the borders of H3K27me3 domains. Genome Res. 2012;22:2176–2187. doi: 10.1101/gr.136788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofueva S, et al. Cohesin-mediated interactions organize chromosomal domain architecture. EMBO J. 2013 doi: 10.1038/emboj.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuin J, et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1317788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seitan VC, et al. Cohesin-based chromatin interactions enable regulated gene expression within preexisting architectural compartments. Genome Res. 2013 doi: 10.1101/gr.161620.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TH, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, et al. Comprehensive identification and annotation of cell type-specific and ubiquitous CTCF-binding sites in the human genome. PloS one. 2012;7:e41374. doi: 10.1371/journal.pone.0041374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong CT, et al. Poly(ADP-ribosyl)ation Regulates Insulator Function and Intrachromosomal Interactions in Drosophila. Cell. 2013;155:148–159. doi: 10.1016/j.cell.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu W, et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet. 2004;36:1105–1110. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, et al. Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res. 2012;22:1680–1688. doi: 10.1101/gr.136101.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, et al. Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res. 2012;22:1680–1688. doi: 10.1101/gr.136101.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukhopadhyay R, et al. The binding sites for the chromatin insulator protein CTCF map to DNA methylation-free domains genome-wide. Genome Res. 2004;14:1594–1602. doi: 10.1101/gr.2408304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldmann A, et al. Transcription factor occupancy can mediate active turnover of DNA methylation at regulatory regions. PLoS Genet. 2013;9:e1003994. doi: 10.1371/journal.pgen.1003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zampieri M, et al. ADP-ribose polymers localized on CTCF–Parp1–Dnmt1 complex prevent methylation of CTCF target sites. Biochem J. 2012;441:645–652. doi: 10.1042/BJ20111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 48.Efroni S, et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell. 2008;2:437–447. doi: 10.1016/j.stem.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Wit E, et al. The pluripotent genome in three dimensions is shaped around pluripotency factors. Nature. 2013;501:227–231. doi: 10.1038/nature12420. [DOI] [PubMed] [Google Scholar]
- 50.Denholtz M, Plath K. Pluripotency in 3D: genome organization in pluripotent cells. Curr Opin Cell Biol. 2012;24:793–801. doi: 10.1016/j.ceb.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apostolou E, et al. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013;12:699–712. doi: 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang P, et al. Higher-order genomic organization in pluripotent stem cells. Protein Cell. 2012;3:483–486. doi: 10.1007/s13238-012-2806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei Z, et al. Klf4 organizes long-range chromosomal interactions with the oct4 locus in reprogramming and pluripotency. Cell Stem Cell. 2013;13:36–47. doi: 10.1016/j.stem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, et al. Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature. 2013;504:306–310. doi: 10.1038/nature12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peric-Hupkes D, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whyte WA, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagey MH, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Handoko L, et al. CTCF-mediated functional chromatin interactome in pluripotent cells. Nat Genet. 2011;43:630–638. doi: 10.1038/ng.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z, et al. Control of embryonic stem cell lineage commitment by core promoter factor, TAF3. Cell. 2011;146:720–731. doi: 10.1016/j.cell.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delgado-Olguín P, et al. CTCF Promotes Muscle Differentiation by Modulating the Activity of Myogenic Regulatory Factors. J Biol Chem. 2011;286:12483–12494. doi: 10.1074/jbc.M110.164574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plasschaert RN, et al. CTCF binding site sequence differences are associated with unique regulatory and functional trends during embryonic stem cell differentiation. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt910. gkt910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teif VB, et al. Nucleosome repositioning links DNA (de)methylation and differential CTCF binding during stem cell development. Genome Res. 2014 doi: 10.1101/gr.164418.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips-Cremins JE, et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–1295. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, et al. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell. 2013;13:30–35. doi: 10.1016/j.stem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Seitan VC, et al. Cohesin, CTCF and lymphocyte antigen receptor locus rearrangement. Trends Immunol. 2012;33:153–159. doi: 10.1016/j.it.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seitan VC, et al. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature. 2011;476:467–471. doi: 10.1038/nature10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shih HY, et al. Tcra gene recombination is supported by a Tcra enhancer- and CTCF-dependent chromatin hub. Proc Natl Acad Sci U S A. 2012;109:E3493–3502. doi: 10.1073/pnas.1214131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Degner SC, et al. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol. 2009;182:44–48. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Degner SC, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci U S A. 2011;108:9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo C, et al. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell. 2011;147:332–343. doi: 10.1016/j.cell.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo C, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011;477:424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ribeiro de Almeida C, et al. The DNA-binding protein CTCF limits proximal Vkappa recombination and restricts kappa enhancer interactions to the immunoglobulin kappa light chain locus. Immunity. 2011;35:501–513. doi: 10.1016/j.immuni.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 73.Guo CG, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011;477:424–U182. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen WV, Maniatis T. Clustered protocadherins. Development. 2013;140:3297–3302. doi: 10.1242/dev.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, et al. CTCF/cohesin-mediated DNA looping is required for protocadherin alpha promoter choice. Proc Natl Acad Sci U S A. 2012;109:21081–21086. doi: 10.1073/pnas.1219280110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monahan K, et al. Role of CCCTC binding factor (CTCF) and cohesin in the generation of single-cell diversity of protocadherin-alpha gene expression. Proc Natl Acad Sci U S A. 2012;109:9125–9130. doi: 10.1073/pnas.1205074109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirayama T, et al. CTCF is required for neural development and stochastic expression of clustered Pcdh genes in neurons. Cell Rep. 2012;2:345–357. doi: 10.1016/j.celrep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Kehayova P, et al. Regulatory elements required for the activation and repression of the protocadherin-α gene cluster. Proc Natl Acad Sci U S A. 2011;108:17195–17200. doi: 10.1073/pnas.1114357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noordermeer D, et al. The dynamic architecture of Hox gene clusters. Science. 2011;334:222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- 80.Andrey G, et al. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science. 2013;340 doi: 10.1126/science.1234167. [DOI] [PubMed] [Google Scholar]
- 81.Berlivet S, et al. Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of HoxA Genes in Developing Limbs. PLoS Genet. 2013;9:e1004018. doi: 10.1371/journal.pgen.1004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montavon T, et al. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147:1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 83.Soshnikova N, et al. Functional analysis of CTCF during mammalian limb development. Dev Cell. 2010;19:819–830. doi: 10.1016/j.devcel.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Kim YJ, et al. Conserved, developmentally regulated mechanism couples chromosomal looping and heterochromatin barrier activity at the homeobox gene A locus. Proc Natl Acad Sci U S A. 2011;108:7391–7396. doi: 10.1073/pnas.1018279108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rousseau M, et al. Hox in motion: tracking HoxA cluster conformation during differentiation. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eskeland R, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]