Abstract
Objective
Child maltreatment is associated with dysregulation of stress-mediating systems and an increased risk of mental and physical health problems. Specifically, disruptions in hypothalamic-pituitary-adrenal (HPA) axis regulation have been reported in maltreated children. The current study investigates whether increased cortisol variability is responsible for inconsistent patterns in the literature.
Method
This study modeled cortisol activity over 20 weeks in 187 maltreated and 154 nonmaltreated children (M = 8.4 years, SD = 1.8 years) in order to capture week-to-week cortisol patterns. Maltreatment was assessed through coding of Department of Human Services records. Children attended an after school program one day per week for 20 weeks where saliva was collected at the same time each day and subsequently assayed for cortisol.
Results
Multiple-group growth curves indicated that maltreated and nonmaltreated children differ in longitudinal cortisol patterns. Maltreated children showed higher variance in the initial cortisol levels and slope over time compared to nonmaltreated children, indicating greater between-person variability in the maltreated group. Maltreated children with higher cortisol at the first assessment showed cortisol suppression over time, indicating potential HPA blunting after chronic high cortisol levels. The severity, timing, and number of subtypes of maltreatment predicted individuals’ cortisol variability, and both maltreatment status and greater cortisol variability predicted more behavior problems.
Conclusion
Interventions for maltreated children may benefit from pre- and post-intervention HPA assessments to determine a component of treatment efficacy. As maltreatment dimensions predicted differential cortisol regulation, assessment of maltreatment experiences is necessary to understand alterations in behavior and HPA regulation post-intervention.
Keywords: maltreatment, middle childhood, cortisol, behavior problems, structural equation modeling
Introduction
Maltreatment during childhood is associated with disruptions in social, emotional, and cognitive development, and an increased risk for psychopathology and physical health problems throughout the lifespan.1–2 Pathways to increased mental and physical health problems are thought to operate through cumulative deterioration of bodily systems caused by chronic stressors, such as the stress of abuse or neglect in childhood. The allostatic load model (ALM) postulates that the body is able to up- and down-regulate vital functions in response to acute stressors (e.g., mobilization of glucose to muscles, suppression of non-essential processes), but that these adaptations that promote survival in the short term are actually harmful if activated chronically.3
The hypothalamic-pituitary-adrenal (HPA) axis is of particular interest in the ALM, as it is a physiological system that mediates the impact of systemic and psychosocial stressors on biological and behavioral responses. The HPA axis has been targeted because of its importance in regulating the body’s response to and recovery from stress, and there is growing evidence that this system may be dysregulated following chronic adversity.4 Specifically, the stress of maltreatment in childhood has been linked to HPA dysregulation around the period of maltreatment as well as in adulthood.5 In psychiatrically healthy adults, those who were maltreated as children tend to exhibit lower levels of cortisol in the evening,6 blunted responsiveness to psychosocial challenge,7 and stronger suppression to dexamethasone challenge.6,8 However, some studies report that adults maltreated as children have increased cortisol and adrenocorticotropic hormone (ACTH) responsiveness to challenge.9 Further, adult psychopathology is an important moderator of cortisol regulation that further complicates interpretation.10–11
The picture is less clear in maltreated children, with children showing elevated, lowered, and similar patterns of cortisol activity compared to nonmaltreated children.5,12 Compared to nonmaltreated children, some physically abused children show low cortisol and a flattened diurnal slope.13 Sexually abused girls have demonstrated a blunted ACTH response following a corticotropin-releasing factor (CRF) stimulation test compared to comparisons,14 and both boys and girls who were maltreated have shown attenuated cortisol responses to stressors.15–16 Researchers have found elevated cortisol levels in maltreated children with internalizing problems even though adults who were maltreated as children often show hyposecretion of cortisol and increased ACTH responsiveness.5 Few longitudinal studies of HPA regulation in maltreated individuals exist. However, one study reported that sexually abused girls with high cortisol levels in childhood showed attenuated cortisol in adolescence and exhibited low cortisol levels in adulthood.17 This finding suggests a switch to hypocortisolism across time that could be the result of receptor down-regulation following a sustained ACTH drive in response to chronic stress.18 One hypothesis is that dysregulation of the HPA axis occurs after repeated exposure to psychosocial stressors. Maltreated children may demonstrate a difficulty in the following: responding appropriately to stressors, returning to baseline post-stressor, and maintaining a regular diurnal rhythm. Evidence supporting this hypothesis exists in the literature on foster children who also experience early adversity. Children in foster care have demonstrated an increasingly blunted diurnal cortisol slope compared to children in the community, suggesting an inability to regulate the HPA axis in response to accumulating stress.19 In addition, children in foster care show higher variability in morning cortisol levels than children not living in foster care, which appears to be related to the type of maltreatment experienced, providing further evidence of cortisol dysregulation for children experiencing chronic stress.20 The current study will examine whether similar cortisol variability occurs in maltreated children not living in foster care.
One challenge associated with investigating maltreatment is that children often experience multiple forms of abuse and neglect, including co-occurring stressors. Despite increased rates of a number of pathological conditions, some maltreated children are remarkably resilient, which may contribute to inconsistencies in the literature on the neurobiological correlates of maltreatment.21–22 Factors such as the type and severity of maltreatment, developmental stages of occurrence, and recency of maltreatment are likely important determinants of HPA activity and may be responsible for inconsistencies. For example, children who have experienced both physical and sexual abuse have demonstrated high morning cortisol levels, and children who experienced multiple types of abuse are more likely to show elevated morning and afternoon cortisol.13 Concurrent psychopathology and behavior problems may also be related to HPA regulation. School-age children who experienced physical and/or sexual abuse before age 5 showed attenuated diurnal cortisol slope but only with concurrent internalizing symptoms.23 Specifically, severity of depressive symptoms may moderate the impact of maltreatment on cortisol levels, with severe depressive symptoms related to blunted cortisol reactivity and mild symptoms related to heighted cortisol reactivity.24
Although effects of child maltreatment on the diurnal rhythm and reactivity of the HPA axis have been reported, there are inconsistencies in the literature that may be due to increased within- and between-person variability in the maltreated group. To our knowledge, there are no studies that examine variability in cortisol levels in maltreated and nonmaltreated individuals measured consistently over time. To fill this gap, the current study models patterns of cortisol over 20 weekly assessments occurring in middle childhood. It was hypothesized that maltreated children would show greater variability in the initial cortisol level and the slope of cortisol levels over time than nonmaltreated children, indicating greater difficulty in regulating cortisol levels. The variance in cortisol levels was expected to be higher in the maltreated group, which would suggest greater variability between individuals in the maltreated group and may explain why consistent cortisol patterns are elusive. In response to research indicating down-regulation of cortisol levels with chronic stress, it was predicted that maltreated children would demonstrate a smaller slope for cortisol levels, especially for children with higher initial cortisol levels. As variability within the maltreated group may be due to differences in maltreatment experiences, differences in the standard error of the cortisol slope over time were examined. It was hypothesized that children with greater duration, earlier onset, more severe, and more subtypes of maltreatment would show greater variability in the cortisol slope over time than children with shorter and less severe maltreatment experiences. Greater variability in the cortisol slope was hypothesized to predict greater behavior problems, especially for maltreated children.
Method
Participants included 341 children (169 females, 172 males) between the ages of 5 and 13 (M = 8.4 years, SD = 1.8 years) living in their biological families. The sample was diverse, including 209 (61.3%) black, 47 (13.8%) Latino, 46 (13.5%) white, and 39 (11.5%) children of multiracial backgrounds. The maltreated group included 187 children, and the nonmaltreated group included 154 children (see Table 1 for demographic information by group). In the maltreated group, 147 children experienced individual maltreatment documented by the Department of Human Services (DHS), and 40 children came from families where DHS documented maltreatment in their families; however, DHS records did not supply details necessary to provide individual maltreatment experience determinations. These children were used in the multiple-group growth models but not in the analyses using specific maltreatment parameters. Over 92% of families received public assistance in both the maltreated and nonmaltreated groups. Of those with documented subtypes of maltreatment, 29.9% experienced physical abuse, 6.4% sexual abuse, 66.3% neglect, and 49.7% emotional maltreatment. Furthermore, 51 experienced 1 subtype of maltreatment (neglect, emotional abuse, physical abuse, sexual abuse), 59 experienced 2 subtypes, 32 with 3 subtypes, and 5 experienced all 4 subtypes.
Table 1.
Family Demographic Characteristics and Maltreatment Parameters
| Maltreated (n = 187)
|
Nonmaltreated (n = 154)
|
|||||
|---|---|---|---|---|---|---|
| M | SD | % | M | SD | % | |
| Marital Status | ||||||
| Never married | 35.2 | 38.9 | ||||
| Married | 31.5 | 26.4 | ||||
| Living with partner | 9.9 | 16.7 | ||||
| No longer married | 23.5 | 18.1 | ||||
| Maternal education | ||||||
| Did not graduate high school | 49.4 | 30.6 | ||||
| Total family income per person | ||||||
| $1000s including public assistance | 5.24 | 3.30 | 5.60 | 3.51 | ||
| Race | ||||||
| Black | 62.6 | 59.7 | ||||
| White | 15.5 | 11.1 | ||||
| Latino | 12.3 | 15.6 | ||||
| Multiracial or Other | 9.6 | 13.6 | ||||
| Maltreatment Subtype | ||||||
| Physical Abuse | 29.9 | |||||
| Sexual Abuse | 6.4 | |||||
| Neglect | 66.3 | |||||
| Emotional Abuse | 49.7 | |||||
| Number of Maltreatment Subtypes | ||||||
| 1 Subtype | 34.7 | |||||
| 2+ Subtypes | 65.3 | |||||
| Maltreatment Severity | ||||||
| Less Severe | 56.5 | |||||
| More Severe | 43.5 | |||||
| Onset/Recency of Maltreatment | ||||||
| Early Onset/Not Recent | 40.8 | |||||
| Early Onset/Recent | 25.9 | |||||
| Late Onset/Recent | 33.3 | |||||
Note: Families with reported maltreatment were less likely to have mothers who graduated high school χ2(1, N = 341) = 8.2, p < .01. No other group contrasts were significant: p > .05. Maltreatment parameters were calculated based on the 147 children with individual maltreatment experiences.
Data were collected in four different cohorts in four separate years of an after-school peer enrichment program that included a research component. Children attended the program once a week for 20 weeks from November through April, with a 5-week winter break that began after the 5th week of the program for the first cohort and after the 6th week for the other 3 cohorts. All children in the program took part in the research component with assent yet were free to decline any research activity.
Child Protective Services (CPS) reports were examined by a DHS liaison in order to identify and recruit children who had experienced maltreatment. The DHS liaison randomly selected families with documented CPS histories with a child in the targeted age range, contacted these eligible families, explained the study, and if parents were interested, obtained signed permission to share their contact information with research staff. Similarly, to recruit demographically comparable nonmaltreated children and hold socioeconomic status (SES) constant across groups, families receiving Temporary Assistance to Needy Families but with no CPS history were randomly selected and contacted to determine their interest in having their child participate. Staff members then obtained informed consent from parents of all children for their child’s participation in the program and for permission to examine any DHS records of the family. The Institutional Review Board of the University of Rochester approved these methods and procedures. After recruitment, trained research staff reviewed all DHS records for each family and coded maltreatment information using the Maltreatment Classification System (MCS).25 Staff members were instructed to code using all information without relying on DHS determinations, and subsequent classifications described below were created using MCS standards. Staff also searched for DHS records of children in the nonmaltreated group to confirm that there was no history of maltreatment. Mothers of nonmaltreated children were interviewed with the Maternal Child Maltreatment Interview to ensure that children did not experience maltreatment.
The number of maltreatment subtypes each child experienced was summed with a possible range from 0–4. Children were then divided into 3 groups: nonmaltreated = 0, 1 subtype = 1, 2+ total subtypes = 2. A 5-point scale from the MCS is used to rate the severity of each of the 4 possible subtypes of maltreatment experienced. The severity ratings for each subtype were added, yielding a severity score ranging from 0–20. Children were divided into 3 groups: nonmaltreated = 0, less severe maltreatment (sum of severity score between 1–6) = 1, and more severe maltreatment (severity sum of 7–20) = 2. The developmental periods during which maltreatment occurred were recorded: infancy (0–18 months), toddlerhood (19–35 months), preschool age (36–59 months), early school age (5–7 years), and late school age (8–12 years). The total number of developmental periods in which maltreatment occurred was summed for each child and ranged from 0–5. Children were divided into 3 groups by duration of maltreatment: nonmaltreated = 0, 1 developmental period of maltreatment = 1, or 2+ periods of maltreatment = 2. Children whose maltreatment onset was in infancy, toddlerhood, or preschool were defined as early onset, and those whose onset was early or late school age were defined as late onset. Children whose most recent experience of maltreatment was in infancy, toddlerhood, or preschool were defined as not recent, and those whose latest experience was early or late school age were defined as recent. Maltreated children were divided into three groups based on timing of maltreatment: early onset/not recent, early onset/recent, and late onset/recent. These 4 maltreatment parameters were all significantly related to one another (r = 0.17–0.64, p < .05).
Cortisol Assessment
Children provided saliva samples shortly after they arrived each day by bus at 4:00 pm. Children did not have food or drink for a minimum of 30 minutes before the sample. Saliva was collected through a straw and directly deposited in a vial that was then stored at −80°C until it was shipped to Pennsylvania State University for cortisol analysis using a high-sensitivity enzyme immunoassay (Salimetrics, PA). Intra- and inter-assay variability was less than 10%, and the minimum detection threshold was 0.007 μg/dL. Due to kurtosis and skew in cortisol values, the assay results were log-transformed, and extreme outliers (> +3 SD) were removed.
Teacher Report Form (TRF)
After school group counselors who worked with the children throughout the program assessed behavioral symptomatology by completing the Teacher Report Form (TRF).26 The TRF is a 118-item assessment that evaluates frequency of behavioral problems. Items load onto eight symptom scales: withdrawn, somatic complaints, anxiety/depression, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior. Items also load onto three summary scales: internalizing behavior, externalizing behavior, and total behavior problems. Counselors evaluated each cohort of children at the end of the program. The TRF was used because counselors could observe classroom behaviors in the after school program context. Counselors were unaware of maltreatment status and study hypotheses.
Data Analytic Plan
Multiple-group growth curve modeling was used to model within-person change and differences in change between persons.27–28 Change in cortisol levels over time was not expected to be linear, so growth modeling provided the best tool for examining non-linear change across the 20 weekly cortisol assessments. As growth models usually assume that all participants come from the same group and share a single set of parameters (e.g., means, variances), multiple-group growth modeling can be used to model patterns of change in observed groups. In this analysis, growth modeling was used to model differences in longitudinal cortisol patterns in maltreated versus nonmaltreated children. A latent basis model was chosen as the baseline model as it is ideally suited for non-linear patterns of change.29 Missing data was handled using maximum likelihood estimation. First, a model was fit with the assumption that there were no differences between groups. Then subsequent models were formulated to test whether groups differed in their pattern of change in cortisol over time in order to determine the model that best accounted for between-person differences in within-person change.28–29 In each of the 5 models, separate growth curves were fitted for the maltreated and nonmaltreated groups to examine differences in parameters by group. After fitting Model 1, which assumes no differences between groups, subsequent models were tested to determine whether maltreated and nonmaltreated children differed over time in progressive models. These models freed additional parameters within each model to determine which model, if any, fit better than Model 1. The parameters freed within each subsequent model include: 2) mean cortisol levels; 3) mean levels, variance, and covariance; 4) mean, variance, covariance, and the pattern or shape of change; 5) mean, variance, covariance, the pattern or shape of change, and the residuals. Chi-square tests were conducted to evaluate which model(s) fit better than Model 1, which assumes both groups are equal.
Analyses were then conducted using maltreatment parameters (duration, timing, severity, number of subtypes) to predict the standard error of the cortisol slope over time for each individual controlling for age and total behavior problems using an analysis of variance (ANOVA) with Bonferroni corrections for each parameter. Gender was not related to the standard error in any analysis and was excluded from final models. For these analyses, each individual’s slope was calculated by running a regression line through all 20 data points in order to produce a standard error of the slope for all participants. T-tests were conducted to test for differences in internalizing problems, externalizing problems, and total behavior problems between maltreated and nonmaltreated children. Finally, a regression was conducted to examine whether maltreatment status, the standard error of the cortisol slope, and their interaction predict total behavior problems. Analyses indicated that there was no difference in cortisol slope or variability before and after the break for either group, so all time points were examined together.
Results
All models were formulated in Mplus Version 7.1.30 As there was a 5-week holiday break during the after school program duration, the timing of the cortisol samples were fixed to ensure accurate spacing of assessments. The first cortisol sample was fixed at 0, and the final sample was fixed at 1. Each sample was fixed at an appropriate numerical value (at intervals of 0.04 between 0 and 1) that corresponded with its timing relative to the first and last assessments. One of the 4 cohorts had their break after the fifth assessment while the other cohorts had a break after the sixth assessment. To account for this difference, the sixth assessment for the first cohort was fixed at 0.2, which corresponds to its pre-break timing, and the assessment for the other cohorts was fixed post-break at 0.44. Thus, the model was able to account for accurate timing of cortisol sampling across the 20 weeks of the after school program and 5 weeks of break by fixing samples at intervals of 0.04 between 0 and 1.
Growth Models by Maltreatment Status
Five growth models were fit, with each progressively freeing more parameters (mean, variance, covariance, pattern of change, residuals). Four chi-square tests were conducted, each comparing whether Models 2–5 fit significantly better than Model 1. Model 5, which allowed for differences between the nonmaltreated and maltreated groups in the mean, variance, covariance, the pattern of change, and residuals of cortisol levels over time, was the only model that fit significantly better than Model 1, χ2(25, N = 341) = 37.4, p = .05. All other chi-square tests were nonsignificant (ps > .09). As a result, it was determined that the best fitting model was Model 5 (see Table 2 for fit statistics). Thus, a model accounting for differences in the means, variances, covariances, and patterns of cortisol regulation between groups, and differences in the residuals between nonmaltreated and maltreated children provides the best fit to the data.
Table 2.
Growth Curve Model Fit Statistics.
|
Model 1: No Difference |
Model 2: Means |
Model 3: Var/Covar |
Model 4: Patterns |
Model 5: Residuals |
|
|---|---|---|---|---|---|
| Free parameters | 25 | 27 | 30 | 49 | 50 |
| AIC | 7,638.5 | 7,642.1 | 7,645.2 | 7,652.6 | 7,651.0 |
| BIC | 7,734.3 | 7,745.6 | 7,760.2 | 7,840.3 | 7,842.6 |
| RMSEA | 0.181 | 0.181 | 0.182 | 0.185 | 0.185 |
| Log-likelihood H0 | −3,794.2 | −3,794.1 | −3,792.6 | −3,777.3 | −3,775.5 |
| χ2 test value | 3,131.8 | 3,131.4 | 3,128.5 | 3,097.9 | 3,094.4 |
Note: Model statistics for each model tested. χ2 analyses indicated that Model 5 was the only model that fit better than Model 1 (p = .05). AIC = Akaike Information Criteria; BIC = Bayesian Information Criteria; Covar = covariance; RMSEA: Root Mean Square Error of Approximation; var = variance.
Mean cortisol levels across the 20 weeks did not differ between maltreated and nonmaltreated children (t = −0.52, p = 0.60). The mean slope over time appeared higher for the nonmaltreated group compared to the maltreated group (see Table 3). The mean slope in the maltreated group did not differ from zero (p = 0.37), and the slope for the nonmaltreated group was marginally higher than zero (p = .07). However, a t-test analyzing differences in individual’s slopes between groups found that this difference was not statistically significant (t = 0.09, ns). A t-test between the maltreated and nonmaltreated groups showed higher cortisol variability for the maltreated group than the nonmaltreated group (t = −2.24, p < .05), indicating that maltreated children have greater within-person variation in cortisol regulation than children in the nonmaltreated group. The absence of significant differences in individuals’ cortisol slopes between groups may be due to the higher cortisol variability in the maltreated compared to the nonmaltreated group. The maltreated group showed higher variance in the intercept and slope of cortisol levels over time as calculated by Mplus, indicating greater between-person variability in cortisol levels in the maltreated group (see Table 3). The covariance of −0.13 between the slope and intercept in the maltreated group indicates that maltreated children with higher levels of cortisol at the onset of the sampling period were significantly more likely to show decreases in cortisol levels over time (p < .05). The same direction of effects was marginally significant for the nonmaltreated group but at a lower value (−0.07, p = 0.07).
Table 3.
Mean, Variance, and Covariance of Cortisol Levels by Maltreatment Group in Mplus
| Parameter | Maltreated | Nonmaltreated |
|---|---|---|
| Mean Intercept | −2.40 | −2.44 |
| Variance of Intercept | 0.20 | 0.16 |
| Mean Slope | 0.04 | 0.10 |
| Variance of Slope | 0.23 | 0.20 |
| Covariance of Slope and Intercept | −0.13 | −0.07 |
Note: The values are in log-transformed units.
Maltreatment Parameters
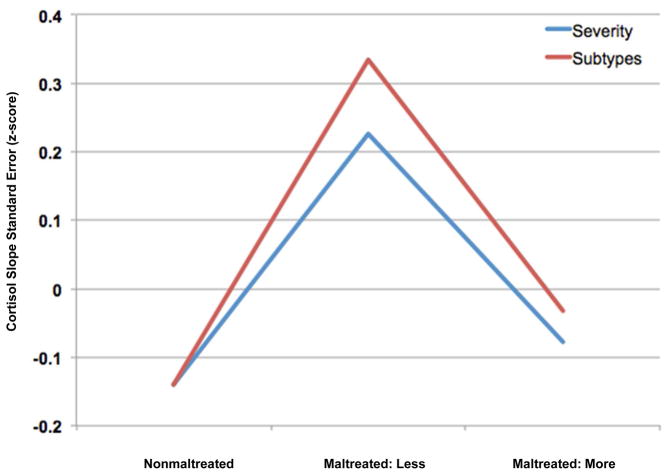
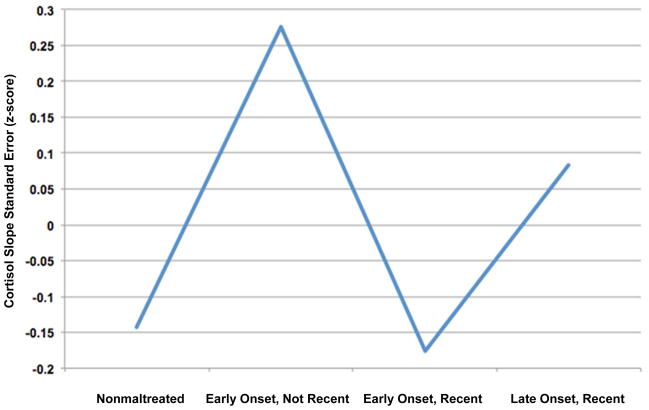
In order to understand which aspects of maltreatment have the greatest effect on within-person HPA variability, 4 ANOVA analyses were conducted examining how the duration, severity, number of subtypes, and timing of maltreatment affect the variation of each individual’s cortisol slope over time, controlling for age and total behavior problems. Bonferroni corrections were used within each ANOVA to correct for multiple pairwise comparisons. The standard errors of the cortisol slopes (cortisol variability) were presented as z-scores for clearer interpretation. An ANOVA with the timing of maltreatment (nonmaltreated, early onset/recent, late onset/recent, early onset/not recent) as the independent variable and the cortisol variability as the dependent variable was significant (F[3, 297] = 2.85, p < .05, partial η2 = 0.03). Children with early-onset maltreatment that was not recent (M = 0.28) had marginally higher cortisol variability than nonmaltreated children (M = −0.14, p = .054), but not children with early-onset, recent maltreatment (M = −0.18, p = .20), or late-onset, recent maltreatment (M = 0.08, p = 1.0). The number of subtypes of maltreatment that children experienced predicted cortisol variability (F[2, 297] = 4.28, p < .05, partial η2 = 0.03). The group that experienced 1 subtype of maltreatment (M = 0.34) had higher cortisol variability than the nonmaltreated group (M = −0.14, p < .05), and neither group differed from those who experienced 2 or more subtypes of maltreatment (M = −0.03, ps > .10). The severity of maltreatment also predicted cortisol variability (F[2, 297] = 3.68, p < .05, partial η2 = 0.03). Specifically, the group with less severe maltreatment (M = 0.23) showed greater cortisol variability than nonmaltreated children (M = −0.14), but neither group differed from the children who experienced more severe maltreatment (M = −0.08, ps > .19). An ANOVA conducted with the duration of maltreatment (nonmaltreated, 1 developmental period, 2 or more developmental periods) did not predict cortisol variability (F[2, 297] = 2.45, p = .14, partial η2 = 0.01). Overall, these findings may indicate greater difficulty with HPA regulation in the groups that experienced 1 subtype, less severe, or early-onset but not recent maltreatment. In order to determine which maltreatment parameter had the greatest effect on cortisol variability, an ANOVA was conducted with the 3 significant maltreatment parameters (subtypes, severity, and onset/recency) as independent variables. The analysis revealed that onset/recency significantly predicted cortisol variability (F[3, 291] = 3.50, p < 0.05), while number of subtypes and severity did not (ps > .38; see Figures 1 and 2).
Figure 1.
Mean standard error of the cortisol slope (z-score) calculated from log-transformed cortisol values. Note: nonmaltreated children, children with fewer subtypes or less severe maltreatment, and children with more subtypes and more severe maltreatment are reported. Analyses indicated that children in the 1 subtype of maltreatment and the less severe maltreatment groups had the highest cortisol variability.
Figure 2.
Mean standard error of the cortisol slope (z-score) calculated for nonmaltreated and maltreated children. Note: maltreated children are reported based on onset and recency of maltreatment. Analyses indicated that children with early-onset but not recent maltreatment had the highest cortisol variability.
Internalizing/Externalizing Problems
Maltreated children (M = 52.3, SE = 0.55) had greater internalizing problems than nonmaltreated children (M = 49.9, SE = 0.66, t[335] = −2.87, p < .01). Maltreated children (M = 56.4, SE = 0.67) exhibited more externalizing problems than nonmaltreated children (M = 53.0, SE = 0.70, t[335] = −3.48, p = .001). Finally, maltreated children (M = 54.4, SE = 0.54) displayed more total behavior problems than nonmaltreated children (M = 50.4, SE = 0.64, t[335] = −4.84, p < .001). Further analyses indicated that maltreated children with different maltreatment experiences did not differ from each other but that both groups had more problems than nonmaltreated children. A regression with maltreatment status, SE of the cortisol slope (cortisol variability), and their interaction term predicting total behavior problems indicated significant effects of maltreatment status (β = 0.24, t(334) = 4.54, p < .001), and cortisol variability (β = 0.11, t[344] = 2.06, p < .05), but not their interaction (t[333] = 0.80, p = .43). As a result, both a history of maltreatment and higher cortisol slope variability predict more behavior problems.
Discussion
The current study used SEM techniques to model multiple-group growth curves describing week-to-week cortisol levels in maltreated and nonmaltreated children, finding that a model that allowed the mean, variance/covariance, shape of the growth curves, and residuals to vary provided the best fit to the data. Maltreated children demonstrated higher variance in both the initial cortisol levels and slope from the first to last sessions compared to nonmaltreated children, indicating greater between-person variation in cortisol levels. In both groups, children with higher cortisol at the first assessment showed increasingly blunted levels of cortisol over time; however, the suppression effect was only significant for maltreated children and marginally significant for nonmaltreated children. Clinically, higher variability in cortisol levels over time could indicate difficulties in regulation at the physiological level that may result in more behavior and emotional problems for maltreated children. Higher variability may also suggest higher levels of stress or unpredictability for maltreated children, which is reflected in difficulty maintaining stable cortisol levels over time.
The high between- and within-person variability in the maltreated group may explain why stable patterns have not been found in studies of maltreated children. Limited within-person stability may be due to trouble with regulation of the HPA axis both diurnally and in response to challenge. This difficulty with maintaining stable patterns of cortisol activity may be physiological (e.g., an adaptation to chronic stressors), psychological (e.g., emotion regulation difficulties or utilization of social support), or environmental (e.g., more stressful events overall). The inability to appropriately respond to and recover from stressors is related to internalizing disorders and symptomology in children,31 so higher HPA variability may signal current psychopathology or be a risk factor for future disorders. Indeed, the finding that higher cortisol variability over time predicted increased total behavior problems indicates that difficulty regulating the HPA axis is related to trouble regulating behavior in children regardless of maltreatment status. However, the direction of the relation between HPA dysregulation and behavior problems is still unclear. This study provides evidence that between-person cortisol variability in the maltreatment group may be due to differences in the type, severity, and timing of maltreatment. Interestingly, the highest levels of variability were found in children with 1 subtype, less severe, and early-onset but not recent maltreatment. It is possible that children with less severe or 1 subtype of maltreatment may have greater trouble with HPA regulation than nonmaltreated children or children with more severe or several types of maltreatment, as they have fewer experiences with maltreatment and may not have developed consistent HPA responses to current life stressors. It is possible that they may not have experienced habituation to maltreatment and are more reactive to a variety of daily stressors. For example, these children may not have enough damage to the HPA axis following less severe maltreatment and thus do not show chronic dysfunction. They may be responding appropriately some of the time and responding in a dysfunctional manner at others, unlike nonmaltreated children who respond normally, or severely maltreated children who respond at a level of chronic dysfunction. In addition, children who experienced maltreatment early in life but not recently had higher within-person cortisol slope variability. They may have difficulty with HPA regulation throughout childhood, especially if they made adaptations to the toxic stress of maltreatment early in life but no longer experience the same type of stressor later in childhood. Exploratory analyses indicated that within the early-onset, not-recent maltreatment group, 85% of children experienced neglect, compared to 55% experiencing emotional maltreatment, 33% physical abuse, and 5% sexual abuse. Future studies should examine early life neglect as a possible cause of later difficulty with cortisol regulation.
Results did not indicate that maltreated children were experiencing blunting of HPA activity at this age. However, habituation of the stress response and lower basal cortisol levels due to glucocorticoid receptor down-regulation may begin in late adolescence and into adulthood.17 Instead, maltreated children with higher initial cortisol levels showed significantly lower levels of cortisol over time, suggesting HPA blunting in those with the highest cortisol levels. For children who have experienced or are currently experiencing maltreatment, the HPA axis may be hypersensitive to elevations in cortisol levels. As a result, high cortisol levels in maltreated children may initiate mechanisms that result in greater blunting over time.
These findings may advance understanding of how early life stress “gets under the skin” to disrupt physiological systems and increase risk for psychopathology and poor health.32 For example, higher HPA variability may be a risk factor for poor health and psychopathology. Furthermore, higher HPA variability may result from difficulty regulating emotions and behavior or from damage to physiological systems due to chronic stress and repeated activation of stress-mediating systems.33 HPA axis dysregulation in particular may affect a number of other systems, including immunological processes critical to well-being.33 As a result, examination of HPA regulation in response to chronic stressors may elucidate both the processes by which the body adapts to unfavorable environments and how these processes affect mental and physical health.34
Our results indicate that psychosocial interventions for maltreated children may benefit from incorporation of well-conceptualized pre- and post-intervention HPA assessments in order to assess treatment efficacy in relation to stress mediating systems. For example, Dozier and colleagues have implemented an attachment-based intervention for infants and toddlers in foster care, which resulted in cortisol levels similar to children not in foster care.35 Intervention studies in maltreated children have examined diurnal cortisol patterns and morning cortisol levels before and after treatment.36 However, no study has assessed variability in cortisol levels as an indicator of HPA axis activity. More stable HPA regulation may suggest positive treatment responses, especially in children with 1 subtype, less severe, or early maltreatment. As maltreatment dimensions predicted different patterns of cortisol regulation, careful assessment of maltreatment parameters is necessary in order to understand alterations in behavior and HPA regulation post-intervention. Maltreated children who have the highest cortisol levels before intervention should be monitored, as they may represent a subset of children that responds differently to treatment. Finally, these analyses indicate that difficulty with cortisol regulation is related to higher levels of problem behavior and that this association is not specific to maltreated children. Evaluations of intervention efficacy would benefit from consideration of the interplay between reductions in behavior problems and improvements in cortisol stability.
There are limitations to this research that must be mentioned. Measures of diurnal cortisol regulation and reactivity to stressors were not obtained due to methodological difficulty of multiple assessments in a vulnerable population. Although the current study utilizes a novel method of collecting saliva samples, future studies should aim to collect samples of diurnal cortisol activity and responses to stressors. Though these were not obtained, the study does provide a methodologically sound assessment of cortisol levels in a real-world context for the children. In addition, current life stress, neighborhood quality, and parental mental health in both the maltreated and nonmaltreated groups were not assessed, so it is unclear whether one group was experiencing greater stress. Increased variability in cortisol levels could be due to greater life stress, poorer neighborhood quality, or possible parental mental illness that may be co-occurring in maltreating families. Finally, measures of pubertal status and body mass index (BMI) were not obtained, so these could not be included as covariates in the analyses.
The multiple-group growth model analyses suggest differences in the HPA growth trajectory between maltreated and nonmaltreated children. Evidence that children under chronic stress exhibit more variable patterns of HPA regulation may provide insight into mechanisms by which stressful experiences are embedded, influencing responses to stress throughout development. Knowledge gained from studying maltreated children may also inform studies of individuals enduring other forms of chronic stress, such as poverty or parental mental illness. These results provide important insights for understanding patterns of HPA axis dysregulation that occur among maltreated children over time. Intervention designs and evaluations should incorporate measurements of improvements in HPA axis regulation as an index of treatment efficacy.
Acknowledgments
Support was provided by grants received from the National Institute of Mental Health (NIMH; 1 R01MH083979-01A1) and the Spunk Fund, Inc., both to D.C. Support for Ms. Doom came from an NIMH training grant (T32MH015755, Principal Investigator: D.C.).
The authors thank Elizabeth Handley, PhD, of the University of Rochester Medical Center for her help with an earlier version of this manuscript.
Footnotes
Disclosure: Drs. Cicchetti and Rogosch and Ms. Doom report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ms. Jenalee R. Doom, Institute of Child Development and the Center for Neurobehavioral Development, University of Minnesota, Minneapolis.
Dr. Dante Cicchetti, Institute of Child Development, University of Minnesota, and Mt. Hope Family Center, Rochester, NY.
Dr. Fred A. Rogosch, Mt. Hope Family Center and the University of Rochester.
References
- 1.Cicchetti D, Toth SL. A multilevel perspective on child maltreatment. In: Lamb M, Garcia Coll C, editors. Handbook of Child Psychology and Developmental Science. 7. Vol. 3. New York: Wiley; Socioemotional process. in press. [Google Scholar]
- 2.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Wingfield JC. What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology. 2011;214:367–75. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiat. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- 10.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrino. 2012;37:317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA-axis Function: A systematic review and meta-analysis. Clin Psychol Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrion VG, Wong SS. Can traumatic stress alter the brain? Understanding the implications of early trauma on brain development and learning. J Adolescent Health. 2012;51(2 Suppl):S23–28. doi: 10.1016/j.jadohealth.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 14.De Bellis M, Chrousos G, Dorn L, et al. Hypothalamic–pituitary–adrenal axis dysregulation in sexually abused girls. J Clin Endocr Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 15.Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Dev Psychopathol. 1995;7:11–26. [Google Scholar]
- 16.Ouellet-Morin I, Odgers CL, Danese A, et al. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiat. 2011;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrino. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrino. 2011;36:531–539. doi: 10.1016/j.psyneuen.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicchetti D. Resilient functioning in maltreated children: Past, present, and future perspectives. J Child Psychol Psyc. 2013;54:402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Dev Psychopathol. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Dev. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrino. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child Abuse, Child Development, and Social Policy. Norwood, NJ: Abex; 1993. pp. 7–73. [Google Scholar]
- 26.Achenbach T. Manual for the Teacher Report Form and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 27.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychol Bull. 1987;101:147–158. [Google Scholar]
- 28.McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- 29.Ram N, Grimm KJ. Using simple and complex growth models to articulate developmental change: Matching theory to method. Int J Behav Dev. 2007;31:303–316. [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus User’s Guide. Vol. 5. Los Angeles: Muthén and Muthén; 1998–2008. [Google Scholar]
- 31.Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrino. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 34.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Dev Psychopathol. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23:789–800. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]