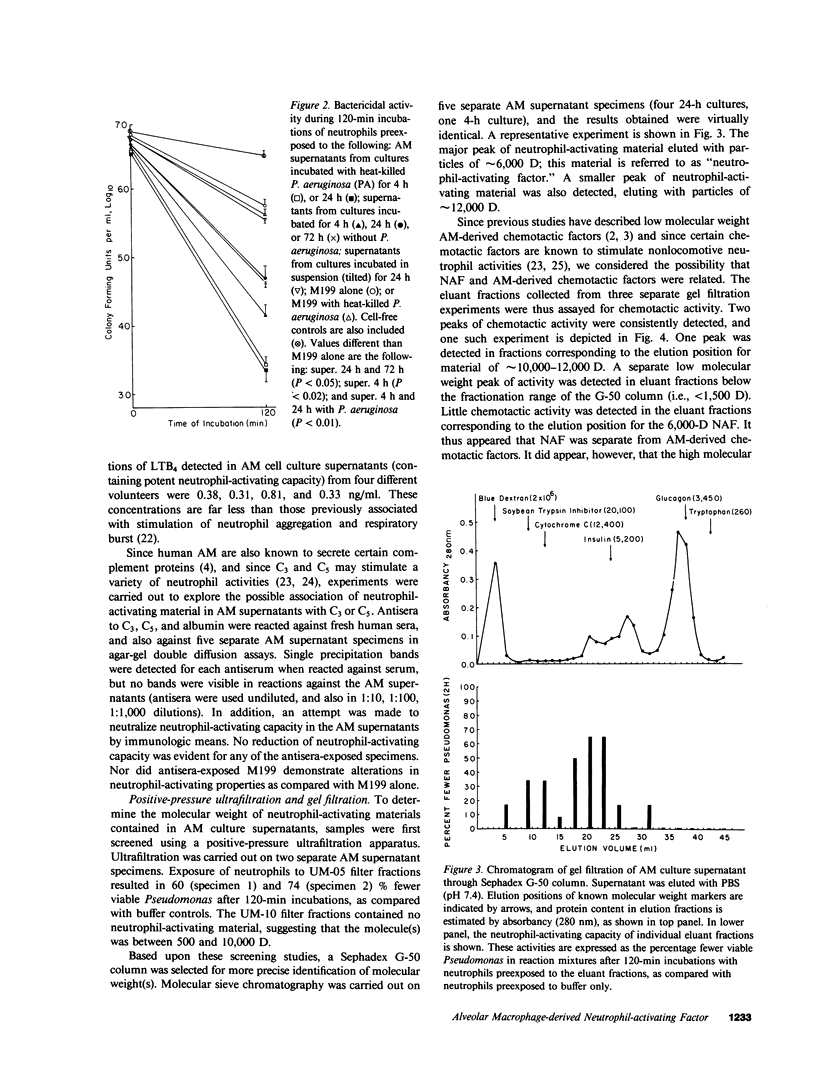
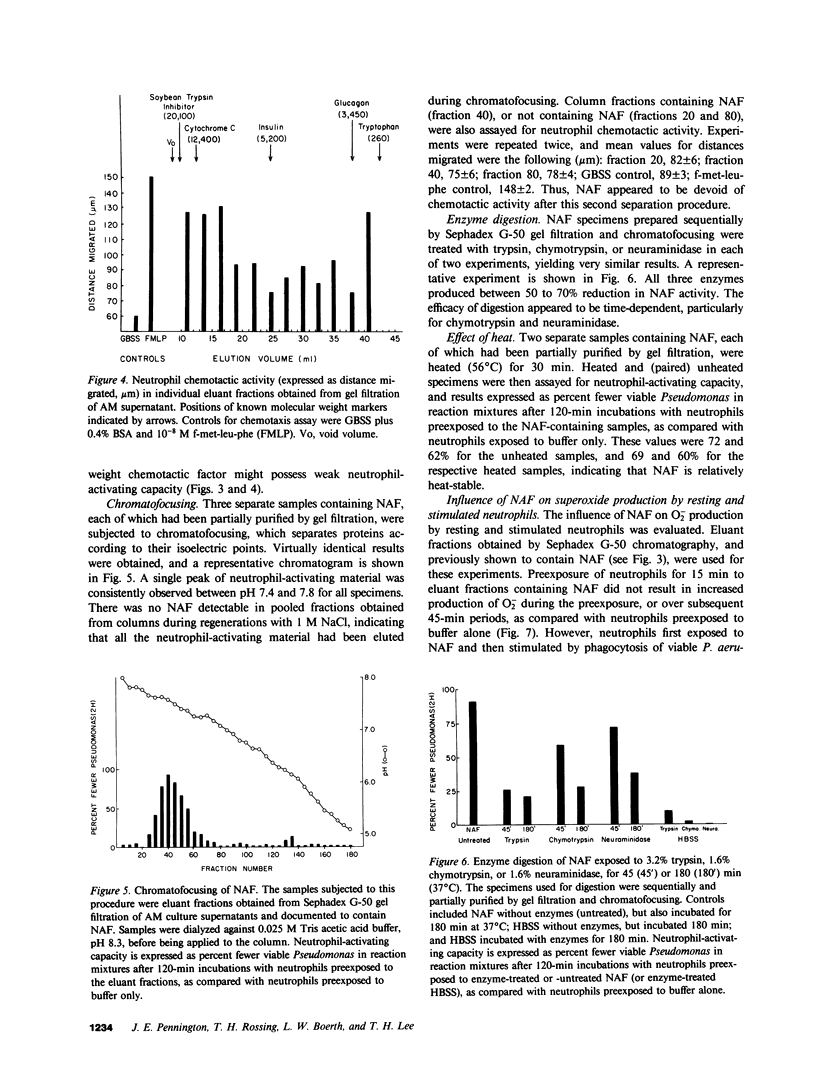
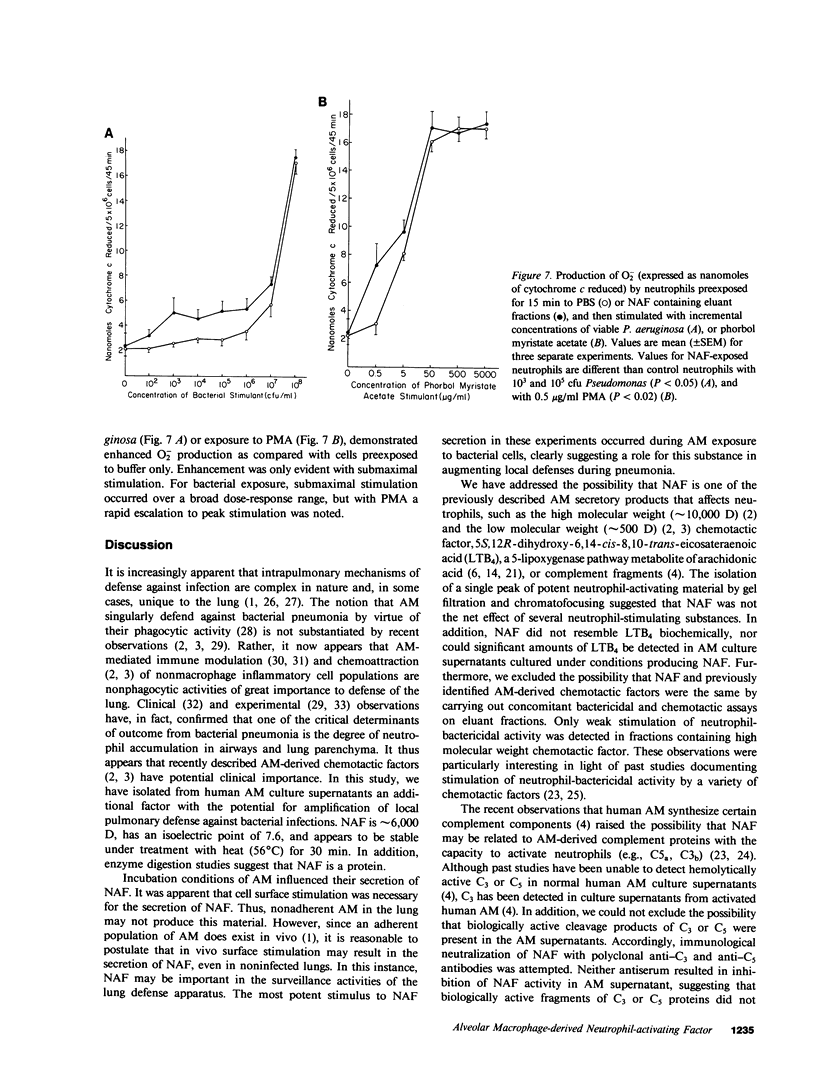
Abstract
Human alveolar macrophages (AM) were obtained from eight normal volunteers using fiberoptic bronchoscopic lavage to explore potential interrelationships among leukocytes in pulmonary defense against infection. AM placed in monolayer tissue cultures released material into culture supernatants with the capacity to enhance the bactericidal capacity of human neutrophils. Neutrophils preexposed to supernatants killed Pseudomonas aeruginosa from 70 to 90% more efficiently than control cells (P less than 0.02). AM culture supernatants contained this material by 4 h of incubation, and in vitro stimulation of AM cultures with heat-killed P. aeruginosa further increased its production. Gel filtration of AM culture supernatants with a G-50 Sephadex column allowed isolation of a 6,000-D neutrophil-activating factor (NAF) that was resistant to heat (56 degrees C, 30 min). The isoelectric point of NAF, as determined by chromatofocusing, was approximately 7.6. Enzyme digestion of NAF specimens, prepared sequentially by gel filtration and chromatofocusing, demonstrated 50-70% loss of activity after incubations with trypsin, chymotrypsin, and neuraminidase. NAF was only minimally chemotactic and eluted from Sephadex G-50 with particles of a different molecular size than those of AM-derived chemotactic factors (i.e., approximately 10,000 D and less than 500 D). Preincubation of neutrophils with NAF resulted in greater release of superoxide anion upon their subsequent stimulation by either bacterial phagocytosis or by phorbol myristate acetate, as compared with control neutrophils stimulated in a like manner. These studies indicate that human AM secrete a heat-stable, low molecular weight basic protein, with the capacity to enhance oxidative microbicidal activity of neutrophils.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F. S., Matthews W. J., Jr, Rossing T. H., Gash D. J., Lichtenberg N. A., Pennington J. E. Complement biosynthesis by human bronchoalveolar macrophages. Clin Immunol Immunopathol. 1983 May;27(2):153–159. doi: 10.1016/0090-1229(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Dale D. C., Reynolds H. Y., Pennington J. E., Elin R. J., Pitts T. W., Graw R. G., Jr Granulocyte transfusion therapy of experimental Pseudomonas pneumonia. J Clin Invest. 1974 Sep;54(3):664–671. doi: 10.1172/JCI107804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Fanta C. H., Pennington J. E. Fever and new lung infiltrates in the immunocompromised host. Clin Chest Med. 1981 Jan;2(1):19–39. [PubMed] [Google Scholar]
- Fels A. O., Pawlowski N. A., Cramer E. B., King T. K., Cohn Z. A., Scott W. A. Human alveolar macrophages produce leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7866–7870. doi: 10.1073/pnas.79.24.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M. The complement system in host defense and inflammation. Rev Infect Dis. 1979 May-Jun;1(3):483–501. doi: 10.1093/clinids/1.3.483. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Issekutz A. C., Lee K. Y., Biggar W. D. Enhancement of human neutrophil bactericidal activity by chemotactic factors. Infect Immun. 1979 May;24(2):295–301. doi: 10.1128/iai.24.2.295-301.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B. Expression of immune mechanisms in the lung. Am Rev Respir Dis. 1976 Mar;113(3):347–379. doi: 10.1164/arrd.1976.113.3.347. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Henderson W. R., Gallin J. I. Stimulation of neutrophil oxygen-dependent metabolism by human leukocytic pyrogen. J Clin Invest. 1979 Oct;64(4):996–1002. doi: 10.1172/JCI109566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Menica-Huerta J. M., Shih C., Corey E. J., Lewis R. A., Austen K. F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984 Feb 25;259(4):2383–2389. [PubMed] [Google Scholar]
- Lewis R. A., Mencia-Huerta J. M., Soberman R. J., Hoover D., Marfat A., Corey E. J., Austen K. F. Radioimmunoassay for leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7904–7908. doi: 10.1073/pnas.79.24.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Albert R. K., Henderson W. R. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am Rev Respir Dis. 1984 Jan;129(1):106–111. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- Mason R., Austyn J., Brodsky F., Gordon S. Monoclonal antimacrophage antibodies: human pulmonary macrophages express HLA-DR (Ia-like) antigens in culture. Am Rev Respir Dis. 1982 May;125(5):586–593. doi: 10.1164/arrd.1982.125.5.586. [DOI] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. M., Goldberger G., Colten H. R., Aden D. P., Knowles B. B. Biosynthesis and processing of a human precursor complement protein, pro-C3, in a hepatoma-derived cell line. Science. 1982 Jan 22;215(4531):399–400. doi: 10.1126/science.7199205. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Pennington J. E., Ehrie M. G. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978 Jun;137(6):764–774. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Harris E. A. Influence of immunosuppression on alveolar macrophage chemotactic activities in guinea pigs. Am Rev Respir Dis. 1981 Mar;123(3):299–304. doi: 10.1164/arrd.1981.123.3.299. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Miler J. J. Evaluation of a new polyvalent Pseudomonas vaccine in respiratory infections. Infect Immun. 1979 Sep;25(3):1029–1034. doi: 10.1128/iai.25.3.1029-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Rossing T. H., Boerth L. W. The effect of human alveolar macrophages on the bactericidal capacity of neutrophils. J Infect Dis. 1983 Jul;148(1):101–109. doi: 10.1093/infdis/148.1.101. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Radin A., Smolen J. E., Korchak H., Samuelsson B., Weissmann G. Leukotriene B4 is a complete secretagogue in human neutrophils: a kinetic analysis. Biochem Biophys Res Commun. 1982 Aug;107(3):1006–1012. doi: 10.1016/0006-291x(82)90622-2. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Dunn M. M., Guzzetta P., Nunez G., Stastny P., Lipscomb M. F. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol. 1984 Jan;132(1):181–186. [PubMed] [Google Scholar]
- Van Epps D. E., Garcia M. L. Enhancement of neutrophils function as a result of prior exposure to chemotactic factor. J Clin Invest. 1980 Aug;66(2):167–175. doi: 10.1172/JCI109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wewers M. D., Rennard S. I., Hance A. J., Bitterman P. B., Crystal R. G. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest. 1984 Dec;74(6):2208–2218. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


