Abstract
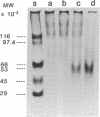
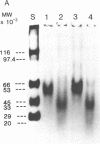
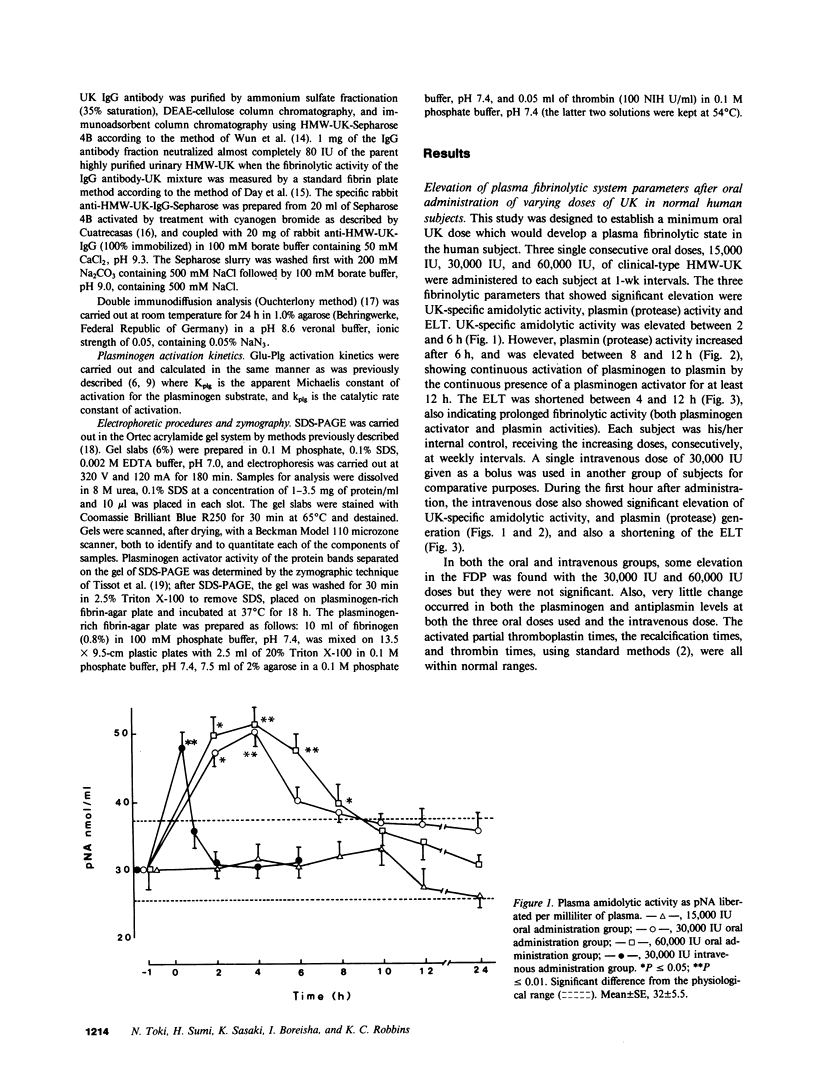
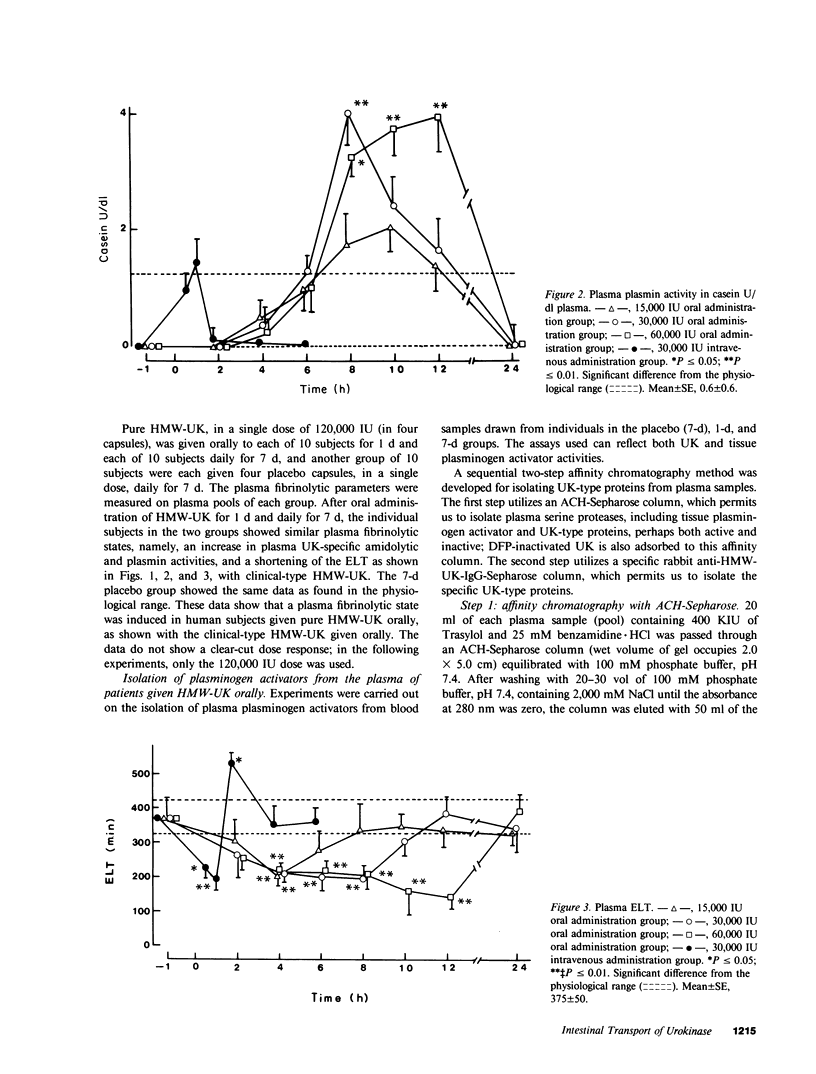
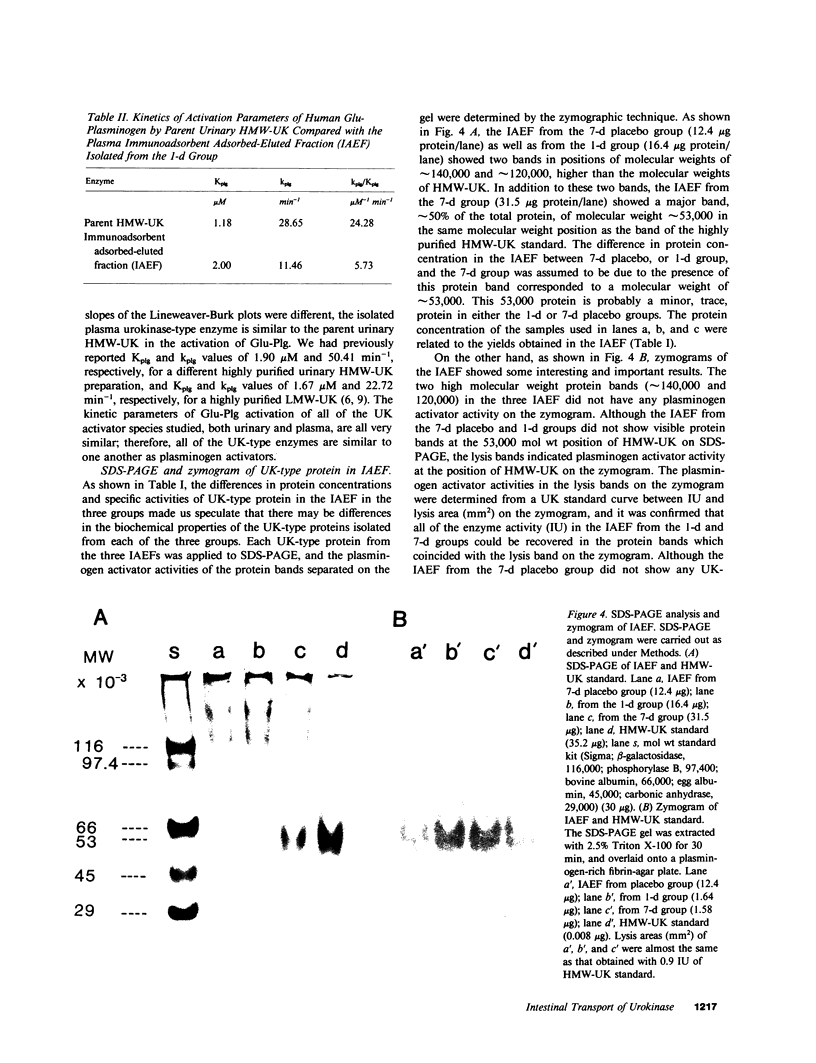
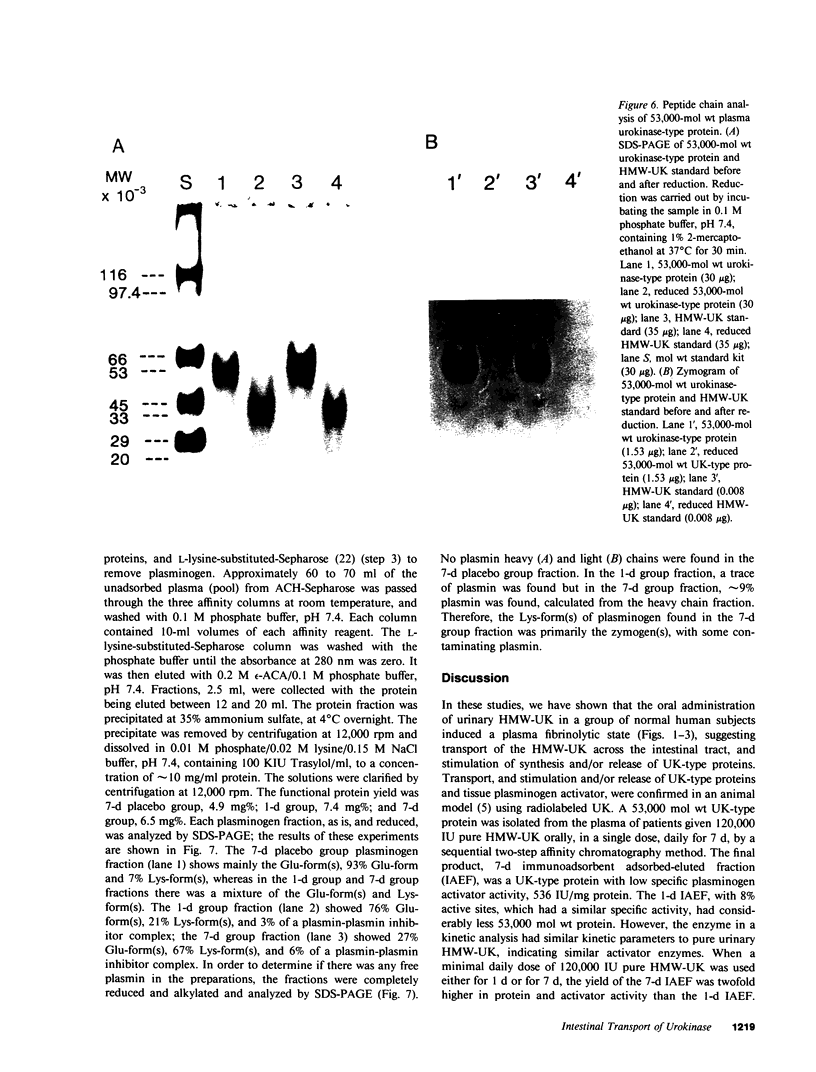
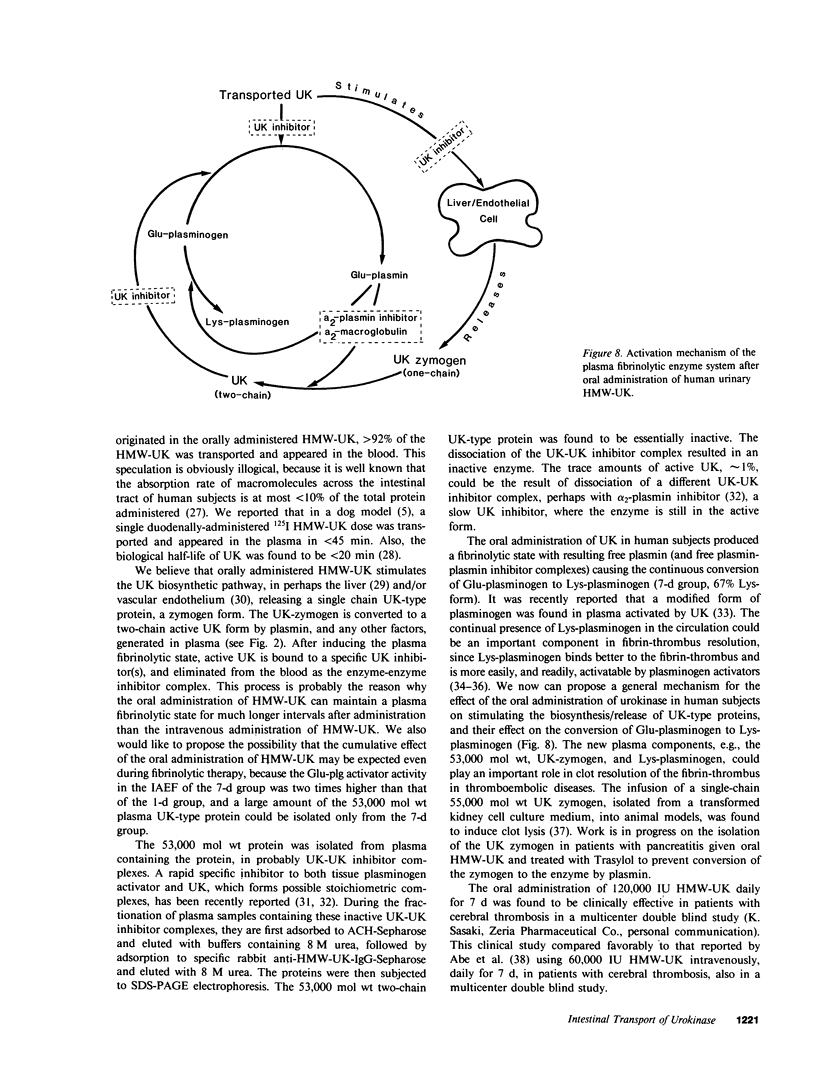
Oral administration of clinical-type high molecular weight urokinase (HMW-UK) in a single dose of 30,000-60,000 International Units (IU) in enteric-coated capsules, in a group of normal human subjects, induced a plasma fibrinolytic state suggesting transport of HMW-UK across the intestinal tract. Other groups of human subjects were given a single dose of 120,000 IU daily of pure HMW-UK for 1 d and 7 d together with a placebo dose, all of the ingredients except urokinase (UK), daily for 7 d. UK-type proteins were isolated from the plasma of blood samples drawn 6 h after administration of the final dose, by a sequential two-step affinity chromatography method first with [N alpha-(epsilon-aminocaproyl)-DL-homoarginine hexylester]-Sepharose and second with a specific rabbit anti-HMW-UK-IgG-Sepharose. The yield of UK-type proteins from the 7-d group, 0.79 mg/dl, was approximately twofold greater than that obtained from either the placebo or 1-d groups. The specific plasminogen activator activity of the 1-d and 7-d groups were similar, 508 and 537 IU/mg protein, respectively; negligible plasminogen activator activity could be detected in the placebo group. The kinetics of activation parameters of human Glu-plasminogen of the UK-type protein, isolated from the 1-d group, were similar to those obtained with urinary HMW-UK. The UK-type proteins isolated only from the 7-d group showed, in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, a major protein band of molecular weight 53,000 in the same position as HMW-UK. In addition, two other major protein bands of approximately 140,000 and approximately 120,000 mol wt were found in the 7-d placebo-, and 1-d groups, and also in the 7-d group. The 53,000 mol wt protein, about 50% of the total protein in the 7-d group, was further purified by preparative SDS-polyacrylamide gel electrophoresis, and found to be a two-chain protein with a specific activity of 1,241 IU/mg protein. The protein showed common antigenic determinants with urinary HMW-UK. The oral administration of 120,000 IU HMW-UK to human subjects for 7 d stimulated the synthesis of a UK-type protein of 53,000 mol wt, probably the zymogen, from either the liver or vascular endothelium, which was released into the circulation, and converted into active UK by circulating plasmin. The induction of the fibrinolytic state produced the conversion of native circulating Glu-plasminogen into the degraded Lys-plasminogen form, which was found in large amounts in the plasma of the 7-d group. The new plasma components, e.g., the 53,000-mol wt UK-zymogen and Lys-plasminogen, could play an important role in clot resolution of the fibrin-thrombus in thromboembolic diseases. Oral administration of HMW-UK has been shown to be clinically effective in patients with cerebral thrombosis in a multicenter double blind study.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow G. H., Firestone S. L., Robbins K. C. Identification of the plasminogen activator(s) produced by the transformed liver cell line, SK-HEP-1. Thromb Res. 1983 Oct 1;32(1):29–34. doi: 10.1016/0049-3848(83)90151-2. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Osikowicz G., Feder S., Scheinbuks J. Isolation and characterization of a urokinase-type plasminogen activator (Mr = 54,000) from cultured human endothelial cells indistinguishable from urinary urokinase. J Biol Chem. 1984 Jun 10;259(11):7198–7205. [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Claeson G., Friberger P., Knös M., Eriksson E. Methods for determination of prekallikrein in plasma, glandular kallikrein and urokinase. Haemostasis. 1978;7(2-3):76–78. doi: 10.1159/000214238. [DOI] [PubMed] [Google Scholar]
- Claeys H., Vermylen J. Physico-chemical and proenzyme properties of NH2-terminal glutamic acid and NH2-terminal lysine human plasminogen. Influence of 6-aminohexanoic acid. Biochim Biophys Acta. 1974 Apr 11;342(2):351–359. doi: 10.1016/0005-2795(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Day E. D., Ball A. P., Jeffords D., Woodard W. T., Jr, Silver D. The immunoassay of human urokinase. Thromb Diath Haemorrh. 1969 Apr 30;21(2):273–286. [PubMed] [Google Scholar]
- Garvey M. B., Black J. M. The detection of fibrinogen-fibrin degradation products by means of a new antibody-coated latex particle. J Clin Pathol. 1972 Aug;25(8):680–682. doi: 10.1136/jcp.25.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurewich V., Pannell R., Louie S., Kelley P., Suddith R. L., Greenlee R. Effective and fibrin-specific clot lysis by a zymogen precursor form of urokinase (pro-urokinase). A study in vitro and in two animal species. J Clin Invest. 1984 Jun;73(6):1731–1739. doi: 10.1172/JCI111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Minowada J., Takita H., Kover L., Markus G. Urokinase-like plasminogen activators of unusually high molecular weight secreted by a cell line derived from a human lung cancer case. J Biol Chem. 1982 May 25;257(10):5645–5651. [PubMed] [Google Scholar]
- Ishihara H., Toki N., Yamura T. Measurement of plasminogen and two antiplasmins by L-lysine sepharose affinity chromatography. Hiroshima J Med Sci. 1974 Dec;23(4):237–246. [PubMed] [Google Scholar]
- Juhan-Vague I., Moerman B., De Cock F., Aillaud M. F., Collen D. Plasma levels of a specific inhibitor of tissue-type plasminogen activator (and urokinase) in normal and pathological conditions. Thromb Res. 1984 Mar 1;33(5):523–530. doi: 10.1016/0049-3848(84)90018-5. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Tran-Thang C., Ransijn A., Bachmann F. Demonstration of a fast-acting inhibitor of plasminogen activators in human plasma. Blood. 1984 Oct;64(4):907–913. [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Porath J., Olin B. Immobilized metal ion affinity adsorption and immobilized metal ion affinity chromatography of biomaterials. Serum protein affinities for gel-immobilized iron and nickel ions. Biochemistry. 1983 Mar 29;22(7):1621–1630. doi: 10.1021/bi00276a015. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Summaria L. Plasminogen and plasmin. Methods Enzymol. 1976;45:257–273. doi: 10.1016/s0076-6879(76)45025-5. [DOI] [PubMed] [Google Scholar]
- Sakata Y., Mimuro J., Aoki N. Differential binding of plasminogen to crosslinked and noncrosslinked fibrins: its significance in hemostatic defect in factor XIII deficiency. Blood. 1984 Jun;63(6):1393–1401. [PubMed] [Google Scholar]
- Sumi H., Robbins K. C. A functionally active heavy chain derived from human high molecular weight urokinase. J Biol Chem. 1983 Jul 10;258(13):8014–8019. [PubMed] [Google Scholar]
- Sumi H., Sasaki K., Muramatu M. A simple and rapid purification method of urokinase using a [Nalpha-(epsilon-aminocaproyl)-DL-homoarginine hexylester]-sepharose column. Nihon Ketsueki Gakkai Zasshi. 1978 Aug;41(4):766–770. [PubMed] [Google Scholar]
- Sumi H., Sasaki K., Toki N., Robbins K. C. Oral administration of urokinase. Thromb Res. 1980 Dec 1;20(5-6):711–714. doi: 10.1016/0049-3848(80)90161-9. [DOI] [PubMed] [Google Scholar]
- Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega-aminocarboxylic acids. Biochim Biophys Acta. 1975 May 30;393(1):55–65. doi: 10.1016/0005-2795(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Tissot J. D., Schneider P., Hauert J., Ruegg M., Kruithof E. K., Bachmann F. Isolation from human plasma of a plasminogen activator identical to urinary high molecular weight urokinase. J Clin Invest. 1982 Dec;70(6):1320–1323. doi: 10.1172/JCI110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]
- Wohl R. C., Summaria L., Robbins K. C. Kinetics of activation of human plasminogen by different activator species at pH 7.4 and 37 degrees C. J Biol Chem. 1980 Mar 10;255(5):2005–2013. [PubMed] [Google Scholar]
- Wohl R. C., Summaria L., Robbins K. C. Physiological activation of the human fibrinolytic system. Isolation and characterization of human plasminogen variants, Chicago I and Chicago II. J Biol Chem. 1979 Sep 25;254(18):9063–9069. [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]
- Yamamoto J., Okamoto U., Morita S., Kikui K., Fujii Y. Production of the modified form of human plasminogen in the plasma activated by urokinase. Jpn J Physiol. 1983;33(3):469–484. doi: 10.2170/jjphysiol.33.469. [DOI] [PubMed] [Google Scholar]