Abstract
Bacteria are killed by a variety of lethal stressors, some of which promote a cascade of reactive oxygen species (ROS). Perturbations expected to alter ROS accumulation affect the lethal action of diverse antibacterials, leading to the hypothesis that killing by these agents can involve ROS-mediated self-destruction. Recent challenges to the hypothesis are considered, particularly with respect to complexities in assays that distinguish primary damage from the cellular response to that damage. Also considered are bifunctional factors that are protective at low stress levels but destructive at high levels. These considerations, plus new data, support an involvement of ROS in the lethal action of some antimicrobials and raise important questions concerning consumption of antioxidant dietary supplements during antimicrobial chemotherapy.
Introduction
Reactive oxygen species (ROS) are emerging as important elements in the bacterial response to lethal stress. Three naturally occurring species, superoxide, hydrogen peroxide, and hydroxyl radical, are receiving the most attention. Superoxide and hydrogen peroxide arise when molecular oxygen adventitiously oxidizes redox enzymes that normally transfer electrons to other substrates [1]. Hydrogen peroxide, which can also be produced from dismutation of superoxide, serves as a substrate for hydroxyl radical formation through Fenton chemistry. This oxidative process can kill cells if hydroxyl radical accumulation is not controlled, since hydroxyl radical breaks nucleic acids, carbonylates proteins, and peroxidates lipids.
Bacteria contain protective proteins that can detoxify ROS (SodA, SodB, SodC, AhpCF, KatG, KatE) and counter damage (e.g., SoxRS, OxyRS, and SOS regulons). However, bacteria may also use ROS to self-destruct when stress is severe. Indeed, no protein-based mechanism has been identified that detoxifies hydroxyl radical. Association of ROS with the lethal action of multiple antibiotic classes (Table 1) has been taken as an example of stress-stimulated self-destruction, but complexities in the system have led to challenges. To clarify issues surrounding a debate that may have clinical importance, we review key aspects of the hypothesis that ROS contribute to antimicrobial lethality.
Table 1.
Relationships between ROS and lethal stressors
| Stressor category | Lethal agent examined | ROS/oxidative stress level increasea | Effect of ROS perturbation on lethality | References | ||
|---|---|---|---|---|---|---|
| Anaerobic conditions | Chemical inhibition of ROS accumulationb | Deficiency in ROS scavenging enzyme | ||||
| Quinolone | Nalidixic acid | Yes, all ROS (H2DCFDA) | Block | Block | Increase by ΔkatG | [11,26] |
| Oxolinic acid | Yes, hydroxyl radical (HPF) | Block | Block | Increase by ΔkatGc | [17] | |
| Norfloxacin | Yes, all ROS (multiple dyes)d | Reduce | Reduce | Increasee (ΔkatG)/decrease (katG over-expression) | [9,12,30,44] | |
| Ciprofloxacin | Yes, all ROS (lucigenin and luminol). Yes, lipid and DNA base oxidation | Reduce or block | Reduce | Increase by ΔkatGc | [5,8,10,11] | |
| Ofloxacin | NAf | Block | NAf | NAf | [10] | |
| Moxifloxacin | Yes, hydroxyl radical (HPF) | Reduce | Reduce | NAf | [13,17] | |
| PD161144 | Yes, hydroxyl radical (HPF) | No effect | No effect | NAf | [11,17] | |
| β-lactam | Ampicillin | Yes, all ROS (multiple dyes)d | Reduce | Reduce | Increase by ΔahpCFe | [9,12,30,44] |
| Oxacillin | NAf | NAf | Reduce | NAf | [13] | |
| Piperacillin, ceftazidime | Yes, increased lipid and DNA base oxidation | NAf | NAf | NAf | [18] | |
| Cefuroxime | NAf | NAf | Reduce | NAf | [21] | |
| Aminoglycoside | Kanamycin | Yes, hydroxyl radical (HPF) | Delay or reducec | Reduce | Increase by ΔahpCFe | [9,12] |
| Gentamicin | Yes, all ROS (multiple dyes)d | Reduce | Reduce | Decrease by katG over-expression) | [22,30,44] | |
| Glycopeptide | Vancomycin | Yes, hydroxyl radical (HPF) | NAf | NAf | NAf | [9] |
| Polymyxin | Polymyxin, colistin | Yes, hydroxyl radical (HPF) | NAf | Reduce (delay) | NAf | [45] |
Intracellular ROS species were detected by various fluorescent dyes: HPF is specific for hydroxyl radical, H2DCFDA reports all ROS, lucigenin is for detection of superoxide, and luminal is for other ROS.
Chemicals used were bipyridyl, thiourea, glutathione, vitamin C, nitric oxide, and hydrogen sulfide.
Authors’ unpublished data.
Increased signal observed with seven fluorescent dyes having various specificities for ROS types [30].
An Hpx− (katG−, katE−, ahpC−) triple mutant showed no increase in lethality with ampicillin, kanamycin, and long but not short exposure to norfloxacin [29], possibly because spontaneous compensatory induction of oxidative stress defense occurs when this mutant grows aerobically [30].
NA, data not available.
Connection of ROS to stress-mediated killing
A link between oxidative stress and antimicrobial action surfaced from work showing that activation of the SoxRS regulon confers resistance to multiple antimicrobial classes [2–4]. Similar conclusions were reached when antioxidants, such as vitamin C and glutathione, raised minimal inhibitory concentration (MIC) and efficiency-of-plating (EOP, fraction of cells that form colonies on drug-containing agar) for quinolones and aminoglycosides [5,6]. Moreover, elevated levels of oxidative stress signals were detected in antimicrobial-treated cells [7,8]. However, an unambiguous connection between ROS and cell death was not established, because measurements such as MIC and EOP primarily report inhibition of bacterial growth rather than cell death.
A specific connection between antimicrobial-mediated killing and ROS emerged when the Collins group found that a surge in hydroxyl radical, detected by intracellular oxidation of a fluorescent dye (HPF), accompanied killing by norfloxacin, ampicillin, and kanamycin; no surge in HPF fluorescence was seen with five different bacteriostatic agents or with bactericidal compounds at sublethal, bacteriostatic concentrations [9]. In addition, hydroxyl radical accumulation and cell death were reduced by treatment with thiourea, an ROS scavenger, and with dipyridyl, an iron chelator that suppresses the Fenton reaction. These observations, plus anaerobic blockage of killing by some quinolones [10,11], indicated that the accumulation of ROS could augment effects of antibiotic-mediated lesions.
We have extended Collins’ finding and uncovered complexities. As expected, a deficiency in catalase/peroxidase increased the lethal action of three diverse antimicrobial classes [12]. In addition, examples were found in which ROS contributed to rapid killing but not to growth inhibition, as measured by MIC, or to slow death associated with long incubation times [12]. Indeed, with Staphylococcus aureus, ROS affected the rate of killing but not the overall extent of killing [13]. Surprisingly, production of sublethal levels of superoxide or the absence of superoxide dismutases reduced rather than increased antimicrobial-mediated killing [12,14,15]. Thus, superoxide appeared to have a protective function in addition to the known destructive one [16]. We even found examples in which ROS was involved in the lethal activity of only some members of an antimicrobial family [17]: apparently the primary damaged caused by some fluoroquinolones is so lethal that ROS-mediated effects are irrelevant. Thus, a contribution of ROS to antimicrobial lethality was solidified, but it turned out to be more complex than initially thought.
The hypothesis that ROS contribute to antimicrobial-mediated lethality also explains why some antimicrobial effects are seemingly unrelated to the primary damage caused by the drugs. For example, treatment of S. aureus with ciprofloxacin, a lethal, topoisomerase-mediated DNA-damaging agent, leads to lipid peroxidation and DNA base oxidation (8-oxo-dG [18]). Furthermore, kanamycin, an inhibitor of protein synthesis, causes protein aggregation that is reduced by over-expression of a peroxide scavenger [19]. In other examples, ampicillin- and kanamycin-mediated lethality is reduced by over-expression of enzymes (MutT and RibA) that are involved in removal of 8-oxo-dGTP, a toxic product generated by ROS-mediated oxidation of the guanine nucleotide pool [20]. Finally, antibiotic-mediated killing is reduced by bacterial production of nitric oxide and hydrogen sulfide. These gases stimulate ROS-scavenging enzymes (catalase and superoxide dismutase) and suppress the Fenton reaction [21,22]. Collectively the data support the idea that primary damage caused by antimicrobials can trigger ROS-mediated effects.
Challenges to ROS involvement in antimicrobial lethality
Several recent reports question the contribution of ROS to antimicrobial-mediated killing. One describes examples in which ROS accumulation and cell death are discordant [23], and another emphasizes that the effect of iron/iron-sulfur clusters on killing by certain antibacterials depends largely on drug uptake with no role for ROS in lethality [24]. A third [25] points out that the Cpx envelope stress-response system increases resistance only to some antimicrobial classes (Cpx is proposed to be important in the control of ROS accumulation [26–28]). Low specificity of dyes and other molecular probes for ROS and their effects are among many criticisms in a fourth report [29]. Thus, the challenges are quite varied.
New work from the Collins group [30] addresses many of the specific, technical issues; consequently, we focus on general aspects of assay design and interpretation. First we emphasize the need to measure lethal action: factors that affect bacterial growth but not survival are by definition irrelevant. Thus, growth-inhibition assays, such as determination of MIC or EOP, which do not specifically measure survival, are inadequate for addressing the involvement of ROS in cell death [5,6]. Indeed, perturbations of ROS can have a profound effect on rapid killing with little effect on MIC [12].
It is also important to distinguish factors affecting formation of primary damage from those affecting the response to that damage. For example, drug uptake, efflux, and target interactions influence primary lesion formation and cell death arising directly from the lesion, but they differ in principle from the cellular response to the lesion, i.e. from the ROS cascade and secondary damage. In the absence of such distinction, lack of correlation between perturbation of ROS and lethality does not refute the ROS-antimicrobial lethality hypothesis [24]. Conversely, ROS-mediated effects on primary lesion formation do not support the hypothesis. Among the latter is superoxide-mediated induction of drug efflux systems [15,31]. One way to focus on the response to a lesion is to normalize lethal antimicrobial concentrations to MIC [12]. MIC is sensitive to drug uptake, efflux, and target interactions, but it can be distinct from concentrations that rapidly kill cells, particularly with quinolones. Failure to focus on response weakens lack-of-correlation arguments.
Examining a broad range of drug concentration and incubation time reduces the chance that ROS-mediated effects are overlooked or misinterpreted. Indeed, the common practice of using single drug concentrations during searches for ROS effects may miss effects occurring at low drug concentrations and short treatment times. For example, anaerobiosis inhibits norfloxacin lethality at low but not high drug concentrations [11,23]. Another problem can arise from combining a single, high antimicrobial concentration with a long incubation time. That may eliminate the major, growing portion of a bacterial population and make persister cell [32] survival the measure for evaluating factors potentially involved in ROS accumulation. Persister cells are metabolically dormant and are less likely than growing cells to exhibit ROS-mediated killing by antibiotics.
Another complexity arises from ROS-dependent, programmed cell death that continues after lethal stress is removed. This phenomenon, which can be seen when quinolone-treated bacteria are applied to drug-free agar [26], makes it difficult to determine whether cells die before or after sampling. Consequently, accurate kinetic correlations, or lack thereof, between hydroxyl radical accumulation and cell death [23] require a more precise way to determine when cells actually die.
An intriguing challenge to the hypothesis that ROS contribute to antimicrobial-mediated killing derives from the observation that the iron chelator dipyridyl, which inhibits aerobic production of hydroxyl radical, also appears to inhibit anaerobic lethality [23,29]. Although several explanations are still under consideration, an interesting possibility is that oxidants or radicals other than ROS also contribute to antibiotic-mediated cell death. Such a result would extend the general principle of stress-mediated self-destruction to anaerobic conditions.
In summary, challenges to the ROS-antimicrobial lethality hypothesis have often relied on work with single drug concentrations or on growth-inhibition measurements. Moreover, they often failed to sharply separate primary lesion formation from responses. We conclude that the hypothesis is strong enough to merit consideration of control elements.
Control of stress-induced, ROS-mediated bacterial cell death
A stress-mediated ROS cascade is expected to be auto-stimulating, since production of hydroxyl radical will cause secondary macromolecular damage and thereby stimulate additional ROS production (Fig. 1). Consequently, the cascade must be controlled to avoid runaway death due to minor, transient stress [26]. One type of control is exerted by ROS-detoxifying enzymes: deletion of katG or ahpCF confers hyperlethality to diverse antibiotics [12], while over-expression of these genes mitigates ROS-mediated damage [19,30].
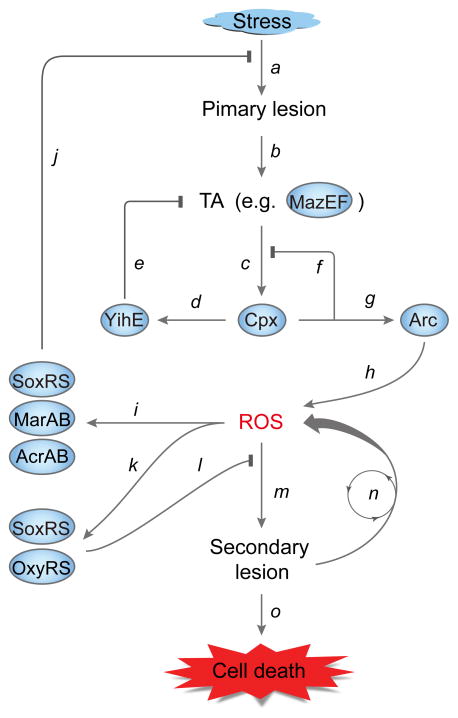
Figure 1.
Stress-induced ROS and the live-or-die bacterial stress response. Stress, such as exposure to lethal antibiotics, generates stressor-specific primary lesions (a) that activate toxin-antitoxin modules (TA, for example MazEF, (b)). Toxin-mediated mRNA cleavage may lead to production of truncated, misfolded peptides that lodge in cell membranes and induce the two-component Cpx envelope stress system (c). Induction of Cpx up-regulates expression of YihE protein kinase (d), which mitigates MazF toxin activity (e). Induction of Cpx can also induce expression of genes encoding proteins that refold and degrade misfolded peptides and suppress Cpx induction (f). Cpx activation and membrane perturbation may activate the Arc two-component system (g), which raises ROS levels (h), possibly through stimulation of the TCA cycle and interference with cytochrome bd oxidase [27,28]. Elevated levels of ROS can induce the SoxRS-MarRAB-AcrAB efflux pump system (i) that exports toxic stressors and thereby suppresses primary lesion formation (j). ROS accumulation also induces the SoxRS-OxyRS regulons (k) that interfere with (l) ROS generating secondary cellular damage (m). Secondary damage, if uncontrolled, is expected to stimulate successive rounds of ROS production (n) that kill cells (o), thereby removing severely damaged cells from bacterial populations.
Another level of control appears to involve a genetic pathway that transmits a signal from the stressor-specific, primary lesion to the ROS-generating system. When bacterial cells are exposed to stress, preferential degradation of the MazE antitoxin occurs, thereby liberating MazF toxin. MazF then cleaves many cellular RNAs [33,34]. Some of the 5′ truncated mRNAs may be translated into truncated peptides that misfold, lodge in cell membranes, and activate the Cpx envelope protein stress system [26–28]. Activation of Cpx is expected to induce expression of YihE [35], a protein kinase that negatively regulates MazF [26]. Cpx activation also induces many other functions that help renature/degrade misfolded proteins in cell envelopes [36]; thus, the Cpx system is protective. However, deletion of CpxR, the response regulator, protects from the lethal action of quinolones, gentamycin, and ampicillin [26,27]. Thus, the wild-type Cpx system appears to have a destructive activity, perhaps through activation of the two-component Arc redox-sensing system [27,28]. Arc could in turn perturb electron transfer complexes, such as cytochrome bd oxidase [28], and thereby increase ROS levels.
If stress is mild and transient, ROS accumulate to a moderate level that is sufficient for ROS to be beneficial mutagens and inducers of protective functions. However, if stress is severe and persistent, Cpx-mediated perturbation of Arc may lead to high levels of ROS that overwhelm protective elements. According to this scenario, the level of stress influences the outcome of the stress response. Such an idea suggests the existence of bifunctional elements and threshold-based cell death responses. One bifunctional factor may be superoxide. It kills bacteria when produced at high concentrations triggered by plumbagin, a metabolic generator of superoxide [16]. However, moderate concentrations of superoxide induce a variety of protective genes, largely through the SoxRS regulon [14,15,31]. Another dual-function element appears to be MazF. Whether MazF promotes stress-mediated programmed cell death or helps stressed cells enter a stress-tolerant metabolic dormancy has been debated [37,38]. The controversy is resolved in part by MazF having both activities -- we found with Bacillus subtilis that a deficiency in a mazF orthologue, ndoA, was either protective or destructive depending on the level of stress [39]. A third bifunctional factor is Cpx, as pointed out above. Such bifunctional elements could serve as part of a live-or-die stress response [26]: they are protective at low stress levels but destructive at high levels (Fig. 1). Within this context the ROS cascade acts as an executioner when stress is high enough to elicit programmed cell death.
Concluding Remarks
The ROS contribution to lethal stress is complex. The live-or-die stress response provides a framework for explaining many complexities associated with ROS, but interesting questions remain to be answered. For example, we do not know how information from the initial stress-induced lesion is transduced to the MazF-Cpx-ROS pathway. Nor do we know why different antibiotic classes elicit different patterns of expression for genes in the TCA cycle [9], which are far downstream from the initial lesion. Additional work is also required to determine whether non-oxygen reactive oxidants play a role similar to that of ROS when bacteria experience lethal stress during anaerobic growth. And from an evolutionary perspective we wonder whether the destructive role of ROS is merely collateral damage arising from protective activities or whether self-destruction confers a selective advantage to bacterial populations, perhaps limiting the amount of energy spent on futile repair.
Relationships between ROS and antimicrobial lethality may also be clinically significant. For example, factors that interfere with antimicrobial lethality are likely to compromise efficacy and contribute to the emergence of antimicrobial resistance. One of those factors may be consumption of antioxidant dietary supplements, since they interfere with antimicrobial lethality [40]. Indeed, consumption of antioxidants is a common practice in the U.S. [41]. ROS may also be clinically significant if ways are found to boost intracellular ROS production, as have been recently suggested with inhibitors of nitric oxide synthase and succinate dehydrogenase [42,43]. Such work could lead to novel strategies for increasing the lethal action of many antimicrobial classes.
Highlights.
Lethal antimicrobial stress triggers accumulation of Reactive Oxygen Species (ROS).
The stress-mediated ROS cascade and its effects are controlled in multiple ways.
At moderate levels, ROS reduce stress by inducing protective regulons.
At high levels, ROS can be destructive by creating a variety of secondary lesions.
Dietary consumption of antioxidants may antagonize antimicrobial therapy.
Acknowledgments
We thank the following for critical comments on the manuscript: Arnold Bendich, Marila Gennaro, Richard Pine, and Issar Smith. The work was supported by grants from National Institutes of Health (1DP2OD007423, 2R01AI073491, and 1R21AI103781).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest published in the past two years have been highlighted as:
*of special interest
**of outstanding interest
- 1.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990;87:6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oethinger M, Podglajen I, Kern WV, Levy SB. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob Agents Chemother. 1998;42:2089–2094. doi: 10.1128/aac.42.8.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koutsolioutsou A, Martins EA, White DG, Levy SB, Demple B. A soxRS-constitutive mutation contributing to antibiotic resistance in a clinical isolate of Salmonella enterica (Serovar typhimurium) Antimicrob Agents Chemother. 2001;45:38–43. doi: 10.1128/AAC.45.1.38-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami M, Mangoli SH, Jawali N. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob Agents Chemother. 2006;50:949–954. doi: 10.1128/AAC.50.3.949-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami M, Mangoli SH, Jawali N. Effects of glutathione and ascorbic acid on streptomycin sensitivity of Escherichia coli. Antimicrob Agents Chemother. 2007;51:1119–1122. doi: 10.1128/AAC.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albesa I, Becerra MC, Battan PC, Paez PL. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun. 2004;317:605–609. doi: 10.1016/j.bbrc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 8.Becerra MC, Albesa I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem Biophys Res Commun. 2002;297:1003–1007. doi: 10.1016/s0006-291x(02)02331-8. [DOI] [PubMed] [Google Scholar]
- 9.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Lewin CS, Morrissey I, Smith JT. The mode of action of quinolones: the paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur J Clin Microbiol Infect Dis. 1991;10:240–248. doi: 10.1007/BF01966996. [DOI] [PubMed] [Google Scholar]
- 11.Malik M, Hussain S, Drlica K. Effect of anaerobic growth on quinolone lethality with Escherichia coli. Antimicrob Agents Chemother. 2007;51:28–34. doi: 10.1128/AAC.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Liu X, Qu Y, Wang X, Li L, Zhao X. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob Agents Chemother. 2012;56:6048–6050. doi: 10.1128/AAC.00754-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger RM, Drlica K. Superoxide protects Escherichia coli from bleomycin mediated lethality. J Inorg Biochem. 2009;103:1273–1277. doi: 10.1016/j.jinorgbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Mosel M, Li L, Drlica K, Zhao X. Superoxide-mediated protection of Escherichia coli from antimicrobials. Antimicrob Agents Chemother. 2013;57:5755–5759. doi: 10.1128/AAC.00754-13. The authors showed that superoxide can protect bacterial cells from antimicrobial action at both the growth inhibition (primary lesion) and killing levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985;164:1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Zhao X, Malik M, Drlica K. Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death. J Antimicrobial Chemother. 2010;65:520–524. doi: 10.1093/jac/dkp486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becerra MC, Paez PL, Larovere LE, Albesa I. Lipids and DNA oxidation in Staphylococcus aureus as a consequence of oxidative stress generated by ciprofloxacin. Mol Cell Biochem. 2006;285:29–34. doi: 10.1007/s11010-005-9051-0. [DOI] [PubMed] [Google Scholar]
- 19.Ling J, Cho C, Guo LT, Aerni HR, Rinehart J, Soll D. Protein aggregation caused by aminoglycoside action is prevented by a hydrogen peroxide scavenger. Mol Cell. 2012;48:713–722. doi: 10.1016/j.molcel.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. The authors demonstrated that 1) ROS facilitate stress-mediated killing by oxidization of the guanine pool, 2) incorporation of 8-oxo-guanine derivatives into DNA and RNA fuels the production of secondary cellular damage, such as double-stranded DNA breaks and misfolding of membrane proteins, and 3) secondary damage in turn triggers more ROS and further damage that eventually kills cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 23**.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. The authors challenged the idea that all antimicrobials kill through ROS-mediated mechanisms by reporting that anaerobic conditions block the lethal action of the quinolone norfloxacin only at low concentration, that hydroxyl radical accumulation fails to always correlate with antimicrobial killing, and that dipyridyl, an iron chelator that inhibits the Fenton reaction, and thiourea, a hydroxyl radical scavenger, protect cells from antimicrobial killing under both aerobic and anaerobic conditions. [DOI] [PubMed] [Google Scholar]
- 24.Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, Su SY, Espinosa L, Loiseau L, Py B, et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science. 2013;340:1583–1587. doi: 10.1126/science.1238328. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney TF, Silhavy TJ. The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics. J Bacteriol. 2013;195:1869–1874. doi: 10.1128/JB.02197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Dorsey-Oresto A, Lu T, Mosel M, Wang X, Salz T, Drlica K, Zhao X. YihE kinase is a central regulator of programmed cell death in bacteria. Cell Rep. 2013;3:528–537. doi: 10.1016/j.celrep.2013.01.026. The authors, for the first time, demonstrated the existence of ROS-mediated post-stress programmed cell death in bacteria; identified the YihE protein kinase as a control element for stress-triggered, ROS-dependent cell death; and proposed an ROS-based live-or-die stress response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies BW, Kohanski MA, Simmons LA, Winkler JA, Collins JJ, Walker GC. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol Cell. 2009;36:845–860. doi: 10.1016/j.molcel.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. The authors challenged the idea that all antimicrobials kill through ROS-mediated mechanisms by pointing out that 1) dyes used as ROS probes may be oxidized in multiple ways, 2) some antibiotics can kill cells grown anaerobically, and 3) dipyridyl, an iron chelator, may have off-target effects that are not ruled out and that dipyridyl interferes with anaerobic antimicrobial-mediated killing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Dwyer DJ, Belenky P, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CTY, Lobritz MA, Braff D, Schwarz EG, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014;111:E2100–2109. doi: 10.1073/pnas.1401876111. The authors used a combination of biophysical, biochemical, and transcriptomic approaches to make point-by-point rebuttal of most of the challenges to the ROS-antimicrobial lethality hypothesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Vulic M, Keren I, Lewis K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. 2012;56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell. 2003;12:913–923. doi: 10.1016/s1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 34.Gerdes K, Christensen SK, Lobner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 35.Pogliano J, Lynch AS, Belin D, Lin EC, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 36.Raivio TL, Silhavy TJ. Periplasmic stress and ECF sigma factors. Annu Rev Microbiol. 2001;55:591–624. doi: 10.1146/annurev.micro.55.1.591. [DOI] [PubMed] [Google Scholar]
- 37.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006;2:e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen K, Christensen SK, Gerdes K. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins. Mol Microbiol. 2002;45:501–510. doi: 10.1046/j.1365-2958.2002.03027.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Wang X, Drlica K, Zhao X. A toxin-antitoxin module in Bacillus subtilis can both mitigate and amplify effects of lethal stress. PLoS One. 2011;6:e23909. doi: 10.1371/journal.pone.0023909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Marathe SA, Kumar R, Ajitkumar P, Nagaraja V, Chakravortty D. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella typhimurium and Salmonella typhi. J Antimicrob Chemother. 2013;68:139–152. doi: 10.1093/jac/dks375. The authors demonstrated that consumption of a common dietary antioxidant (curcumin) antagonizes antimicrobial killing. [DOI] [PubMed] [Google Scholar]
- 41.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 42*.Holden JK, Li H, Jing Q, Kang S, Richo J, Silverman RB, Poulos TL. Structural and biological studies on bacterial nitric oxide synthase inhibitors. Proc Natl Acad Sci U S A. 2013;110:18127–18131. doi: 10.1073/pnas.1314080110. The authors reported that inhibitors of nitric oxide synthase may serve as an antimicrobial potentiator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Brynildsen MP, Winkler JA, Spina CS, MacDonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat Biotechnol. 2013;31:160–165. doi: 10.1038/nbt.2458. The authors showed that multiple metabolic pathways can be perturbed to enhance endogenous ROS for potentiation of antimicrobial killing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeom J, Imlay JA, Park W. Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. J Biol Chem. 2010;285:22689–22695. doi: 10.1074/jbc.M110.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother. 2012;56:5642–5649. doi: 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]