Summary
Reminders of happy memories can bring back pleasant feelings tied to the original experience, suggesting an intrinsic value in reminiscing about the positive past. However, the neural circuitry underlying the rewarding aspects of autobiographical memory is poorly understood. Using fMRI, we observed enhanced activity during the recall of positive relative to neutral autobiographical memories in corticostriatal circuits that also responded to monetary rewards. Enhanced activity in the striatum and medial prefrontal cortex was associated with increases in positive emotion during recall and striatal engagement further correlated with individual measures of resiliency. Striatal response to the recall of positive memories was greater in individuals whose mood improved after the task. Notably, participants were willing to sacrifice more tangible monetary rewards in order to reminisce about positive past experiences. Our findings suggest that recalling positive autobiographical memories is intrinsically valuable, which may be adaptive for regulating positive emotion and promoting better well-being.
Introduction
Sometimes it can be good to live in the past. Autobiographical memories serve a variety of adaptive functions, such as bolstering a sense of self identity (Bluck et al., 2005; D’Argembeau and Van der Linden, 2005) or planning for the future (Schacter and Addis, 2007). When it comes to remembering positive life events, however, one adaptive function of autobiographical memories may be its most prominent: it feels good to remember the good times. That is, reminders of the past can bring back emotions tied to the original experience (e.g., Bower, 1981; Westermann et al., 1996). For example, remembering a game winning goal in a championship game may trigger a re-experience of positive emotions associated with that day. In this way, the recall of positive autobiographical memories may be intrinsically valuable to an individual by increasing or maintaining positive feelings that contribute to one’s general well-being.
The capacity to savor or maintain positive feelings is associated with an increased ability for emotion regulation (Carl et al., 2013), which in turn may promote successful adaptation to stress (i.e., resilience; Philippe et al., 2009). Individuals afflicted with depression tend to recall fewer autobiographical memories, especially positive ones (Young et al., 2013), and have difficulties sustaining positive emotions (Carl et al., 2013; Heller et al., 2009). Critically, these patients also show aberrant activation patterns in neural circuits involved in reward processing (Delgado, 2007; Haber and Knutson, 2010; O’Doherty, 2004), such as reduced striatum responses to rewards (Pizzagalli et al., 2009) and positive feedback (Elliott et al., 1998), as well as difficulty sustaining reward-related activity in the ventral striatum in response to positive stimuli (Heller et al., 2009). Together, these observations highlight the benefits of savoring events that elicit positive emotions to one’s well-being, and underscore the importance of understanding the neural mechanisms underlying changes in positive emotion.
One potential way of eliciting positive emotions that engage reward-related circuitry may be to recall pleasant autobiographical memories. Such memories tend to be vivid and rich (e.g., Schaefer and Philippot, 2005; Talarico et al., 2004) and benefit from having a positive value attached to it during encoding (Scimeca and Badre, 2012) that is re-experienced during recall (Westermann et al., 1996). In fact, a positive, arousing event (e.g., the potential to earn money) can impact the encoding of memory in general (e.g., a neutral scene), engaging activity in reward-related regions such as the midbrain and the ventral striatum at the time of memory formation that promotes better subsequent recall (Adcock et al., 2006), potentially via interactions with memory-related regions such as the hippocampus (Wittmann et al., 2005). Thus, it is plausible that increased dopamine release during encoding influences dopaminergic targets (such as the striatum) and deem the memories more relevant, strengthening them over time and making them easier to access in the future (for review see Shohamy and Adcock, 2010).
Autobiographical memories may also be adaptive in the sense that recalling the past is something we already do naturally. Importantly, we may be able to capitalize on the positive emotion we feel when reminiscing about happy memories, which unlike other forms of emotion regulation (e.g., cognitive reappraisal) may be a proactive rather than reactive strategy for increasing positive emotion. Indeed, the rewarding aspects of autobiographical memories are a likely component of affective well-being and protection from disorder, but their underlying neural substrates are poorly understood. That is, do autobiographical memories display properties in common to other rewards? In particular, how does the brain represent rewarding aspects of memories (i.e., positive valence, positive emotion during recall)? Further, can autobiographical memories be assigned a value that is comparable with other rewards (i.e., will people sacrifice a tangible reward for a more highly valued autobiographical memory)?
In this experiment, we utilized functional magnetic resonance imaging (fMRI) to examine neural activity during the recall of autobiographical memories, particularly memories with positive content, which can lead to the re-experience of positive emotion. Participants first participated in Day 1, where they were presented with common life event cues (e.g., Family Vacation) and asked to describe specific memories of positive (e.g., visiting Disneyland) and neutral (e.g., packing for a trip) content, but not negative (e.g., lost luggage). Participants who met criteria (i.e., had enough memories) were then invited to participate in Day 2 – the fMRI session. In the scanner, they recalled (i.e., brought the details of the original event to mind) a number of positive and neutral memories prompted by the cues from the first session, and provided ratings of valence (positive, neutral), feeling and intensity. We hypothesized that recalling a positive memory would a) evoke positive feelings and b) engage reward-related neural circuitry including the striatum, which is involved in the processing of primary, secondary and abstract rewards (e.g., food, money and pleasant music, Salimpoor et al., 2011, respectively; see for review Delgado, 2007; Haber and Knutson, 2010; O’Doherty, 2004) as well as active coping processes related to dealing with negative affect (Delgado et al., 2009; Ledoux and Gorman, 2001). Finally, we conducted a behavioral experiment on a separate cohort of participants to probe for the value of recalling such positive memories. We hypothesized that participants would value the experience of recalling positive autobiographical memories and consequently choose such an experience over a more tangible reward (i.e., money).
Results
The recall of autobiographical memories elicits positive emotion
Nineteen healthy participants (10 females; mean age = 26.1, SD = 7.78) performed a cued recall task while undergoing fMRI. Prior and following the task, participants were given the Positive and Negative Affective Scale (PANAS; Watson et al., 1988) to assess mood change during the experiment. They concluded the fMRI session by performing a card task for monetary rewards (Delgado et al., 2000) which served as an independent localizer of reward-related brain activity.
The autobiographical memory recall task involved the presentation of 21 positive and 21 neutral cues for a total of 42 trials. On each trial (Figure 1), participants were presented with a written cue (e.g., Family Vacation) which prompted a memory – a personal experience from the past that was deemed as positive (e.g., visiting Disneyland) or neutral (e.g., packing for a trip). The memories were previously validated in an interview three days earlier by participants’ descriptions and ratings of emotional intensity and feeling (Day 1 – see procedures in Methods). Memory cues selected for the recall task were those in which the valence rating (e.g., positive) matched their emotion ratings (e.g., high intensity, high feeling). Upon seeing a cue (e.g., family vacation) participants had 14 seconds to recall a memory. They made a button press to indicate the onset of the recall experience, and another button press to indicate when they were done recalling the memory – a procedure similar to (Daselaar et al., 2008; Sharot et al., 2007). Participants were then asked to rate the memory they just recalled with respect to valence (positive or neutral), level of emotion or feeling (i.e., how did you feel when you recalled this memory; 1-4: 1 = neither good nor bad, 4 = very good) and emotional intensity (i.e., how intense was the particular memory; 1-4: 1 = not intense, 4 = very intense).
Figure 1.
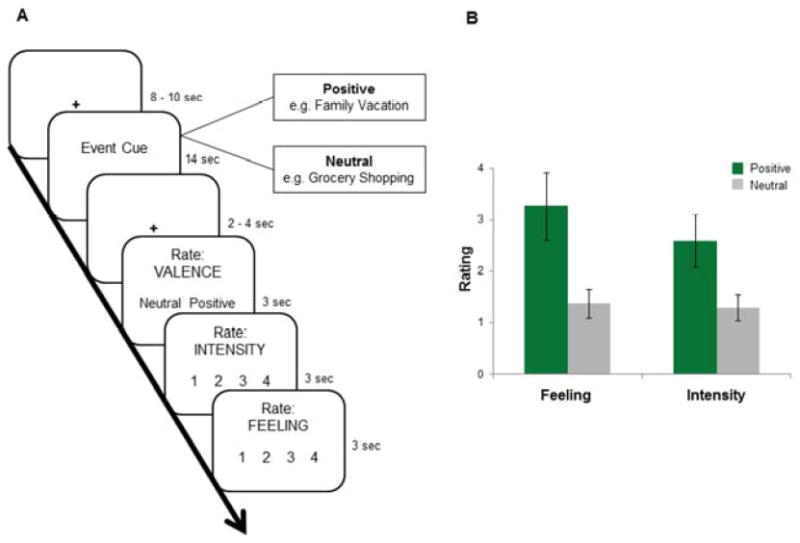
fMRI autobiographical memory task and behavior. (A) On each trial, participants recalled either a positive or neutral memory while an event cue was displayed for 14s. Participants indicated the beginning and end of the memory with button presses during the recall period. Then, they gave subjective ratings of the memory’s valence (i.e., positive or neutral), emotional intensity, and feeling. (B) Positive memories were rated as significantly greater in positive feeling and emotional intensity than neutral memories. P < 0.05, two-tailed test. Error bars represent ±1 s.e.m.
During performance in the memory cued recall task, we observed that positive memories elicited an increase in experienced positive emotions. Recalled memories that were rated as positive evoked greater emotional intensity (t(18) = 9.51, p < .001) and positive feeling (t(18) = 20.0, p < .001) compared to memories rated as neutral. Perhaps unsurprisingly, participants spent more time during the recall of positive (M =6.7 s, SD = 3.0 s) compared to neutral memories (M = 5.6 s, SD = 2.6 s; t(18) = -4.91, p < .001). There was no significant relationship between feeling ratings and duration for positive (r(18) = -.18, p = .47) or neutral memories (r(18) = .23, p = .34), however, suggesting that the observed enhanced emotion cannot necessarily be due to a longer period of recall. Importantly, the onset of memory recall did not differ between positive (M = 3.0 s, SD = 0.9 s) and neutral memories (M = 2.9 s, SD = 1.0 s), suggesting that neither memory valence was easier or harder to remember.
Neural activity represents the valence and emotion of autobiographical memory recall
We were interested in investigating the neural correlates underlying the recall of autobiographical memories which elicited such subjective increases in positive emotion. We performed 3 key analyses (see Methods). The first analysis was a random-effects whole brain general linear model (GLM) and focused on the period of recall. We used the cluster level statistical threshold plugin in BrainVoyager to correct for multiple comparisons (Goebel et al., 2006). Resulting statistical maps were set to a threshold of p<0.005 and corrected to a whole brain cluster correction threshold of p < .05 with a threshold of 6 contiguous voxels (162mm3 as determined by the plugin).
A simple contrast of positive compared to neutral autobiographical memories revealed increased activity in corticostriatal circuits involved in reward-related processing (Balleine et al., 2007; Delgado, 2007; Haber and Knutson, 2010; O’Doherty, 2012). Specifically, this analysis identified voxels within the striatum (including bilateral head of the caudate), ventral medial prefrontal cortex, orbitofrontal cortex and anterior cingulate as showing greater activity for positive compared to neutral autobiographical memories during time of recall (see Table S1; Figure 2A-B; Figure S1). These ROIs were then used in a subsequent follow-up analysis to test relationships between neural activity and emotion ratings collected during the autobiographical memory task in the scanner.
Figure 2.
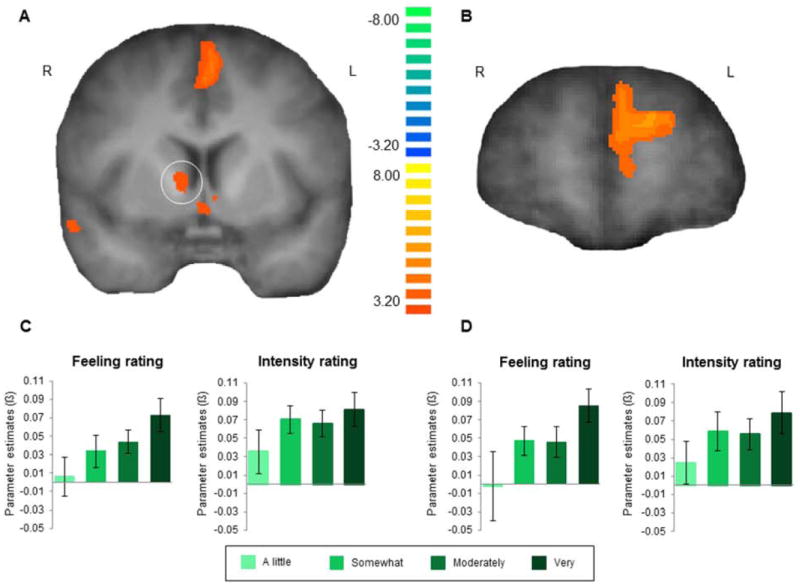
Neural activity during the recall of autobiographical memory. ROIs defined by contrasting positive and neutral memory recall revealed activity in (A) the striatum, including the head of the caudate, and (B) the medial prefrontal cortex. P < 0.05; corrected. (C) BOLD signal in the right caudate (peak at x, y, z: 11, 1, 6) and (D) medial prefrontal cortex (peak at x, y, z: -13, 58, 24) during the recall of positive versus neutral memory was parametrically modulated by positive feeling ratings on each trial. The plots show BOLD signal parameter estimates for positive memory recall according to the subjective ratings of feeling and emotional intensity. Error bars represent ±1 s.e.m. See also Figure S1, Table S1.
Of particular interest was the involvement of the striatum and prefrontal cortex, given their involvement in reward processing, in the recall of positive autobiographical memories. We hypothesized that such regions would be modulated by the degree of positive emotion evoked by the specific memory (i.e., feeling ratings on a trial by trial basis). We also explored the possibility that these neural regions may be modulated by the degree of arousal evoked by the memory (as indicated by emotional intensity ratings). To test this, we conducted two additional tests (one for feeling ratings and one for emotional intensity ratings during the memory task) on regions of interest (ROIs) in the striatum and prefrontal cortex identified in the main contrast of positive and neutral memories. Specifically, we included subjective ratings (of either feeling or emotional intensity) as a parametric modulator for positive memory trials in a GLM and extracted parameter estimates from these ROIs. This test was not biased by the ROI selection procedure because the parametric modulator in each model was not correlated with the positive and neutral memory regressors.
We observed that positive emotion (i.e. feeling ratings) significantly modulated activity in the caudate bilaterally (right: t(18) = 2.43 p = .03; left: t(18) = 2.38, p = .03; Figure 2C) and medial prefrontal cortex (mPFC; t(18) = 2.40, p = .03; Figure 2D). This suggests that, during the recall of positive life events, neural activity in these regions increases to the degree that positive emotions were generated. In contrast, the second GLM with emotional intensity ratings as a parametric regressor for positive memory trials did not significantly modulate activity in these regions. These results suggest that activity in the striatum and mPFC during the recall of positive autobiographical memories is associated with the magnitude of positive emotions experienced as opposed to merely reflecting an arousal response.
Given this linear relationship between positive emotion and activity in regions previously implicated in reward processing, one hypothesis is that the level of such engagement during recall may be related to individual differences in a stress-protective factor, such as resilience. That is, the ability to recall positive autobiographical memories in a manner that increases positive emotion and recruits activity in these regions may benefit successful adaptation from adversity. Highly resilient individuals tend to report greater levels of subjective wellbeing, which may be the result of experiencing more positive emotion (e.g. optimism) or engaging in active coping mechanisms (e.g. humor, mindfulness; Wu et al., 2013) – a process that has been associated with striatum function during aversive learning (Delgado et al., 2009; Ledoux and Gorman, 2001). We observed that participants with higher self-reported resiliency (Connor-Davidson Resiliency scale; Connor and Davidson, 2003) exhibited greater activity in the right caudate ROI – defined by the contrast of positive and neutral memory recall – during the recall of positive autobiographical memories (correlation between resilience scores and parameter estimates from the right caudate ROI: r(19) = 0.458, p = .05).
Changes in mood after recalling autobiographical memories are related to striatal activity
In a second key analysis, we explored whether remembering positive memories would also lead to mood improvements. We asked participants to report their current mood state using the Positive and Negative Affective Scale (PANAS; Watson et al., 1988) before and after the memory task. No significant changes in mood were observed in the sample (t(18) = 0.500, p = .62). However, there were individual differences as some participants’ mood’s improved (n = 7), some worsened (n = 9) and some showed no change (n = 3). We then conducted an exploratory analysis comparing behavioral and neural changes underlying the mood-increase and the mooddecrease groups. Both groups were similar in terms of task performance during recall, showing no differences in emotional ratings (positive memories: t(14) = 0.798, p = .44; neutral memories: t(14) = 1.70, p = .11), or recall duration (positive memories: t(14) = 0.076, p = .94; neutral memories: t(14) = 0.200, p = .84). Thus a question remained about what was underlying the changes in mood across the experiment for these individuals.
Based on a specific hypothesis that striatum activity underlies mood increases brought about by positive autobiographical memory recall, we tested neural differences within this region between individuals whose mood improved and individuals whose mood worsened after recalling memories. To avoid potential biases within ROIs previously defined by the contrast of positive and neutral memories in our first analysis, we instead applied a small volume correction (SVC) in a structurally defined striatum mask (including putamen, caudate, and nucleus accumbens using the Harvard-Oxford probabilistic atlas; Desikan et al., 2006), and then performed a Valence (positive/neutral) by Mood Change (mood-increase/mood-decrease) ANOVA. We observed a significant interaction in the right ventral striatum (x, y, z: 10, 11, 1; F(1,14) = 17.55, p < .05, SVC; Figure 3), driven by a marked increase in activity for positive relative to neutral memories in the mood-increase group (t(6) = 11.46, p < .001), but no significant change for the mood-decrease group (t(6) = 0.32, p = .76). We also observed a significant interaction in the left putamen (x, y, z: -25, 1, -3; F(1,14) = 18.64, p < .05, SVC), with the mood-increase group showing a similar increase for positive compared to neutral memories (t(8) = 4.29, p = .005), and the mood-decrease group trending in the opposite direction (t(8) = 1.75, p = .12). Although the results should be deemed exploratory, given the small sample size of the groups, these relationships suggest that, in certain individuals, an increase in striatum activity during the recall of positive life events can accompany an improvement in mood.
Figure 3.
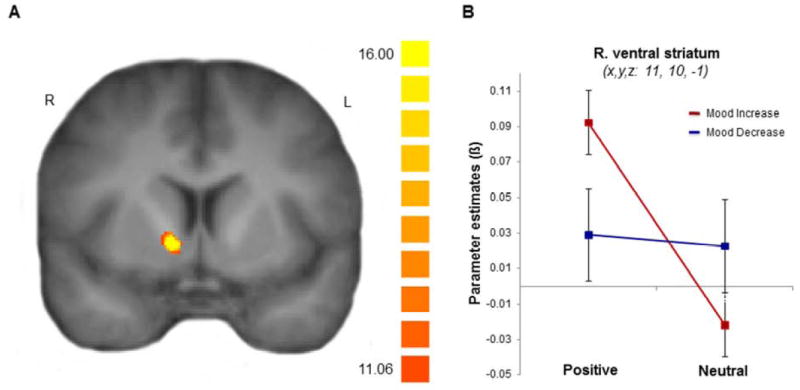
Ventral striatum activation during memory recall and subsequent mood change. (A) A valence (positive/neutral) by mood change group (increase/decrease) ANOVA revealed a significant interaction in the right ventral striatum. P < 0.05; SVC. (B) Mean parameter estimates from the right ventral striatum are displayed showing greater activity for positive compared to neutral memory in the mood-increase group (n = 7), but no significant change in the mooddecrease group (n = 9). P < 0.05, two-tailed test. Error bars represent ±1 s.e.m.
Ventral striatum responses distinguish between the valence of monetary outcomes and memory recall
The third key analysis performed addressed an interesting question of whether brain regions that actually distinguish between more tangible rewards (i.e., money) during reward paradigms would also distinguish between positive and neutral autobiographical memories during recall. We asked participants to play a surprise card game for monetary rewards (adapted from Delgado et al., 2000) at the conclusion of the memory task. Consistent with previous findings using this reward paradigm (see for review, Delgado, 2007), a contrast of gain and loss outcomes revealed robust activation in the striatum (Figure 4; Table S2). We defined this reward-based ROI in the right ventral striatum and extracted parameter estimates for positive and neutral memories. An increase in activity related to the recall of positive compared to neutral autobiographical memories was observed in the right ventral striatum (t(18) = 2.33, p = .03). Thus, voxels in a ventral striatum ROI functionally defined by a contrast between monetary gains and losses (i.e. tangible, extrinsic reward) were also sensitive to the valence of autobiographical memories in the same individuals.
Figure 4.
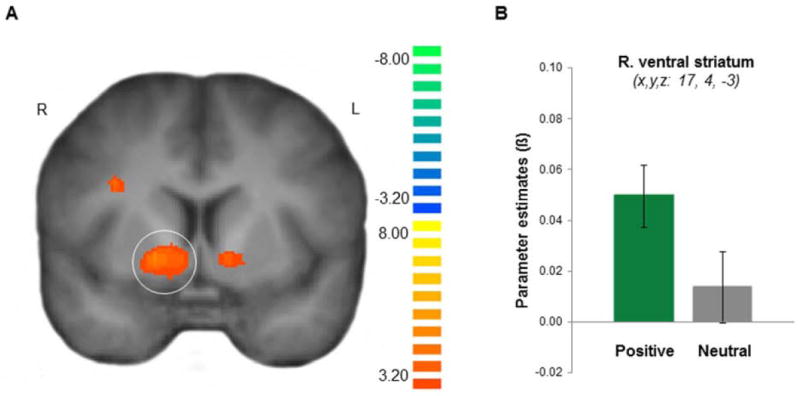
Voxels within the ventral striatum that respond to the receipt of monetary reward also distinguish between the valence of autobiographical memories during recall. (A) Monetary reward ROIs defined by contrasting gain and loss outcomes in a card guessing task revealed activity in the ventral striatum bilaterally. P < 0.05; corrected. (B) A monetary reward ROI (i.e. the right ventral striatum) showed significantly greater activity for the recall of positive memory as compared to neutral memory. P < 0.05, two-tailed test. Error bars represent ±1 s.e.m. See also Table S2.
Positive autobiographical memories are valuable
Our findings suggest that recalling positive experiences from the past increases one’s positive emotion and engages reward-related neural circuitry, such as the striatum and medial prefrontal cortex. One implication of these results is that reminiscing about positive experiences is intrinsically valuable to an individual. If this is the case, however, then people should be willing to forgo another, more tangible reward (i.e., money) for the opportunity to recall positive as compared to neutral autobiographical memories. We tested this hypothesis in a behavioral experiment using a separate cohort of participants.
The behavioral study adopted the same design as the fMRI study with one important difference: prior to recalling a memory, participants had a choice of which memory to conjure up. They chose between a positive (e.g., “Family Vacation”) or a neutral (“Grocery Shopping”) memory cue (Figure 5A). Both options were accompanied by a specified monetary payoff that ranged from $0.01 to $0.04 (every combination of monetary payoffs was represented in option pairs as in similar paradigms; Hayden et al., 2007; Tamir and Mitchell, 2012). Thus, some choices required participants to choose to forego a larger monetary payoff in order to recall their preferred memory in the task. Participants then recalled the selected memory as in the fMRI study, and at the end of the task received a monetary payoff according to the sum of their choices.
Figure 5.
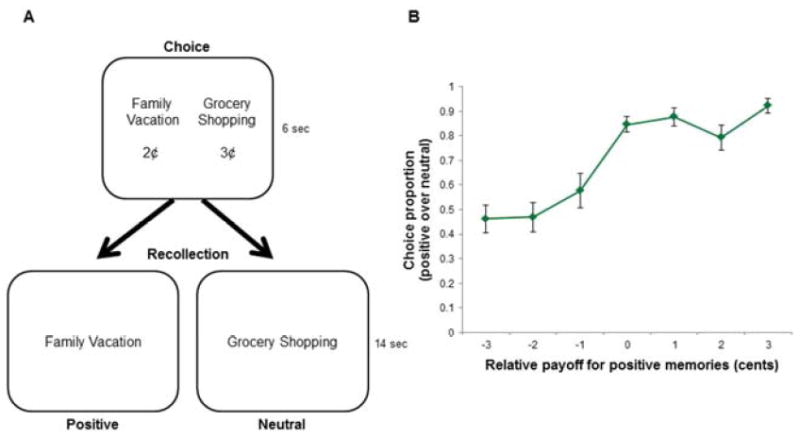
Memory choice task and behavior. (A) On each trial, participants made a choice between two life event cues (positive, neutral) to recall, where each choice was associated with a monetary payoff ranging from $0.01 to $0.04. The rest of a trial was the same as the fMRI memory task. Participants recalled a memory while viewing the (selected) event cue for 14s, made button presses to indicate the memory’s beginning and end, and then gave subjective ratings for the memory. (B) The graph represents the monetary value of positive autobiographical memory for all participants in the behavioral study. Each dot represents the percentage of trials where participants chose to recall a positive over a neutral memory (y-axis) for each relative payoff (x-axis). Relative payoffs were the difference between the monetary value of the two options on a particular trial (i.e. positive – neutral). We measured the relative monetary value of recalling positive autobiographical memory by finding the point where the cumulative normal distribution, fit to the data, passed 50% (Deaner et al., 2005; Tamir and Mitchell, 2012). Across the sample, participants were willing to forgo 1.94 cents (SD = 1.22) to recall a positive over a neutral memory. See also Figure S2.
Twenty-six healthy participants (19 females; mean age 20.0, SD = 2.98) preferred to recall positive over neutral memories. Compared to chance (50%), specifically, participants chose positive memories 85% of the time when choices were of equal value (t(25) = 10.8, p < .001) and 70.7% of the time across all relative payoffs (t(25) = 7.45, p < .001). To measure the subjective value associated with choosing a positive over a neutral memory, we calculated the point of subjective equity (PSE) for each participant by fitting a cumulative normal distribution to their choice behavior (adapted from Tamir and Mitchell, 2012; Figure 5B). The PSE represents the monetary payoff foregone to choose to recall a preferred memory. That is, PSE is the difference in monetary payoffs between the two options when a participant was equally likely to choose a positive or neutral memory (i.e. where the cumulative normal distribution passed 50% and limited to the range of relative payoffs: -$0.03 to +$0.03). Participants were willing to forgo an average of 1.94 cents (SD = 1.22) to recall a positive instead of a neutral memory, (t(25) = -8.16, p < .001), which was still significant when controlling for gender (t(25) = 2.66, p = .014). That is, participants sacrificed 28% of potential monetary earnings to recall positive rather than neutral memories.
Given the known deficits in reward-related processing and positive emotion observed in depressed individuals (Carl et al., 2013; Pizzagalli et al., 2009), we also tested whether participants’ willingness to forgo monetary rewards to recall positive memories was related to symptoms of depression. Interestingly, there was a significant positive correlation between PSE and depression scores (Beck Depression Inventory; Beck et al., 1961; r(25) = .39, p = .047, and when controlling for gender, partial r(25) = .40, p = .048). Individuals with greater symptoms of depression were less willing to give up money to recall a positive experience.
Discussion
Autobiographical memory is remarkable not only for the ability to re-experience the past, but also for allowing us to rekindle emotions captured during the original experience. Our findings suggest that reminiscing about positive past experiences produces internally generated positive emotions, which may be rewarding in and of itself. In the current study, the recall of positive autobiographical memories elicited an increase in positive emotion, as measured by subjective ratings of feelings, and recruited neural circuits involved in reward-related processing. Activity in the striatum and medial prefrontal cortex was modulated by the strength of positive emotion experienced and striatal activity in particular correlated with individual measures of resiliency. Further, re-living these happy memories in one’s mind led some participants to improve their mood during the experiment, with such individuals also showing greater ventral striatum activity when recalling positive compared to neutral autobiographical memories. Finally, a separate cohort of participants chose to recall positive compared to neutral memories even if it came at a financial cost; that is, they were willing to choose recalling a positive memory over a neutral one even when the neutral memory was accompanied by a monetary reward. This behavior suggests that individuals value positive memories to the point of sacrificing more tangible rewards. Taken together, the findings suggest that positive autobiographical memories can be intrinsically valuable to an individual, serving adaptive functions such as bolstering positive emotion which is significant for an individual’s well-being and ability to cope with negative affect (Carl et al., 2013).
The recruitment of reward-related regions such as the striatum and medial prefrontal cortex during the recall of positive autobiographical memories and association with increased self-reported positive emotion is in line with our hypothesis that such memories are valuable and elicit positive emotions attached to the original experience. Specifically, the striatum has been involved in processing the subjective value of typical (Delgado, 2007; Haber and Knutson, 2010; O’Doherty, 2004) and atypical (Delgado et al., 2009; Ledoux and Gorman, 2001; Salimpoor et al., 2011; Tamir and Mitchell, 2012) rewards, and decreases in self-reported positive emotion correlate with decreases in striatal signal in depressed individuals (Heller et al., 2009).
There are alternative interpretations of striatum recruitment during memory recall worth considering, however. For instance, it is possible that striatal activity solely reflects emotional arousal, which is a component of an emotional response. While it is conceivable that positive emotional responses will elicit a higher arousal than neutral feelings, arousal per se did not explain the modulation of the striatum activation. In particular, this region was modulated by subjective ratings of emotional feelings, but not emotional intensity. It is also possible that the striatum activity is due to re-assigning value to a recalled stimulus, consistent with findings of striatum activity in post-choice changes in preference (Sharot et al., 2009), when an increase in perceived value of a stimulus is observed after it is chosen. This interpretation is not inconsistent with our account that such memories carry value and serve to increase positive emotion and perhaps improve mood in select individuals.
The prefrontal cortex has been more consistently observed among studies of autobiographical memory retrieval (Markowitsch et al., 2003; Piefke, 2003), but it is also plausible that striatum activity is related to aspects of the memory process itself, such as adaptive updating or the cognitive control of episodic retrieval (Scimeca and Badre, 2012), rather than the account proposed in the current paper. Our design attempted to control for aspects of the memory process itself by asking participants to recall, describe, and emotionally rate all memories, both neutral and positive, three days prior to scanning. Accordingly, there was no difference in the amount of time it took to bring a positive or a neutral memory to mind in the subsequent scanning session, suggesting similar ease of recall, and thus similar retrieval success, between positive and neutral memories. Further, the current findings show a specific role for striatal activity in representing affective aspects of memory and lesser engagement when recalling neutral memories. Although it is worthy to note that participants may have re-encoded the memories with additional value during the initial retrieval process three days prior to the scanning, consistent with Scimeca and Bradre’s framework of adaptive encoding (2012).
One interesting aspect of the observed striatal activity was the consistent observation of greater responses during the recall of positive compared to neutral autobiographical memories regardless of the location of the ROI within the striatum. The whole-brain contrast of positive and neutral memories elicited activation in the caudate nucleus, part of the dorsal striatum and involved in affective and cognitive aspects of behavior (Balleine et al., 2007; Delgado, 2007; Haber and Knutson, 2010). However, we also observed greater responses to the recall of positive memories in the ventral striatum. Specifically, we observed an interaction between memory valence and mood increase in a structurally defined striatum ROI during memory task performance. We also observed greater activation to positive compared to neutral memory recall in a functionally defined independent ROI in the ventral striatum related to reward processing (i.e., showing responses to monetary gains compared to losses). These results highlight the involvement of both dorsal and ventral striatum in the recall of positive autobiographical memories.
There are several important implications of our findings. First, we reinforce the idea that positive autobiographical memories have an adaptive function to increase positive emotion, which can potentially benefit one’s ability to use emotion regulation (Carl et al., 2013). Consistent with the idea that positive autobiographical memories carry intrinsic value, we demonstrated that participants had a significant preference for recalling past, positive events, at the point of forgoing a more tangible reward. This is somewhat surprising considering that participants have access to their own positive memories at any time, and therefore are not at a loss if they waive an opportunity to recall a positive memory in order to earn a larger monetary reward. However, it fits with the idea that people tend to value experiences over material possessions (e.g., Dunn et al., 2011), and that looking at the past positively, or more nostalgically, can make people happier (Zhang and Howell, 2011). This may be a unique feature of increasing positive emotions via autobiographical memories instead of other methods. For instance, participants who are asked to create a positive mental image (e.g., a baby laughing) have enhanced positive emotions much like those elicited by positive memories, but are less willing to sacrifice money to imagine positive situations in comparison to participants who forgo rewards to recall the positive past (see Supplemental Information; Figure S2). Although this is just one example, and more research is necessary to investigate other ways in which positive emotions can be elicited, these results support an inherent value attached to the recall of autobiographical memories.
Second, we highlight a neural mechanism involved in increases in positive emotion and mood related to recalling positive autobiographical memories – a potentially important emotion regulation strategy to cope with negative affect (Rusting and Dehart, 2000). Not surprisingly, patients with depression have difficulties recalling positive autobiographical memories (Young et al., 2013) and using this type of strategy (Dalgleish et al., 2013), which may be related to an inability to sustain activity in the striatum during positive emotion (Heller et al., 2009). We observed individual differences in mood improvement after recalling positive memories, Although this finding is exploratory given the small samples, it lends support to the idea that some people are able to benefit more than others when recalling the positive past, in accordance with individual differences in emotion regulation success (e.g., Martin and Delgado, 2011). Interestingly, we also observed that resilience was associated with greater striatal activity during the recollection of positive memories, consistent with the thought that cultivation of positive emotions builds resources to enhance life satisfaction, for example, by increasing the likelihood of experiencing positive emotion in the future and even garnering one’s resilience against negative affect (Tugade and Fredrickson, 2004). Savoring positive events from our past may be an adaptive tool for combating life’s troubles. Therefore, it is intriguing that resilient individuals, who have a greater tendency to experience positive emotions, are also those who exhibit more reward-related activity in the striatum when remembering happy moments. In a similar vein, individuals who showed a boost in mood from before to after the memory task had more striatal activation when recalling positive memories. Although we cannot test a causal relationship, this raises the question of whether purposefully engaging in positive recollection of the past increases one’s resilience, and perhaps mood, over time.
We also observed a pattern linked to depression in our behavioral study. Individuals with greater depressive symptoms were less willing to give up money to recall a positive event. Depression has been linked to not only functional anatomical deficits in autobiographical recall (Young et al., 2013), but also a diminished ability to recall specific, positive events (Williams and Scott, 1988). One explanation is a lack of normative positivity bias in depression rather than a negativity bias, which our results seem to support (Suslow et al., 2001). Therefore, it is possible that if recalling pleasant memories feels effortful and evokes only a small, fleeting positive feeling, then depressed individuals may feel like the payoff is too small. Our results suggest that reminiscing about past positive experiences may have an adaptive role in regulating and maintaining positive emotion, which may promote better well-being, particularly in the case of affective disorders like depression.
While experiencing positive emotion when reminiscing about happy memories is not an entirely new idea, the uniqueness in regulating emotions using autobiographical recall is, in part, that memories are an abundant resource we already have available to us, whereas other emotion regulation strategies are typically stimulus-driven. For instance, studies examining the cognitive regulation strategy of reappraisal (e.g., Wager et al., 2008) require the reinterpretation of something negative to something less negative or positive. In contrast, recalling the past is a natural phenomenon that people choose to do quite frequently in their daily lives (Killingsworth and Gilbert, 2010) and therefore does not require instruction, reinterpretation, or necessarily a cue. In this way, positive memories may uniquely represent a proactive, rather than reactive method of generating positive emotion. Our findings demonstrate not only that something naturally-occurring like recalling the past can successfully influence mood in some individuals, but it is linked with activity in reward-related circuitry – which fails to be sustained in depressed patients during experience of positive emotions (Heller et al., 2009) – and leads people to forgo an extrinsic reward (money) in order to experience it.
Methods
Participants
Twenty-eight healthy right-handed individuals were recruited via flyers posted on the Rutgers campus to participate in a 2-day study (13 males, 15 females). Given our experimental requirements (e.g., meeting criteria for number of available memories), we had a number of exclusions prior to scanning. Specifically, participants completed an autobiographical memory questionnaire on Day 1 and returned for the fMRI session on Day 2 only if they met criteria for inclusion (see procedures for Day 1; 23 met criteria). Five participants did not make it to the fMRI session on Day 2 based on these criteria. Additionally, we excluded 2 participants for excessive head motion (more than 4mm in any direction), 1 for performance (i.e., 46% missed responses), and 1 withdrew before completing the scanning session (claustrophobia). Final analysis was therefore performed on 19 participants (9 males, 10 females; mean age = 26.1, SD = 7.78). Our slightly higher attrition rate is due to the potential for participants to be excluded after Day 1 as well as Day 2. All participants were free of neurological or psychological conditions, and were not taking any medications at the time of testing. Participants gave informed consent in accordance with the Rutgers University Institutional Review Board for the Protection of Human Subjects in Research.
Experimental design
Day 1: Autobiographical Memory Questionnaire
Participants wrote about real memories prompted by each of 70 common life event cues (e.g., Family Vacation). The written cues were a compilation of cues created for this study and cues used in prior studies (Markowitsch et al., 2003; Piefke, 2003; Sharot et al., 2007). For each cue, participants selected a memory in which they had been personally involved and had occurred at a specific place and time. For each memory, participants reported a brief description, the location, the date, and gave subjective ratings for valence (positive or neutral), emotional intensity (i.e., how intense was the particular memory; 1-4: 1 = not intense, 4 = very intense), and feeling (i.e., how did you feel when you recalled this memory; 1-4: 1 = neither good nor bad, 4 = very good). Importantly, participants were instructed to select memories that were positive (e.g. visiting Disneyland) or neutral (e.g. packing for a trip), but not negative memories (e.g., lost luggage).
To be invited back to the imaging session (Day 2), individuals had to report at least 21 positive and 21 neutral memories. For each participant who met criteria, the 21 most positive memories (i.e. high intensity, high feeling) and the 21 most neutral memories (i.e. low intensity, low feeling) were selected as unique event cues to recall in the scanner.
Additionally, participants filled out the following questionnaires related to resiliency and emotion: the Connor-Davidson Resiliency scale (CD-RISC; Connor and Davidson, 2003) and the Beck Depression Inventory (BDI; Beck et al., 1961).
Day 2: fMRI session
Participants returned for the second session (Day 2) three days later. While in the MRI scanner, participants first reported their current mood state via the PANAS (Positive and Negative Affective Schedule; Watson et al., 1988), then completed a cued recall autobiographical memory paradigm.
In the memory task, participants re-accessed 21 positive and 21 neutral memories triggered by event cues from their questionnaire on Day 1. Each trial (Figure 1) included one written event cue displayed for 14s. Participants were asked to recall the same memory from Day 1 and to elaborate on it for as long as they could or until the 14s were up. Participants made a button press to indicate the ‘start’ of a memory (i.e. when it began to form in their mind) and another button press to indicate the ‘end’ of a memory (if they finished elaborating on the memory before time was up). After a delay of 2-4s, participants rated the memory on valence, emotional intensity, and feeling (3s for each rating). An 8-10s delay separated one trial from the next. The task was administered over the course of three functional runs, each containing 7 positive and 7 neutral event cues. Cues were pseudorandomly presented such that no more than 4 consecutive events of the same valence were displayed in a row. Immediately afterwards, participants again rated their current mood state via the PANAS. The pre- and post-task ratings of mood allowed for an assessment of mood change. Specifically, mood change was calculated as the change in positive affect subscale (10 adjectives) from pre-autobiographical memory task to post- autobiographical memory task.
While still in the scanner, participants performed a surprise monetary reward task (the card-guessing game adapted from Delgado et al. (2000). This task occurred without warning as to not influence the memory task and particularly the mood ratings post-memory task. The purpose of this paradigm was to identify reward-related regions of interest (ROIs) to serve as independent ROIs to test with positive and neutral memory regressors. In each trial of the card task, participants saw a card with a question mark inside for 2s. They guessed whether the card’s value was higher or lower than the number 5 by making a button press. After a short 2-4s delay, the card and monetary outcome were displayed. A correct response earned a green checkmark signifying a gain of $1.00 whereas an incorrect response earned a red X signifying a loss of $0.50. Unbeknownst to participants, outcomes were predetermined to control schedule of reinforcement and number of gain and loss trials (20 each for a total of 40 trials).
At the conclusion of the scanning session participants were debriefed and compensated for their time in the scanner and bonus money earned in the card game.
fMRI Data Acquisition
A 3T Siemens Magnetom Trio scanner was used for acquisition of T1-weighted MPRAGE structural images (256 x 256 matrix, FOV = 256 mm, 176 1-mm sagittal slices). Additionally, functional images were acquired in 35 contiguous oblique-axial slices (3 x 3 x 3 mm voxels) prescribed parallel to the AC-PC plane with a single shot gradient echo EPI sequence (TR = 2 s, TE = 25 ms, FOV = 192, flip angle 90, bandwidth = 2232 Hz/Px, echo spacing = 0.51) in three runs of the memory task and one run of the monetary reward task.
Data were preprocessed and analyzed using BrainVoyager QX (v2.3, Brain Innovation). Functional images were motion-corrected (six parameters), slice-timing corrected using a cubic spline interpolation, and spatially smoothed using a Gaussian kernel of 8 mm FWHM. Further, the data were temporally smoothed with voxelwise linear detrending and high-pass filtering of frequencies (three cycles per time course). The images were spatially normalized to the Talairach stereotaxic space (Talairach and Tournoux, 1988) in BrainVoyager.
fMRI Data Analysis
Functional data were analyzed using a whole brain random-effects general linear model (GLM). The memory task was modeled using two valence regressors representing positive and neutral trials during memory recall and a regressor representing missed trials (i.e., no valence rating given for the memory, 2.1% missed trials). The memory regressors begin at memory formation and end after elaboration, with this period defined by participants’ own button presses in each trial (for onset and conclusion of memory recall). The monetary reward task was modeled using two regressors representing gain and loss trials during the 2s outcome phase along with a regressor representing missed trials (no response, 3.0%). For both the memory and monetary reward tasks, regressors were convolved with a canonical double-gamma hemodynamic response function and six regressors for motion parameters were included in the model.
To correct for multiple comparisons, we used the cluster level statistical threshold plugin in Brain Voyager (Goebel et al., 2006) which uses Monte Carlo simulations in order to determine the likelihood of observing clusters of various sizes in a given map at a set threshold. After correction, the map automatically applies the minimum cluster size threshold that produces the desired cluster-level false-positive alpha rate (5% was chosen). We applied a voxel cluster threshold of 6 contiguous voxels (162mm3 as determined by the plugin) defined at a threshold of p<0.005 to obtain a corrected alpha < 0.05. We then performed 3 main analyses with the neuroimaging data.
1) Whole brain contrast of positive > neutral memories
We conducted a whole brain contrast between positive and neutral memories during memory recall to examine the effect of memory valence. From this simple contrast, we identified ROIs in regions previously implicated in reward processing, namely the right caudate nucleus in the striatum and the mPFC (for review see Haber and Knutson, 2010), and conducted follow-up analyses. First, we tested whether positive emotion (i.e. feeling ratings or emotional intensity ratings) modulated neural activity in the striatum during the recall of positive memories on a trial by trial basis. Specifically, we conducted two separate analyses that included subjective ratings (of either feeling or of emotional intensity during the recall task) as a parametric modulator for positive memory trials in the GLM (orthogonalized with respect to the original positive memory regressor). To test the relation of positive emotion to neural activity during recall, a t-test was conducted on striatum and prefrontal cortex ROI parameter estimates for the subjective feeling parametric modulation and the subjective emotional intensity parametric modulation. Second, we examined individual differences in neural activity during the recall of positive memories. In particular, we extracted parameter estimates from the striatum and prefrontal cortex ROIs defined by the whole brain contrast of positive > neutral memories and tested for correlation with self-reported scores of resiliency as measured with the Connor-Davidson Resiliency scale (CD-RISC; Connor and Davidson, 2003).
2) Mood improvement and memory valence ANOVA using striatum mask
We probed the relationship between neural activity during memory recall and mood change from beginning to the end of the autobiographical memory task (i.e. post-PANAS – pre-PANAS). Participants were grouped into mood-increase (n = 7) and mood-decrease (n = 9) groups, while participants with no mood change were excluded (n = 3). Based on a specific hypothesis that striatum activity underlies mood increases brought about by positive autobiographical memory recall we performed a Valence (positive/neutral) by Mood Change (mood-increase/mood-decrease) ANOVA within a striatum ROI. We used a non-biased structurally defined striatum mask (including putamen, caudate and nucleus accumbens using the Harvard-Oxford probabilistic atlas; Desikan et al., 2006) and applied a small volume correction (p < 0.05, corrected).
3) Monetary reward task contrast of Gains > Losses
We conducted a contrast of gain and loss outcomes in the monetary reward task to identify reward-related ROIs (see Table S2). The goal of this analysis was to confirm that a “reward-related” functionally defined ROI would show an independent effect of memory valence. We used the functionally defined reward ROIs in the striatum based on the contrast of gain > loss and ran a GLM using positive and neutral memory recall regressors to examine overlap in neural activity for positive autobiographical memory and a more tangible reward (i.e., money).
Behavioral Study
Participants
Thirty-two healthy individuals were recruited via the Rutgers psychology student subject pool to participate in a 2-day study (9 males, 23 females). Similar to the fMRI study, participants completed an autobiographical memory questionnaire on Day 1 and returned for Day 2 only if they met criteria for inclusion (see procedures for Day 1; 26 met criteria). The final sample included twenty-six participants (7 males, 19 females; mean age 20.0, SD = 2.98). Participants gave informed consent in accordance with the Rutgers University Institutional Review Board for the Protection of Human Subjects in Research.
Experimental design day 1: Autobiographical memory questionnaire
The first session followed the same procedure as the fMRI study except the autobiographical memory questionnaire included 84 (rather than 70) life events. Participants returned for Day 2 only if they described at least 35 positive and 35 neutral memories on their questionnaire. For each participant who met criteria, the 35 most positive (i.e. high intensity, high feeling) and 35 most neutral (i.e. low intensity, low feeling) memories were used in the memory choice task on Day 2.
Experimental design day 2: Memory choice task
Participants returned three days later to perform the autobiographical memory choice task. On each of 35 trials, participants made a choice between two options presented simultaneously: 1) recalling a positive memory or 2) recalling a neutral memory; both options were accompanied by a specified monetary payoff. The payoffs ranged from $0.01 to $0.04 and were presented in increments of $0.01 such that every combination of monetary payoffs was represented in option pairs (as in previous studies Hayden et al., 2007; Tamir and Mitchell, 2012). Thus, a participant’s choice of an option was based on a combination of preference for recalling the cued memory as well as preference for receiving the specified monetary payoff (in comparison to preference for the cued memory and monetary payoff in the other option). Similar to the fMRI study, participants then recalled the selected memory for 14s making button presses to indicate recall duration, followed by ratings of valence, emotional intensity, and feeling.
Behavioral Data Analysis
To measure the subjective value associated with choosing a positive over a neutral memory, we calculated the point of subjective equity (PSE) for each participant by fitting a cumulative normal distribution to their choice behavior (adapted from Deaner et al., 2005; Tamir and Mitchell, 2012). We first calculated the percentage of time participants chose a positive over a neutral memory for each of the monetary differences between those two options (i.e. relative payoffs of -$0.03, -$0.02, -$0.01, $0, +$0.01, +$0.02, and +$0.03). For each participant, a cumulative normal curve was fit to the resulting seven data points using the Matlab curve fitting toolbox. The PSE was calculated as the point where the cumulative normal distribution passed 50%. This represents the relative monetary value (gain or loss) when a participant was equally likely to choose a positive or neutral memory. PSE calculations were limited to the range of relative payoffs (i.e. -$0.03 to +$0.03).
Supplementary Material
Acknowledgments
This research was supported by funding from the National Institute on Drug Abuse to M.R.D (DA027764) and a National Science Foundation SBE Postdoctoral Research Fellowship to J.P.B. (1305994). We would like to thank Elizabeth Tricomi, Susan Ravizza and Dominic Fareri for helpful comments and discussion, and three anonymous reviewers for the constructive suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bluck S, Alea N, Habermas T, Rubin DC. A tale of three functions: Self-reported uses of autobiographical memory. Soc Cogn. 2005;23:91–117. [Google Scholar]
- Bower GH. Mood and memory. Am Psychol. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Carl JR, Soskin DP, Kerns C, Barlow DH. Positive emotion regulation in emotional disorders: a theoretical review. Clin Psychol Rev. 2013;33:343–360. doi: 10.1016/j.cpr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Influence of emotion on memory for temporal information. Emotion. 2005;5:503–507. doi: 10.1037/1528-3542.5.4.503. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Navrady L, Bird E, Hill E, Dunn BD, Golden A-M. Method-of-Loci as a Mnemonic Device to Facilitate Access to Self-Affirming Personal Memories for Individuals With Depression. Clin Psychol Sci. 2013;1:156–162. [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dunn EW, Gilbert DT, Wilson TD. If money doesn’t make you happy, then you probably aren’t spending it right. J Consum Psychol. 2011;21:115–125. [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc Biol Sci. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A Wandering Mind Is an Unhappy Mind. Science. 2010;(80):330–932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Gorman JM. A call to action: Overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhove MMP, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39:643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Martin LN, Delgado MR. The influence of emotion regulation on decision-making under risk. J Cogn Neurosci. 2011;23:2569–2581. doi: 10.1162/jocn.2011.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Beyond simple reinforcement learning: the computational neurobiology of reward-learning and valuation. Eur J Neurosci. 2012;35:987–990. doi: 10.1111/j.1460-9568.2012.08074.x. [DOI] [PubMed] [Google Scholar]
- Philippe FL, Lecours S, Beaulieu-Pelletier G. Resilience and positive emotions: examining the role of emotional memories. J Pers. 2009;77:139–175. doi: 10.1111/j.1467-6494.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- Piefke M. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Pizzagalli Da, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusting CL, Dehart T. Retrieving Positive Memories to Regulate Negative Mood: Consequences for Mood-Congruent Memory. J Pers Soc Psychol. 2000;78:737–752. doi: 10.1037//0022-3514.78.4.737. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Philippot P. Selective effects of emotion on the phenomenal characteristics of autobiographical memories. Memory. 2005;13:148–160. doi: 10.1080/09658210344000648. [DOI] [PubMed] [Google Scholar]
- Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75:380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Sharot T, De Martino B, Dolan RJ. How choice reveals and shapes expected hedonic outcome. J Neurosci. 2009;29:3760–3765. doi: 10.1523/JNEUROSCI.4972-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Percept Mot Skills. 2001;92:857–868. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Talarico JM, LaBar KS, Rubin DC. Emotional intensity predicts autobiographical memory experience. Mem Cognit. 2004;32:1118–1132. doi: 10.3758/bf03196886. [DOI] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci U S A. 2012;109:8038–8043. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist Ma, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. Eur J Soc Psychol. 1996;26:557–580. [Google Scholar]
- Williams J, Scott J. Autobiographical memory in depression. Psychol Med. 1988;18:689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathé Aa. Understanding resilience. Front Behav Neurosci. 2013;7:1–15. doi: 10.3389/fnbeh.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Bellgowan PSF, Bodurka J, Drevets WC. Behavioral and Neurophysiological Correlates of Autobiographical Memory Deficits in Patients With Depression and Individuals at High Risk for Depression. JAMA Psychiatry. 2013:1–10. doi: 10.1001/jamapsychiatry.2013.1189. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Howell RT. Do time perspectives predict unique variance in life satisfaction beyond personality traits? Pers Individ Dif. 2011;50:1261–1266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


