Abstract
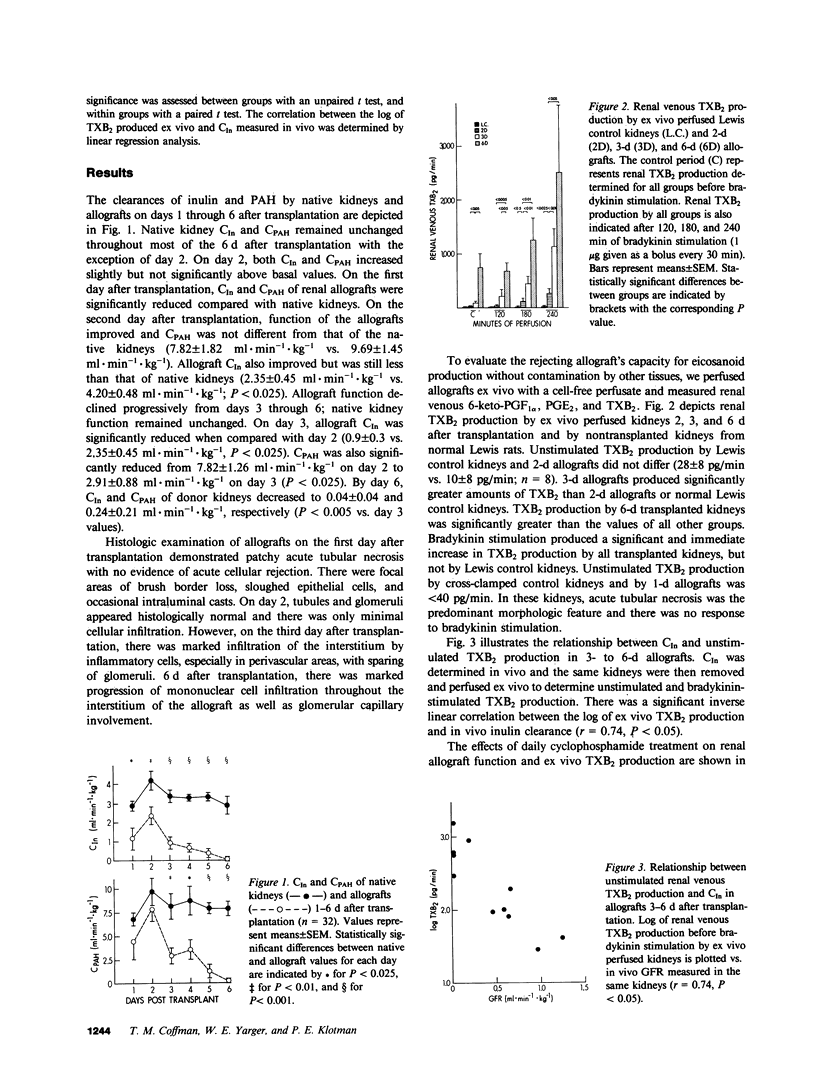
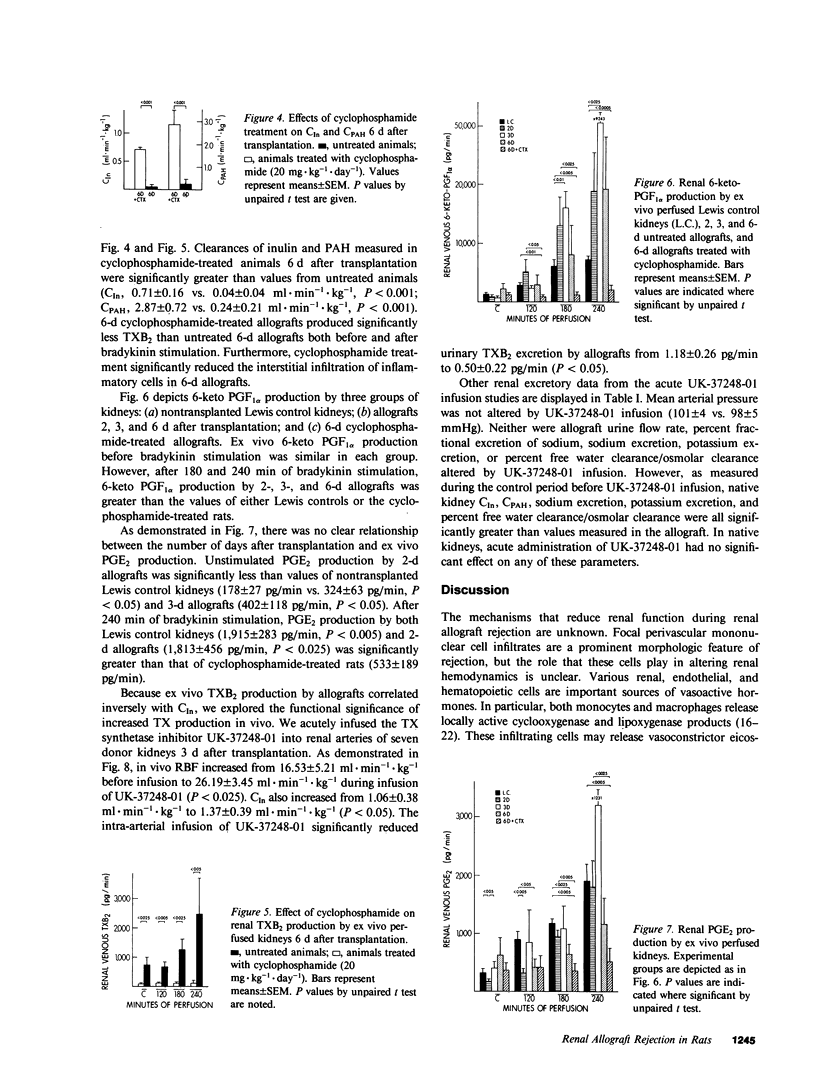
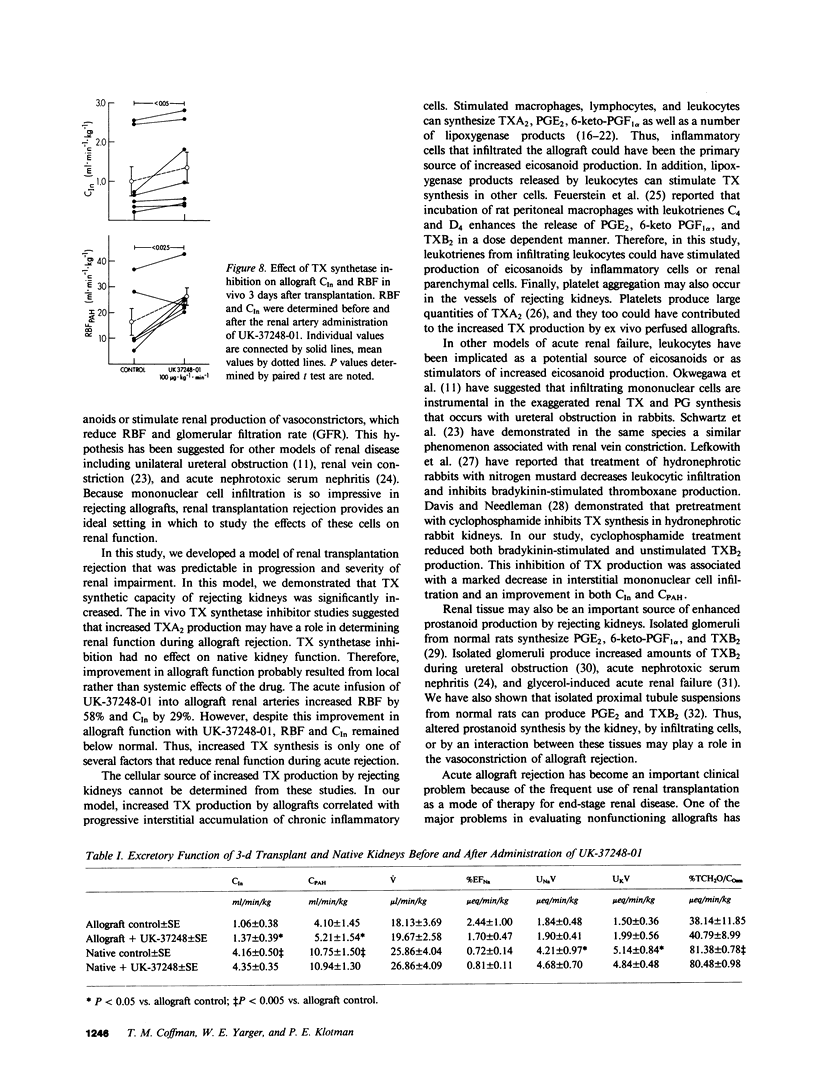
We investigated the role of thromboxane in mediating the reduction in renal function and renal blood flow characteristic of acute renal allograft rejection. We transplanted kidneys from Lewis rats to Brown-Norway recipients. By the third day after transplantation, histologic changes that were consistent with cellular rejection occurred in the kidney. These changes were associated with a moderate reduction in renal function. By day 6, histologic changes of rejection were advanced and included interstitial and perivascular infiltration by mononuclear cells. The clearances of inulin and para-aminohippuric acid were also markedly reduced. As renal function deteriorated, thromboxane B2 (TXB2) production by ex vivo perfused renal allografts increased progressively from 2 to 6 d after transplantation. However, prostaglandin (PG) E2 and 6-keto PGF1 alpha production remained essentially unchanged. There was a significant inverse correlation between the in vivo clearance of inulin and the log of ex vivo TXB2 production. Infusion of the thromboxane synthetase inhibitor UK-37248-01 into the renal artery of 3-d allografts significantly decreased urinary TXB2 excretion and significantly increased renal blood flow (RBF) and glomerular filtration rate (GFR). Although renal function improved significantly after the acute administration of UK-37248-01, GFR and RBF did not exceed 33 and 58% of native control values, respectively. In other animals, daily treatment with cyclophosphamide improved the clearances of inulin and para-aminohippuric acid and reduced thromboxane production by 6-d renal allografts. These studies demonstrate that histologic evidence of rejection is associated with increased renal thromboxane production. Inhibition of thromboxane synthetase improves renal function in 3-d allografts. Cytotoxic therapy improves renal function, reduces mononuclear cell infiltration, and decreases allograft thromboxane production. Thus, the potent vasoconstrictor thromboxane A2 may play a role in the impairment of renal function and renal blood flow during acute allograft rejection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockman R. S. Prostaglandin production by human blood monocytes and mouse peritoneal macrophages: synthesis dependent on in vitro culture conditions. Prostaglandins. 1981 Jan;21(1):9–31. doi: 10.1016/0090-6980(81)90192-1. [DOI] [PubMed] [Google Scholar]
- Carnuccio R., Di Rosa M., Flower R. J., Pinto A. The inhibition by hydrocortisone of prostaglandin biosynthesis in rat peritoneal leucocytes is correlated with intracellular macrocortin levels. Br J Pharmacol. 1981 Oct;74(2):322–324. doi: 10.1111/j.1476-5381.1981.tb09974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J. S. Improved techniques for acute and chronic cannulation of renal artery in the rat. Kidney Int. 1982 Jul;22(1):75–79. doi: 10.1038/ki.1982.135. [DOI] [PubMed] [Google Scholar]
- Doig M. V., Ford-Hutchinson A. W. The production and characterisation of products of the lipoxygenase enzyme system released by rat peritoneal macrophages. Prostaglandins. 1980 Dec;20(6):1007–1019. doi: 10.1016/0090-6980(80)90055-6. [DOI] [PubMed] [Google Scholar]
- Fabre J., Lim S. H., Morris P. J. Renal transplantation in the rat: details of a technique. Aust N Z J Surg. 1971 Aug;41(1):69–75. [PubMed] [Google Scholar]
- Feuerstein N., Foegh M., Ramwell P. W. Leukotrienes C4 and D4 induce prostaglandin and thromboxane release from rat peritoneal macrophages. Br J Pharmacol. 1981 Mar;72(3):389–391. doi: 10.1111/j.1476-5381.1981.tb10988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegh M. L., Winchester J. F., Zmudka M., Helfrich G. B., Cooley C., Ramwell P. W., Schreiner G. E. Urine i-TXB2 in renal allograft rejection. Lancet. 1981 Aug 29;2(8244):431–434. doi: 10.1016/s0140-6736(81)90772-8. [DOI] [PubMed] [Google Scholar]
- Folkert V. W., Schlondorff D. Altered prostaglandin synthesis by glomeruli from rats with unilateral ureteral ligation. Am J Physiol. 1981 Sep;241(3):F289–F299. doi: 10.1152/ajprenal.1981.241.3.F289. [DOI] [PubMed] [Google Scholar]
- Franke H., Barlow C. H., Chance B. Fluorescence of pyridine nucleotide and flavoproteins as an indicator of substrate oxidation and oxygen demand of the isolated perfused rat kidney. Int J Biochem. 1980;12(1-2):269–275. doi: 10.1016/0020-711x(80)90083-x. [DOI] [PubMed] [Google Scholar]
- Gardner L. B., Guttmann R. D., Merrill J. P. Renal transplantation in the inbred rat. IV. Alterations in the microvasculature in acute unmodified rejection. Transplantation. 1968 May;6(3):411–418. doi: 10.1097/00007890-196805000-00012. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg N. K., Retik A. B., Rosen S. M., Murray J. E., Merrill J. P. The role of vasoconstriction in the ischemia of renal allograft rejection. Transplantation. 1968 Jan;6(1):59–69. doi: 10.1097/00007890-196801000-00006. [DOI] [PubMed] [Google Scholar]
- KOUNTZ S. L., WILLIAMS M. A., WILLIAMS P. L., KAPROS C., DEMPSTER W. J. MECHANISM OF REJECTION OF HOMOTRANSPLANTED KIDNEYS. Nature. 1963 Jul 20;199:257–260. doi: 10.1038/199257a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M. S., Stobo J. D., Goldyne M. E. In vitro synthesis of prostaglandins and related lipids by populations of human peripheral blood mononuclear cells. Prostaglandins. 1980 Jul;20(1):135–145. doi: 10.1016/0090-6980(80)90013-1. [DOI] [PubMed] [Google Scholar]
- Lefkowith J. B., Okegawa T., DeSchryver-Kecskemeti K., Needleman P. Macrophage-dependent arachidonate metabolism in hydronephrosis. Kidney Int. 1984 Jul;26(1):10–17. doi: 10.1038/ki.1984.127. [DOI] [PubMed] [Google Scholar]
- Lianos E. A., Andres G. A., Dunn M. J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. Effects on renal hemodynamics. J Clin Invest. 1983 Oct;72(4):1439–1448. doi: 10.1172/JCI111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Wyche A., Bronson S. D., Holmberg S., Morrison A. R. Specific regulation of peptide-induced renal prostaglandin synthesis. J Biol Chem. 1979 Oct 10;254(19):9772–9779. [PubMed] [Google Scholar]
- Okegawa T., Jonas P. E., DeSchryver K., Kawasaki A., Needleman P. Metabolic and cellular alterations underlying the exaggerated renal prostaglandin and thromboxane synthesis in ureter obstruction in rabbits. Inflammatory response involving fibroblasts and mononuclear cells. J Clin Invest. 1983 Jan;71(1):81–90. doi: 10.1172/JCI110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. M., Truniger B. P., Kriek H. R., Murray J. E., Merrill J. P. Intrarenal distribution of blood flow in the transplanted dog kidney: effect of denervation and rejection. J Clin Invest. 1967 Jul;46(7):1239–1253. doi: 10.1172/JCI105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D., DeSchryver-Kecskemeti K., Needleman P. Renal arachidonic acid metabolism and cellular changes in the rabbit renal vein constricted kidney: inflammation as a common process in renal injury models. Prostaglandins. 1984 Apr;27(4):605–613. doi: 10.1016/0090-6980(84)90096-0. [DOI] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Bonser R. W., Cuatrecasas P. The production of 5-HETE and leukotriene B in rat neutrophils from carrageenan pleural exudates. Prostaglandins. 1981 Jan;21(1):123–132. doi: 10.1016/0090-6980(81)90202-1. [DOI] [PubMed] [Google Scholar]
- Sraer J. D., Moulonguet-Doleris L., Delarue F., Sraer J., Ardaillou R. Prostaglandin synthesis by glomeruli isolated from rats with glycerol-induced acute renal failure. Circ Res. 1981 Sep;49(3):775–783. doi: 10.1161/01.res.49.3.775. [DOI] [PubMed] [Google Scholar]
- Svensson J., Hamberg M., Samuelsson B. On the formation and effects of thromboxane A2 in human platelets. Acta Physiol Scand. 1976 Nov;98(3):285–294. doi: 10.1111/j.1748-1716.1976.tb10313.x. [DOI] [PubMed] [Google Scholar]
- Tyler H. M., Saxton C. A., Parry M. J. Administration to man of UK-37,248-01, a selective inhibitor of thromboxane synthetase. Lancet. 1981 Mar 21;1(8221):629–632. doi: 10.1016/s0140-6736(81)91551-8. [DOI] [PubMed] [Google Scholar]
- Vesterqvist O., Gréen K. Urinary excretion of 2,3-dinor-thromboxane B2 in man under normal conditions, following drugs and during some pathological conditions. Prostaglandins. 1984 Apr;27(4):627–644. doi: 10.1016/0090-6980(84)90098-4. [DOI] [PubMed] [Google Scholar]
- Zipser R. D., Smorlesi C. Regulation of urinary thromboxane B2 in man: influence of urinary flow rate and tubular transport. Prostaglandins. 1984 Feb;27(2):257–271. doi: 10.1016/0090-6980(84)90078-9. [DOI] [PubMed] [Google Scholar]


