Abstract
Atopic dermatitis (AD) is a chronic, relapsing, highly pruritic skin condition resulting from disruption of the epithelial barrier and associated immune dysregulation in the skin of genetically predisposed hosts. AD generally develops in early childhood, has a characteristic age-dependent distribution and is commonly associated with elevated IgE, peripheral eosinophilia and other allergic diseases. Staphylococcus aureus colonization is common and may contribute to disease progression and severity. Targeted therapies to restore both impaired skin barrier and control inflammation are required for optimal outcomes for patients with moderate to severe disease. Pruritus is universal among patients with AD and has a dominant impact on diminishing quality of life. Medications such as anti-histamines have demonstrated poor efficacy in controlling AD-associated itch. Education of patients regarding the primary underlying defects and provision of a comprehensive skin care plan is essential for disease maintenance and management of flares.
Keywords: atopic dermatitis, eczema, allergy, Netherton syndrome, hyper-IgE syndrome
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, and highly pruritic dermatitis, which generally develops in early childhood, and has a characteristic age-dependent distribution. AD is relatively common, affecting 10-20% of children in developed countries.(1) Patients with AD commonly have elevated total IgE levels, sometimes markedly elevated, the level of which appears to correlate with disease severity.(2) AD patients can also have elevated allergen-specific IgE levels, indicating sensitization, but not necessarily clinical allergy, an area of great confusion for patient management, particularly in regard to food allergy.(3) The major medical co-morbidities associated with AD are infections, including Staphylococcus aureus superinfection and eczema herpeticum; however, chronic pruritus and sleep loss, as well as the time and expense associated with treatment, are often most distressing for patients and families. AD has been associated with poor school performance, poor self-esteem, and family dysfunction.(4-7)
The causes of AD are still poorly understood, although genetic predisposition in the setting of inciting environmental factors appears critical. Similar to asthma and other complex, chronic disorders, AD should be viewed as a common end manifestation of many different genetic defects, resulting in impaired epidermal barrier function and immune dysregulation. Additional identification and characterization of genetic defects among patients with AD is needed; this may lead to better characterization of the disease and the development of more effective therapies.
For now, management is based on targeting the known defects in AD, namely skin barrier dysfunction and cutaneous inflammation, along with treatment (in some cases, prophylactically) of associated infections. The pruritus associated with AD is often the most distressing symptom and is treated with skin hydration and topical anti-inflammatories, but is poorly responsive to antihistamines in most patients. Behavioral interventions, such as biofeedback and relaxation techniques, can also be helpful in controlling scratching. Although a comprehensive treatment plan with extensive education is effective is controlling atopic dermatitis in most patients, better treatments are needed, particularly disease-modifying therapies that can be initiated in early childhood.
Clinical Features
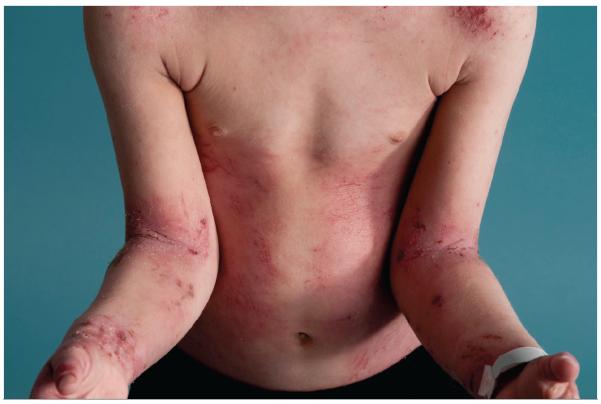
Atopic dermatitis is characterized by a chronic, relapsing dermatitis that is pruritic, begins in the first 5 years of life in 90% of patients (but not in the first weeks of life, as seen in the autosomal dominant hyper-IgE syndrome), and usually presents in a characteristic age-dependent distribution with facial, scalp, and extensor involvement in infants and young children, and predominant flexural involvement in older children and adults. Pruritus is universal and xerosis is a common feature in children with atopic dermatitis. Acute lesions are characterized by pruritic papules with erythema, excoriations and serous exudate, while chronic AD is characterized by areas of lichenification and fibrotic nodules, often accompanied by acute lesions. (Figure 1)
Fig 1.
Typical distribution of skin lesions in a child with atopic dermatitis.
Since pathognomonic lesions are not present to definitively diagnose atopic dermatitis, diagnostic criteria have been described; the most widely cited being the ‘Hanifin and Rajka’ criteria(8) and subsequent modifications, including the UK Working Party’s Diagnostic Criteria for Atopic Dermatitis.(9) (Box 1) Five major clinical features based on these criteria are: (1) pruritus; (2) a chronic, relapsing course; (3) typical distribution; (4) family or personal history of atopy; (5) onset before 2 years of age. In addition, associated minor criteria are frequently observed in patients with AD and aid in diagnosis.
Box 1. Clinical features of atopic dermatitis.
Major features
Pruritus
Characteristic morphology and distribution:
Facial and extensor involvement in infants and children; flexural involvement with lichenification in adults
Chronic or chronic, relapsing course
Personal or family history of atopy, including asthma, allergic rhinitis, atopic dermatitis
Minor features
Early age of onset
Xerosis
Palmar hyperlinearity, ichthyosis, keratosis pilaris
Immediate skin test reactivity, elevated serum IgE
Cutaneous infection, including Staphylococcus aureus and Herpes simplex virus
Nipple eczema
Cheilitis
Pityriasis alba
White dermatographism, delayed blanching
Perifollicular accentuation
Anterior subcapsular cataracts
Itch when sweating
Non-specific hand or foot dermatitis
Recurrent conjunctivitis
Dennie-Morgan folds
Keratoconus
Facial erythema or pallor
Data from Hanifin, JM, Rajka, G. “Diagnostic features of atopic dermatitis”. Acta Dermatovener (Stockholm) Suppl 92:44-7. 1980.
Common triggers for flares include heat, sweating, anxiety, frustration, and infections. Additionally, in a subset of patients with moderate to severe disease refractory to standard therapy, food allergy may play a role in exacerbations, particularly in younger children.(10) Testing for food allergy in children should be limited due to the low positive predictive value of both skin testing and in vitro serum assays for allergen-specific IgE.(11) Food allergy appears to be greatly overdiagnosed in children with AD, so elimination diets should be approached cautiously to avoid unnecessary restrictions.(3) Likewise blind panel food allergy testing or avoidance of foods in the absence of a history suggestive of a food-specific IgE-mediated reaction is not recommended.
Infectious complications
Staphylococcus aureus colonization is common in patients with atopic dermatitis, affecting >90% of AD patients, and the density of S. aureus on the skin correlates directly with AD severity.(12, 13) Patients with severe AD, even in the absence of clear signs of infection, may improve with antibiotics.(14, 15) Clinical signs of S. aureus infection requiring treatment with topical or systemic antibiotics include honey-colored crusting, pustules, and folliculitis. Colonization of the nares with S. aureus and transmission with hands may be an important reservoir for cutaneous S. aureus colonization.(16) In addition, S. aureus strains isolated from children with AD and their parents are identical based on pulse field electrophoresis, suggesting intra-familial transmission is a source of recolonization following antibiotic treatment.(17) Increased colonization rates in children with AD have been observed and may be related to skin barrier disruption; exposed binding sites for the bacteria in the extracellular matrix, disruptions in innate immunity, and cellular immune dysfunction with predominant Th2 responses likely contribute.(18, 19) Specific IgE to staphylococcal enterotoxin has also been found in sera of AD patients and is associated with disease severity in children. (20, 21)
Viral infections that occur in patients with AD include eczema herpeticum, eczema coxsackium, eczema vaccinatum, and molluscum contagiosum. Eczema herpeticum (EH) results from dissemination of herpes simplex virus (HSV-1 or HSV-2) in AD patients commonly with the first exposure, and is characterized by punched-out erosions and vesicles, occasionally complicated by secondary infection with staphylococcal or streptococcal species. Fever, lymphadenopathy and malaise are common with EH. EH can be severe with keratoconjunctivitis and multi-organ involvement leading to fatality, and requires prompt diagnosis and initiation of anti-viral medication. Risk factors for EH include early-onset and severe AD, marked elevations in total IgE, elevated allergen-specific IgE levels, peripheral eosinophilia, and the presence of filaggrin mutations.(22) Disseminated coxsackie A6 viral infections with eruptions at sites of AD lesions have recently been described, and termed ‘eczema coxsackium’, but data on this infectious complication are limited.(23) For patients with AD, even with quiescent disease, smallpox vaccination or contact with persons vaccinated with smallpox can result in the potentially fatal complication of eczema vaccinatum from dissemination and poor immune control of the virus.(24)
Differential Diagnosis
A number of diseases may present with eczematous rashes and can be misdiagnosed as AD. (Box 2) Several primary immunodeficiency diseases exist with prominent allergic inflammation, elevated total IgE levels, eosinophilia, and an eczematous rash that can be mistaken for typical AD. The autosomal-dominant hyper-IgE syndrome presents with an eczematous rash in the first weeks of life, which is atypical for atopic dermatitis (25). DOCK8 deficiency, which is a combined immunodeficiency with prominent cutaneous viral susceptibility, is associated with rash that is usually more dramatic than that seen in typical AD (26). PGM3 deficiency, a congenital disorder of glycosylation resulting in a hyper-IgE phenotype with unusual features including neurologic and skeletal anomalies and increased circulating Th17 cells can also present with an eczematous-like rash (27). Wiskott-Aldrich syndrome, in which the dermatitis is variable, is characterized by thrombocytopenia with small platelets (28). IPEX syndrome presents in the first weeks of life with both prominent allergic inflammation and dermatitis, as well as autoimmune diseases (29). Netherton syndrome is a genetic disease affecting primarily the barrier function of the skin and is characterized by ichthyosis, diffuse erythroderma, severe atopy with elevated total IgE levels and eosinophilia, and trichorrhexis invaginatum.(30) Finally, genetic defects in corneodesmosin and desmoglein-1 have also been reported and are associated with eczematous dermatitis, elevated IgE levels, and clinical allergic disease.(31, 32)
Box 2. Differential diagnosis for atopic dermatitis.
Contact dermatitis
Seborrheic dermatitis
Drug reactions
Infantile psoriasis
Scabies
Nutritional deficiencies – zinc/biotin
Acrodermatitis enteropathica
Netherton syndrome
Icthyosis vulgaris
Peeling skin disorder, type B
Severe dermatitis, multiple allergies and metabolic wasting (SAM) syndrome
Primary immunodeficiency diseases* & Omenn syndrome
Lymphocytic-variant Hypereosinophilic syndrome (HES)
Cutaneous T cell lymphoma
With all of these disorders, although diffuse eczematous dermatitis, elevated IgE levels, eosinophilia, and allergic diseases are present, there are distinguishing ‘syndromic’ features characteristic of each disease that may aid in diagnosis. (Box 2) Severe and extensive skin involvement, particularly beginning near or at birth, may suggest the presence of a genetic cause for disease. Salient associated features usually necessitating additional clinical investigation include: recurrent or severe infections – particularly recurrent abscesses, lymphadenitis, or pneumonia, late onset of disease (after second decade of life), absence of concomitant allergic disease, and persistent blood eosinophilia (absolute eosinophil count >1000 cells/μL) particularly in the setting of appropriate skin care.
Clonal diseases, including cutaneous T cell lymphoma and lymphocytic variant hypereosinophilic syndrome, should be considered in patients presenting with diffuse eczematous rashes after 5 years of age, but these are uncommon in children.
Other considerations in the differential diagnosis include: contact dermatitis, particularly in older children presenting with eczematous rashes that begin in late childhood and adolescence; nutritional deficiencies; scabies, which can be distinguished by a characteristic distribution; seborrheic dermatitis; psoriasis.
Pathophysiology
Atopic dermatitis (AD) is associated with both disruption of the epithelial barrier of the skin and allergic inflammation in the skin of hosts whose genetic background results in a predisposition to atopy. Atopic dermatitis, along with food allergy present in the first years of life and are the initial steps in the ‘atopic march’. Broad environmental modifiers that are poorly characterized appear to be critical for the development of atopic dermatitis in genetically susceptible children. This section will review defects in pathways that are fundamental to AD development in humans, including barrier disruption and immune dysregulation. The focus is to reveal patterns in the early pathogenesis of AD that guide treatment strategies and that suggest targets for novel therapeutics.
Genetics
Twin concordance studies demonstrate that genetic factors are the primary determinant for the development of atopic dermatitis, with estimates for genetic contribution to disease being approximately 80%.(33, 34) Despite these findings, a common defective pathway which gives rise to the clinical phenotype of AD in the human host has not been identified. Genome-wide association studies have identified a number of genetic susceptibility loci in patients with AD. Many susceptibility loci enriched within the AD population are within or near genes critical to innate immunity, Th2-mediated inflammation, and skin barrier function, highlighting the importance of these pathways in AD pathogenesis.(34)
A small number of heritable genetic atopic syndromes, which are included in the differential diagnosis of common AD, have been characterized that have shed additional light on the pathogenesis of the disease. (Table 1) Loss of skin barrier integrity appears essential for the development of AD; several genetic diseases affecting skin barrier function have been described, including Netherton syndrome and Peeling skin syndrome, type b, all of which have features of allergic inflammation. In concert with barrier dysfunction, virtually every lineage of immune cell has an implicated role in the immunopathogenesis of AD, with certain populations such as CD4+ T cells clearly being necessary for disease development.
Table 1.
Heritable genetic syndromes resulting in atopic dermatitis
| Syndrome | Gene | Primary Defect | Common distinguishing features | |
|---|---|---|---|---|
| Immune Pathway Defects | Autosomal dominant hyper-IgE syndrome |
STAT3 | Abnormal cytokine signaling |
Bacterial pneumonias; lung bullae/bronchiectasis; absent TH17 |
| Autosomal recessive hyper-IgE syndromes |
DOCK8 | Cytoskeletal dysfunction | Cutaneous viral susceptibility; progressive combined immunodeficiency |
|
| PGM3 | Abnormal glycosylation | Marked neurologic impairment; reduced branching glycans; leukopenia; increased TH17 |
||
| Wiskott-Aldrich syndrome |
WAS | Cytoskeletal dysfunction | X-linked; thrombocytopenia; progressive combined immunodeficiency |
|
| IPEX syndrome | FOXP3 | Absent Tregs | Endocrine abnormalities; chronic diarrhea | |
| Omenn syndrome | RAG1/21 | Lymphopenia; oligoclonal T cells |
“leaky” SCID; oligoclonal Tcell expansion; thymus present | |
| Atypical Complete DiGeorge2 |
del22q11.2 | Lymphopenia; oligoclonal T cells |
“leaky” SCID; velo-cardio-facial abnormalities; oligoclonal Tcell expansion; absent thymus |
|
|
|
||||
| Skin Barrier Defects | Icthyosis vulgaris | FLG | Impaired skin hydration and barrier maintenance |
Palmar hyperlinearity; keratosis pilaris |
| Netherton Syndrome | SPINK5 | Inappropriate protease activation |
Erythroderma; icthyosis; trichorrhexis invaginatum (“bamboo hair”) |
|
| Peeling skin syndrome type B |
CDSN | Impaired intercellular adhesion |
Erythroderma; icthyosis; thin/fine hair | |
| SAM syndrome | DSG1 | Impaired intercellular adhesion |
Erythroderma; hypotrichosis; growth retardation due to metabolic disturbance |
|
IPEX - immune dysregulation, polyendocrinopathy, enteropathy, X linked; SAM - severe dermatitis, multiple allergies and metabolic wasting; SCID - severe combined immunodeficiency; 1Multiple hypomorphic mutations leading to a “leaky” – presence of a few T cells – SCID phenotype may result in Omenn syndrome. 2Atypical Complete DiGeorge is due to a chromosomal deletion, not a single genetic mutation; it also has a “leaky” phenotype.
Barrier Defects in the Skin
A number of proteins contribute to the structure and primary function of the skin as a barrier – preventing water loss and impeding the penetration of irritants, immunogens, and pathogens, most notably Staphylococcus aureus. Damaging mutations in genes critical to normal barrier function of the skin have been shown to strongly segregate with early and severe forms of atopic dermatitis and icthyosis, usually associated with elevations in total serum IgE levels. Among these are mutations in filaggrin (FLG), desmoglein-1 (DSG1), corneodesmosin (CDSN) and serine protease inhibitor Kazal-type 5 (SPINK5). (Box 2)
Skin barrier function and filaggrin (FLG)
Filaggrin is a polyfunctional protein, present in the epidermis, which undergoes complex proteolytic processing during normal desquamation. This processing contributes to the numerous roles FLG has in skin barrier maintenance during its life cycle, and terminates in the release of Natural Moisturizing Factor (NMF) which contributes to water retention and skin hydration.(35) In the last decade, several loss-of-function mutations in FLG have been shown to result in deficiency of the protein, reduced levels of NMF, and severe early-onset atopic dermatitis and icthyosis, with marked elevations of total IgE levels.(36, 37) In addition to its effects on the structure and function of skin, FLG haploinsufficiency may contribute to AD pathogenesis in multiple additional ways including: effects on skin pH, promotion of pro-inflammatory cytokine expression, and unimpeded growth of Staphylocccus aureus.(38-40) FLG mutations also convey major risk for development of peanut allergy as well as asthma, with the latter only occurring among patients with co-morbid AD, supporting a role for epicutaneous sensitization in systemic atopic disease.(41)
The corneodesmosome: corneodesmosin (CDSN) and desmoglein-1 (DSG1) deficiencies
Skin barrier integrity is dependent upon intact cell-cell adhesion and resistance of shearing forces. The desmosome is a junctional protein complex in skin that acts as an anchor for the keratin cytoskeleton and facilitates cell-cell adhesion, resisting mechanical stress.(42, 43) Mutations in two desmosome proteins in human skin – desmoglein-1 and corneodesmosin – have been found to cause severe AD. Complete loss of corneodesmosin expression due to mutations in CDSN result in Peeling skin syndrome, type B, a diffusely icthyotic and erythrodermic skin condition with associated severe pruritus and atopy.(31) Genetic deficiency of desmoglein-1 results in severe dermatitis, multiple allergies and metabolic wasting (SAM) syndrome, associated with elevated IgE levels.(32) Of note, autoantibodies directed at desmoglein-1 in skin result in the bullous disease Pemphigus foliaceous (PF). Remarkably, despite a similar skin defect, atopy is not reported as a common feature of PF.(44)
Protease activity in the skin and Netherton syndrome
During normal desquamation, desmosomes must be processed and degraded by proteases in a regulated manner near the skin surface. Lympho-epithelial Kazal-type-related inhibitor (LEKTI) encoded by the serine protease inhibitor Kazal-type 5 (SPINK5) gene is a major inhibitor of endogenous skin proteases, and under low pH, releases kallikrein family proteases (KLK5, KLK7, KLK14) to facilitate normal exfoliation.(45) In this way, the epidermal pH gradient is able to limit extensive proteolysis to the outermost layers of epidermis. (Figure 2b) Damaging autosomal recessive mutations in SPINK5 lead to loss of LEKTI and enhanced serine protease activity deep in the epidermis with increased desmosome destruction and increased skin permeability. This is clinically manifest as Netherton syndrome, characterized by generalized erythroderma, icthyosis, trichorrhexis invaginatum (bamboo hair), elevated IgE and atopy.(30) Genetic variants in KLK7 have also been reported in association with atopic dermatitis.(46)
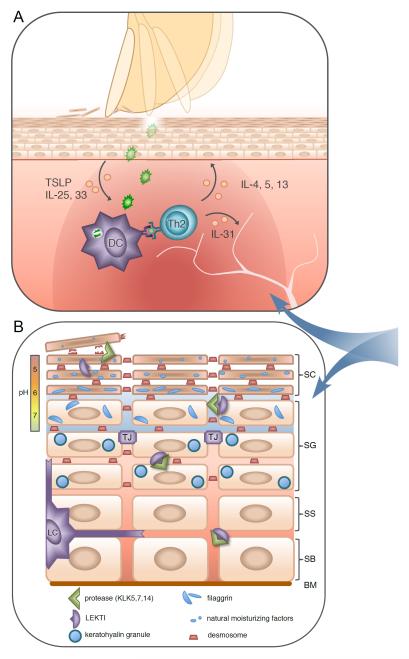
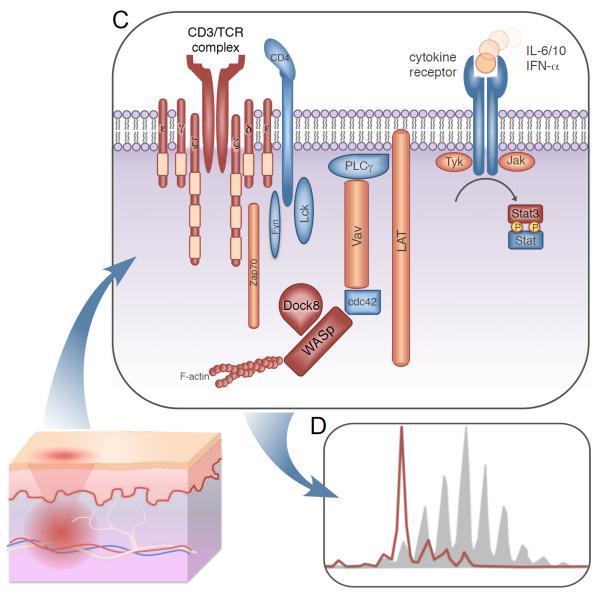
Fig 2. Defective pathways contributing to the pathogenesis of atopic dermatitis.
(A) Intense pruritus develops in lesional skin; defective barrier function leads to immune activation by irritants and microbes, with IL-25, IL-33, and TSLP contributing to Th2-mediated inflammation. IL-4 and IL-13 induce class switching to IgE, and further impair skin barrier function by reducing both filaggrin and anti-microbial peptide expression. Highly pruritogenic IL-31 is also elaborated leading to additional excoriation, further skin barrier degradation and immune activation, thus potentiating disease.
(B) Mutations in several proteins expressed in the epidermis result in severe atopic dermatitis; these include filaggrin (FLG), lympho-epithelial Kazal-type-related inhibitor (LEKTI), as well as corneodesmosin and desmoglein-1 (components of desmosomes). FLG is critical to skin barrier integrity and hydration. Inactive profilaggrin polymers exist inside keratohyalin granules within the stratum granulosum (SG) layer. As epithelial cells transition into the stratum corneum (SC), adjacent proteases (eg. KLK5) are released by LEKTI in a pH dependent manner, acting on these granules to yield FLG monomers which contribute to skin barrier function, as well as on desmosomes to facilitate normal exfoliation. Loss of LEKTI function results in inappropriate protease activity deep within the SG resulting in the severe skin barrier defect characteristic of Netherton syndrome. Similar disease results from absence of proteins necessary for desmosome function. Further proteolysis of FLG releases small hygroscopic molecules and free amino acids that comprise Natural Moisturizing Factor (NMF). Decreased FLG results from null mutations or Th2 mediated inflammation, and leads to impaired skin hydration, dysregulated pH, and barrier dysfunction. SS – stratum spinosum; SB – stratum basale; BM – basement membrane; TJ – tight junction; LC – Langerhans cell.
(C) Multiple heritable immune defects lead to AD; defective proteins or pathways are colored in red. Genetic defects leading to severe limitation of CD3/T cell receptor (TCR) diversity may result in AD. Loss of TCR diversity may also contribute to the prominent AD phenotypes observed in Wiskott-Aldrich syndrome protein (WASp) defects. Defects in Stat3 and Dock8 pathways lead to the autosomal dominant and recessive forms of hyper-IgE syndromes (HIES) characterized by allergy and eczema.
(D) Representative spectratype of a patient with oligoclonal T cell expansion (red) compared to a normal polyclonal TCR repertoire (shaded); typical of abnormality seen in Omenn syndrome or Atypical Complete DiGeorge.
Innate immune contributions to AD
Innate immune cells play a major role in the immunopathogenesis of AD. (Figure 2a) Once a defect in the skin barrier is established, dendritic cells (DC) and denuded epithelium are exposed to exogenous irritants, danger signals, and pathogens. This leads to dendritic cell (DC) and keratinocyte activation via innate immune receptor ligation by damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). In the genetically susceptible host, activated epithelial cells prime DCs to promote Th2 programs through elaboration of TSLP, IL-33, and IL-25.(47) Intense pruritus associated with AD lesions may lead to further activation through mechanical epithelial disruption by vigorous scratching, and promote disease progression. Numerous inherited immunodeficiency diseases exist that are caused by innate immune defects. However, atopy has not been well characterized among these syndromes. Despite this, multiple susceptibility loci have been identified within innate immune receptors which are over-represented among atopic individuals, some segregating with more severe AD phenotypes.(34) For example, single nucleotide polymorphisms (SNPs) have been reported in toll-like receptor-2 (TLR-2), which appear to result in loss of function and impaired sensing of staphylococcus. One SNP R753Q is thought to impair host defense and increase staphylococcal colonization; it is found in over 10% of adult AD patients and segregates with more severe disease.(48)
Discrete immune pathway defects leading to AD
Despite clear evidence that intrinsic defects in skin barrier function predispose to an early and often severe AD, additional factors appear to be necessary for AD development. A significant number of individuals carrying null FLG alleles fail to develop AD.(49) Additionally, patients with pemphigus foliaceous have not been reported to have increased allergic sensitization or disease, despite their skin barrier defect.(44) Recent data generated from whole exome sequencing have led to the revelation that defects in more than one known disease-causing gene among individuals with primary immunodeficiency diseases may occur more frequently than predicted.(50) One can speculate that hypomorphic mutations or SNPs with functional consequences in genes critical to immune function, particularly Th2-mediated inflammation, might provide the necessary background for skin barrier defects to manifest as AD. We will review several discrete genetic defects resulting in immune dysregulation, AD, and allergic disease, which illuminate mechanisms relevant to AD pathogenesis. (Box 2)
Hyper-IgE Syndromes
Common among patients with atopic dermatitis is an increase in IgE, sometimes to very high levels. This increase results from Th2-mediated skin inflammation, and IgE levels significantly decrease with improved control of skin inflammation. Patients with a series of genetic defects resulting in elevations of IgE (commonly called hyper-IgE syndromes, or HIES) – resulting from dominant negative mutations in STAT3, as well as hypomorphic autosomal recessive mutations in DOCK8 or PGM3 – all have AD as a major feature of their clinical phenotypes.(25-27) Despite the common finding of AD, the genetic causes of HIES affect remarkably disparate pathways; one affecting cytokine receptor signaling, one cellular cytoskeletal rearrangement, and one global cellular glycosylation patterns. Mechanisms underlying allergic phenotypes among forms of HIES remain an area of active investigation; however, immune dysregulation resulting in enhanced Th2-mediated inflammation appears common.
Effect of Th2 inflammation on skin barrier function
While barrier defects appear to require immunologic factors to manifest as AD, exuberant Th2 inflammation may itself cause defective skin barrier integrity. FLG can be directly modulated by the Th2 cytokines, IL-13 and IL-4.(51) Additionally, IL-10, IL-4, and IL-13 have been shown to reduce anti-microbial peptide (AMP) expression in keratinocytes, and patients with AD have been shown to have a relative deficiency in AMP production.(19, 52, 53) This may contribute to staphylococcal colonization, increased risk for viral and bacterial superinfections, and increased disease severity.
Impaired CD4+ T cell repertoire and Tregs
CD4+ T cells are essential to development of AD. Diversity of T cell receptors (TCR) has been estimated at 2.5×107 and represents the repertoire human CD4+ T cells have to recognize non-self and inform adaptive immune responses.(54) When this diversity is limited, such as seen in pediatric patients with HIV following immune reconstitution, an increased incidence of atopic dermatitis is observed.(55) More severe repertoire deficits may result from hypomorphic genetic mutations in genes such as IL2RG, JAK3, or RAG, in which patients have a “leaky” phenotype – so-called because a few T cells are present.(56) In this setting massive expansion of one or a few clonal populations may occur leading to the striking phenotype of Omenn syndrome characterized by erythroderma, severe eczema, high IgE, and eosinophilia frequently from birth.(57) Atypical complete DiGeorge represents a similar “leaky” process and shares many clinical features with Omenn syndrome, including severe atopic dermatitis.(58) A reduction in TCR diversity can also be seen in progressive combined immunodeficiencies such as DOCK8 deficiency and Wiskott-Aldrich syndrome, which are both associated with moderate to severe AD.(59, 60) (Figure 2c,d)
How impaired TCR diversity may result in atopic dermatitis remains unproven. However, evidence suggests that a loss of CD25brightCD127negFoxP3+CD4+ regulatory T cell (Treg) diversity may contribute to immunopathogenesis.(28) Primary support for this hypothesis comes from Immunodysregulation Polyendocrinopathy Enteropathy X-linked (IPEX) syndrome due to FoxP3 deficiency, in which patients have a selective loss of Tregs.(29) Affected individuals present with severe atopic dermatitis, high IgE, and eosinophilia, in addition to diarrhea and autoimmunity. WAS patients have also been shown to have specific Treg functional defects that likely contribute to their clinical phenotype, which includes AD and allergic disease.(61) Lastly, eczematous dermatitis and high IgE are both prominent features of acute graft-versus-host disease (aGvHD).(62) While the pathogenesis of aGvHD is complex and results from inappropriate and deleterious alloreactivity of donor T cells, reduced TCR diversity and oligoclonality are observed, with a specific loss of Tregs.(63, 64) Remarkably, donor Treg infusion has been shown to prevent development of eczema and the other clinical features of aGvHD.(65)
Physiology of pruritus and IL31RA/OSMR complex mutations
Intense pruritus is a hallmark of AD lesions. While complete discussion of the neurologic, physical, and immunologic contributions to itch are beyond the scope of this review, skin excoriation due to chronic and severe pruritus contributes to progression of skin lesions and promotes super-infection. Unlike allergic rhinitis or urticaria, histamine receptors 1 and 2 do not appear to be significant mediators of pruritus in AD.(66)
IL-31 is a cytokine expressed by Th2 cells and is a strong pruritogen.(67) (Figure 2a) IL-31 signals through a cognate heterodimeric receptor complex consisting of IL-31 receptor alpha (IL31RA) and oncostatin M receptor beta (OSMR). Increased IL-31 expression has been observed in AD lesions, and injection of IL-31 causes intense pruritus.(67, 68) Mutations in both OSMR and IL31RA result in familial primary localized cutaneous amyloidosis, a syndrome characterized by severe cutaneous pruritus.(69, 70) Despite chronic excoriation resulting in some features consistent with chronic AD lesions, there is no report of increased IgE or atopy within this population.
Hyperalgesia via nociceptive pathways has also been implicated in multiple animal models of pruritus. Recently, human sensory neurons have been shown to express IL-31RA and signal following exposure to IL-31 in vivo and in vitro; this process appears to be transient receptor channel potential cation channel ankyrin subtype 1 (TRPA1)-dependent in murine models.(71) Interestingly, TRPA1 is necessary for both histamine-independent itch mediated by bradykinin and sensing of noxious environmental irritants.(72, 73)
Chronic Management
General Approach
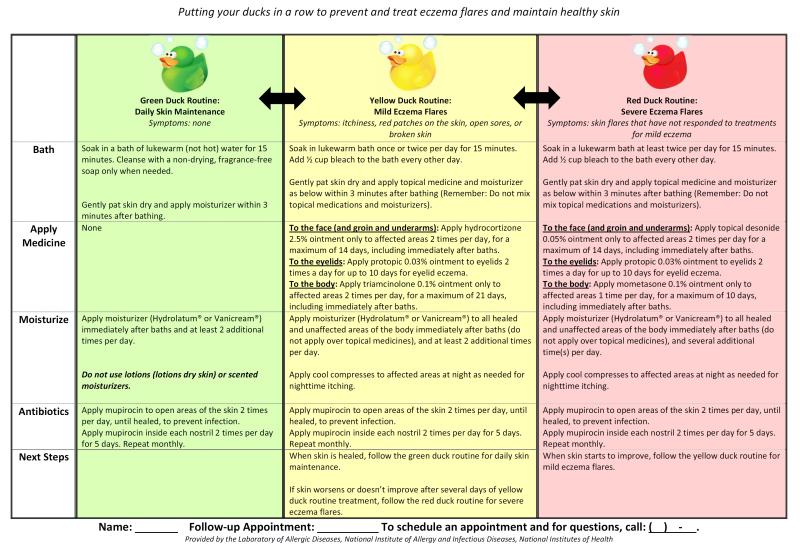
The goals of AD management are to improve quality of life and prevent infectious complications, while minimizing potential medication side effects. Optimal control of all aspects of AD morbidity, including pruritus, is best achieved through hydration, restoration of the skin barrier and control of skin inflammation. Since AD is a chronic, relapsing disorder with flares occurring at variable intervals, a comprehensive home treatment plan is critical to successful management, including steps to manage an acute flare. Chronic management of AD requires extensive patient education on the clinical features and associations of the disorder, its natural history, review of potential triggers for disease flares, discussion of medications and potential side effects, and provision of an individualized and comprehensive treatment plan that is based on the underlying pathophysiology. (Figure 3) Treatment plans should be directed at underlying defects: skin hydration and emollients to address barrier dysfunction and topical (or rarely systemic immunosuppressants) to quell skin inflammation. Anti-microbials should also be included in patients with recurrent infection, and methods to reduce exposure to potential triggers should be addressed. Compulsive attention to the details of the treatment plan, regular follow-up for adjustment of treatment plans for moderate to severe cases, and extensive education at each visit are critical for successful management. Multidisciplinary treatments teams are helpful in managing moderate to severe cases.(74)
Fig 3. Example of eczema management plan.
Provided by the Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Hydration and use of occlusive topical moisturizers
As has been discussed, disruption of the skin barrier is a central feature of AD, leading to transepidermal water loss and xerosis.(75) Diminished levels of ceramide observed in AD skin reduce water-binding capacity and potentiate this problem.(76, 77) Treatment guidelines recommend regular skin hydration with soaking baths and use of occlusive topical ointments and creams for optimal control. This strategy has demonstrated efficacy in reducing the requirement for topical and systemic immunosuppressants (corticosteroid-sparing effect). (10, 78, 79) While increased use of topical occlusive treatments is associated with improved AD control, (80) the optimal regimen for use of topical occlusives has not been extensively studied.(81) In our experience, regular, daily soaking baths of 15 minutes in lukewarm plain water, with immediate application of occlusive ointments (soak-and-seal), (82) and application of occlusive treatments through the day, are the most important aspects of treatment. For facial and neck involvement, a towel gently draped over the head and neck can be used in the bath to assist with hydration. Frequency of baths can be increased to two or three times daily during severe flares. Showers are not as effective at hydrating the skin and are not a satisfactory alternative to soaking baths in moderate to severe cases.
Application of emollients to dry skin immediately after soaking baths functions to create a barrier impeding water loss, restoring the stratum corneum, reducing the requirement for topical corticosteroids to control flares, and reducing pruritus.(81, 83, 84) Occlusion is the primary desired function of emollients, with ointments being more effective than creams. Lotions are not effective in atopic dermatitis; high water content leads to evaporative drying and irritants, such as fragrances and preservatives, may irritate or inflame non-intact skin. While ointments, such as petroleum jelly or hydrated petrolatum, are more occlusive than creams and generally more effective, ointments may be poorly tolerated in some patients and can lead to occlusive folliculitis. Creams are frequently better tolerated during hot, humid days and during school, thus promoting compliance. There is no clear evidence that the newer ceramide-containing creams improve patient outcomes. In general, we provide both an ointment and a cream and educate patients on the relative benefits of each.
Topical corticosteroids
To address the inflammatory component of atopic dermatitis, topical corticosteroids are the most effective treatment, used on an as needed basis to treat acute flares, and in more severe cases, to maintain control. Topical corticosteroids diminish inflammation, pruritus, and Staphylococcus aureus colonization of the skin. Particularly in children, great care must be taken to balance use of the lowest potency topical corticosteroid necessary to achieve control in order to minimize potential side effects, while not undertreating the inflammation. For moderate to severe atopic dermatitis, instruction for stepwise adjustments in the topical corticosteroid potency to be used, based on severity of flare and the area of the body involved, should be included in the comprehensive eczema action plan. (Figure 3) When mid- to high-potency corticosteroids are used for flares, step-wise decreases in potency are necessary to prevent rebound exacerbations. Education of patients and their families on the relative strengths of topical corticosteroids prescribed, potential systemic and local side effects, and strategies for dose adjustments is critical to treatment success. Any concerns of ‘steroid phobia’ that may limit compliance should be openly discussed. As previously noted, aggressive skin hydration and use of emollients is steroid-sparing and should be emphasized in discussions with families.
Topical corticosteroids are available in a wide range of potencies, from the least potent Group 1 preparations (e.g. hydrocortisone 1% ointment), to the most potent Group 7 preparations (e.g. clobetasol propionate 0.05% ointment). The greater the potency of topical corticosteroid used, the greater the risk of systemic and local side effects, particularly when used on large areas of the body over extended periods. For each corticosteroid, there are multiple vehicles, including ointments, creams and lotions. Due to differences in level of occlusion, the vehicle affects potency through degree of absorption; for a given topical corticosteroid, ointments are more potent than creams, which are more potent than lotions. In general, ointments are preferred since they provide a more occlusive barrier for maintaining skin hydration and promote better absorption of the corticosteroid. Ointments also contain fewer preservatives.
Lower potency topical corticosteroids should be used for areas on the body with thinner skin, greater chance for absorption, and higher risk for local side effects. These include the face, eyelids, genitalia and intertriginous areas. For other areas of the body, short courses of higher potency corticosteroids may be needed to achieve control of flares. Choice of topical corticosteroid is based on the severity of disease, distribution, and age of patient. For infants and toddlers, lower potency corticosteroids should be used for all areas of the skin. Of note, fluticasone 0.05% cream is approved for short-term use in children ≥3 months and mometasone cream and ointment are approved for children ≥2 years old; both can be used once daily as needed for flares.
Discussion of the details of application of the topical corticosteroids and topical emollients to the skin is key to successful outcomes. The topical corticosteroid should be applied to areas of flare first, with application of a thin layer. Topical emollient should be applied second in a thick layer to all unaffected areas of skin, avoiding application over areas with topical steroid. Application of emollient over the areas of topical corticosteroid application dilutes the corticosteroid and unnecessarily spreads it to unaffected areas of skin. Particularly in young children, there is a tendency to vigorously rub in both the topical steroid and emollient; rubbing in should be avoided. Provision of sufficient amounts of topical corticosteroids is also important, with attention to the severity and extent of disease.
Local side effects include atrophy, striae, acne, telangiectasias, and secondary infections. The major systemic side effect is adrenal suppression, the risk of which is greater with higher potency topical corticosteroids and with use of occlusive dressings.
Systemic corticosteroids should be avoided in the treatment of atopic dermatitis, even with severe disease. Although systemic corticosteroids provide rapid improvement in atopic dermatitis flares, discontinuation generally results in a significant rebound inflammatory response resulting in another, often more severe, disease flare. Given the chronic relapsing course of AD and significant side effects from prolonged systemic corticosteroid treatment, there is rarely a role for these medications in the treatment of AD. Although systemic corticosteroids are sometimes perceived to be more potent, topical corticosteroids likely achieve higher concentrations at the site of inflammation within the superficial layers of the skin.(85) For children with atopic dermatitis and persistent asthma requiring systemic corticosteroids for asthma exacerbations, we recommend anticipatory treatment of the atopic dermatitis with higher potency topical corticosteroids as the systemic corticosteroids are weaned or stopped, in order to blunt the rebound inflammatory response.
Topical calcineurin inhibitors
The anti-inflammatory effects of topical calcineurin inhibitors result from selective blocking of cytokine transcription in activated T cells. Use of topical calcineurin inhibitors does not result in skin atrophy, making this class of medication effective particularly in controlling eyelid and facial dermatitis. Calcineurin inhibitors may also be useful as a steroid-sparing agent for patients requiring long-term anti-inflammatory treatment. Efficacy as a maintenance medication when applied to areas of frequent flares three times weekly has also been demonstrated, although these medicines are not FDA-approved for this use.(86) Two topical calcineurin inhibitors are available:
Pimecrolimus (Elidel) is available as a 1% cream and is approved for use in children 2 years of age and older.
Tacrolimus (Protopic) is available as a 0.03% or 0.1% ointment. The 0.03% ointment is approved for children 2 years of age and older, while the 0.1% ointment is approved for children ≥16 years.
The major side effect of both calcineurin inhibitors is transient burning that generally subsides after a few days of use. A black box warning was added to this class of medications in 2005 due to concerns of association with specific cancers. A review of the data by the Topical Calcineurin Inhibitor Task Force of the AAAAI and ACAAI, however, did not identify a clear association.(87)
Antimicrobial treatments
Staphylococcus aureus colonization is common in patients with AD. Treatment of infection should be guided by anti-microbial sensitivities to ensure coverage of potentially antibiotic resistant organisms. Use of bleach baths has been reported in the treatment of dermatitis associated with hyper-IgE syndromes and is included in pediatric treatment guidelines for atopic dermatitis, but there is little data supporting efficacy in widespread use.(88-90) However, among children 6 months to 17 years of age with moderate-severe atopic dermatitis and evidence of secondary S. aureus infection, use of dilute bleach baths twice weekly and application of intranasal mupirocin twice daily for 5 days, then repeated monthly, over a 3 month period, resulted in significant improvement of atopic dermatitis severity compared to placebo, (91) and should be considered for any patient who has required more than one course of systemic antibiotics for S. aureus skin infections. Mupirocin can also be applied to excoriated areas of skin twice daily to further prevent infection. Due to evidence for intra-familial transmission of staphylococcus, treatment of family members with intranasal mupirocin should also be considered.
Antihistamines are generally ineffective in the treatment of pruritus associated with atopic dermatitis
Pruritus is the most common feature of atopic dermatitis and the most detrimental for quality of life. Scratching perpetuates cutaneous inflammation through release of TSLP and other mediators, feeding the cycle of continued inflammation and pruritus. As already discussed, the pruritus in atopic dermatitis results from a number of mediators, including neuropeptides and cytokines, namely IL-31. As a result, antihistamines are generally ineffective in controlling the pruritus of atopic dermatitis. Double-blind randomized cross-over trials have demonstrated a lack of efficacy in treating itch by oral antihistamine,(92) and histamine stimulation failed to evoke pruritus in skin of AD patients.(93) While some sleep benefit may be derived from first generation sedating oral antihistamines, this may be largely negated by the resulting ‘hangover’ effect and impairment in cognitive performance, particularly among children.(94). Until more effective therapies are developed, treatment of pruritus should be focused on addressing skin inflammation and barrier dysfunction.
Wet wrap therapy
Wet wrap therapy was initially described more than 20 years ago and consists of application of dilute topical corticosteroids and emollients after a soaking bath, followed by a layer of wet dressing or cloths, then dry clothes.(95) Wet wraps can be used in patients with moderate to severe disease that responds poorly to standard skin care. Wet wraps soothe the skin, promote hydration, prevent scratching, and increase absorption of topical corticosteroids. Wet wraps with dilute topical corticosteroids are effective in clinical trials for controlling flares and maintaining control over short periods of several weeks.(96-98) In patients with severe atopic dermatitis, we have found that wet wraps result in remission of atopic dermatitis, when followed by a routine skin care regimen, over a period of 1 year. (Stone, et al, in preparation) Wet wraps can result in maceration of skin, folliculitis and enhanced absorption of topical corticosteroids, and thus should only be used with close medical supervision. Routine skin care is critical between wet wrap treatments, particularly liberal use of emollients.
Systemic immunosuppressants
For patients with severe atopic dermatitis that is refractory to standard therapies, with careful attention to compliance, systemic immunosuppressants are a treatment option. Systemic immunosuppressants that have been reported include cyclosporine, mycophenolate mofetil, azathioprine, and methotrexate.(99, 100) None of these therapies have a direct effect on restoring barrier function. In our experience, systemic immunosuppressants are not optimally effective unless careful attention to hydration and aggressive use of emollients is also promoted.
Conclusion
AD is a complex disorder that requires both a genetic predisposition and exposure to poorly defined environmental factors. Disease results from a defective skin barrier and immune dysregulation. Effective treatment requires therapies targeted to both restoring barrier function and controlling inflammation. Treating both defects is crucial to optimal outcomes for patients with moderate to severe disease. Education of patients regarding the underlying defects and provision of a comprehensive skin care plan is essential.
Key points.
AD is a complex disorder resulting from gene-environment interactions
Defective skin barrier function and immune dysregulation are paramount to disease pathogenesis
Pruritus is universal, is a major co-morbidity, and is poorly responsive to anti-histamines
Effective treatment requires therapies targeted to restore both barrier function and to control inflammation
Education of patients regarding the principle defects and provision of a comprehensive skin care plan is essential
Acknowledgments
We would like to thank Krista T. Townsend for her contributions to Figure 2.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. The Journal of allergy and clinical immunology. 1999 Jan;103(1 Pt 1):125–38. doi: 10.1016/s0091-6749(99)70536-1. PubMed PMID: 9893196. [DOI] [PubMed] [Google Scholar]
- 2.Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? The Journal of allergy and clinical immunology. 2004 Jul;114(1):150–8. doi: 10.1016/j.jaci.2004.04.027. PubMed PMID: 15241359. [DOI] [PubMed] [Google Scholar]
- 3.Fleischer DM, Bock SA, Spears GC, Wilson CG, Miyazawa NK, Gleason MC, et al. Oral food challenges in children with a diagnosis of food allergy. The Journal of pediatrics. 2011 Apr;158(4):578–83. e1. doi: 10.1016/j.jpeds.2010.09.027. PubMed PMID: 21030035. [DOI] [PubMed] [Google Scholar]
- 4.Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Archives of disease in childhood. 1997 Feb;76(2):159–62. doi: 10.1136/adc.76.2.159. PubMed PMID: 9068310. Pubmed Central PMCID: 1717083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paller AS, McAlister RO, Doyle JJ, Jackson A. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clinical pediatrics. 2002 Jun;41(5):323–32. doi: 10.1177/000992280204100505. PubMed PMID: 12086198. [DOI] [PubMed] [Google Scholar]
- 6.Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004 Sep;114(3):607–11. doi: 10.1542/peds.2004-0374. PubMed PMID: 15342828. [DOI] [PubMed] [Google Scholar]
- 7.Beattie PE, Lewis-Jones MS. An audit of the impact of a consultation with a paediatric dermatology team on quality of life in infants with atopic eczema and their families: further validation of the Infants’ Dermatitis Quality of Life Index and Dermatitis Family Impact score. The British journal of dermatology. 2006 Dec;155(6):1249–55. doi: 10.1111/j.1365-2133.2006.07525.x. PubMed PMID: 17107397. [DOI] [PubMed] [Google Scholar]
- 8.Hanifin JMRG. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(suppl):44–7. [Google Scholar]
- 9.Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. The British journal of dermatology. 1994 Sep;131(3):383–96. doi: 10.1111/j.1365-2133.1994.tb08530.x. PubMed PMID: 7918015. [DOI] [PubMed] [Google Scholar]
- 10.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. The Journal of allergy and clinical immunology. 2013 Feb;131(2):295–9. e1–27. doi: 10.1016/j.jaci.2012.12.672. PubMed PMID: 23374261. [DOI] [PubMed] [Google Scholar]
- 11.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. The Journal of allergy and clinical immunology. 2010 Dec;126(6):1105–18. doi: 10.1016/j.jaci.2010.10.008. PubMed PMID: 21134568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. The British journal of dermatology. 1974 May;90(5):525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. PubMed PMID: 4601016. [DOI] [PubMed] [Google Scholar]
- 13.Williams RE, Gibson AG, Aitchison TC, Lever R, Mackie RM. Assessment of a contact-plate sampling technique and subsequent quantitative bacterial studies in atopic dermatitis. The British journal of dermatology. 1990 Oct;123(4):493–501. doi: 10.1111/j.1365-2133.1990.tb01455.x. PubMed PMID: 2095181. [DOI] [PubMed] [Google Scholar]
- 14.Leyden JJ, Kligman AM. The case for steroid--antibiotic combinations. The British journal of dermatology. 1977 Feb;96(2):179–87. doi: 10.1111/j.1365-2133.1977.tb12541.x. PubMed PMID: 843453. [DOI] [PubMed] [Google Scholar]
- 15.Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DY. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. The Journal of allergy and clinical immunology. 2001 Oct;108(4):651–2. doi: 10.1067/mai.2001.118598. PubMed PMID: 11590398. [DOI] [PubMed] [Google Scholar]
- 16.Williams JV, Vowels BR, Honig PJ, Leyden JJ. S. aureus isolation from the lesions, the hands, and the anterior nares of patients with atopic dermatitis. Pediatric dermatology. 1998 May-Jun;15(3):194–8. doi: 10.1046/j.1525-1470.1998.1998015194.x. PubMed PMID: 9655314. [DOI] [PubMed] [Google Scholar]
- 17.Bonness S, Szekat C, Novak N, Bierbaum G. Pulsed-field gel electrophoresis of Staphylococcus aureus isolates from atopic patients revealing presence of similar strains in isolates from children and their parents. Journal of clinical microbiology. 2008 Feb;46(2):456–61. doi: 10.1128/JCM.01734-07. PubMed PMID: 18077648. Pubmed Central PMCID: 2238135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. The Journal of allergy and clinical immunology. 2001 Aug;108(2):269–74. doi: 10.1067/mai.2001.117455. PubMed PMID: 11496245. [DOI] [PubMed] [Google Scholar]
- 19.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. The New England journal of medicine. 2002 Oct 10;347(15):1151–60. doi: 10.1056/NEJMoa021481. PubMed PMID: 12374875. [DOI] [PubMed] [Google Scholar]
- 20.Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, et al. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. The Journal of allergy and clinical immunology. 1999 Jan;103(1 Pt 1):119–24. doi: 10.1016/s0091-6749(99)70535-x. PubMed PMID: 9893195. [DOI] [PubMed] [Google Scholar]
- 21.Lin YT, Shau WY, Wang LF, Yang YH, Hwang YW, Tsai MJ, et al. Comparison of serum specific IgE antibodies to staphylococcal enterotoxins between atopic children with and without atopic dermatitis. Allergy. 2000 Jul;55(7):641–6. doi: 10.1034/j.1398-9995.2000.00523.x. PubMed PMID: 10921463. [DOI] [PubMed] [Google Scholar]
- 22.Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral research. 2013 May;98(2):153–7. doi: 10.1016/j.antiviral.2013.02.010. PubMed PMID: 23439082. Pubmed Central PMCID: 3773952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathes EF, Oza V, Frieden IJ, Cordoro KM, Yagi S, Howard R, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013 Jul;132(1):e149–57. doi: 10.1542/peds.2012-3175. PubMed PMID: 23776120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 May 15;46(10):1555–61. doi: 10.1086/587668. PubMed PMID: 18419490. [DOI] [PubMed] [Google Scholar]
- 25.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. The New England journal of medicine. 2007 Oct 18;357(16):1608–19. doi: 10.1056/NEJMoa073687. PubMed PMID: 17881745. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. The New England journal of medicine. 2009 Nov 19;361(21):2046–55. doi: 10.1056/NEJMoa0905506. PubMed PMID: 19776401. Pubmed Central PMCID: 2965730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Yu X, Ichikawa M, Lyons JJ, Datta S, Lamborn IT, et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. The Journal of allergy and clinical immunology. 2014 May;133(5):1400–9. e5. doi: 10.1016/j.jaci.2014.02.013. PubMed PMID: 24589341. Pubmed Central PMCID: 4016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. The Journal of allergy and clinical immunology. 2008 Dec;122(6):1054–62. doi: 10.1016/j.jaci.2008.10.023. quiz 63-4. PubMed PMID: 19084106. [DOI] [PubMed] [Google Scholar]
- 29.d’Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo CA. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. Journal of medical genetics. 2012 May;49(5):291–302. doi: 10.1136/jmedgenet-2012-100759. PubMed PMID: 22581967. [DOI] [PubMed] [Google Scholar]
- 30.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nature genetics. 2000 Jun;25(2):141–2. doi: 10.1038/75977. PubMed PMID: 10835624. [DOI] [PubMed] [Google Scholar]
- 31.Oji V, Eckl KM, Aufenvenne K, Natebus M, Tarinski T, Ackermann K, et al. Loss of corneodesmosin leads to severe skin barrier defect, pruritus, and atopy: unraveling the peeling skin disease. American journal of human genetics. 2010 Aug 13;87(2):274–81. doi: 10.1016/j.ajhg.2010.07.005. PubMed PMID: 20691404. Pubmed Central PMCID: 2917721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nature genetics. 2013 Oct;45(10):1244–8. doi: 10.1038/ng.2739. PubMed PMID: 23974871. Pubmed Central PMCID: 3791825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomsen SF, Ulrik CS, Kyvik KO, Hjelmborg J, Skadhauge LR, Steffensen I, et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2007 Sep-Oct;28(5):535–9. doi: 10.2500/aap2007.28.3041. PubMed PMID: 18034971. [DOI] [PubMed] [Google Scholar]
- 34.Morar N, Willis-Owen SA, Moffatt MF, Cookson WO. The genetics of atopic dermatitis. The Journal of allergy and clinical immunology. 2006 Jul;118(1):24–34. doi: 10.1016/j.jaci.2006.03.037. quiz 5-6. PubMed PMID: 16815134. [DOI] [PubMed] [Google Scholar]
- 35.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. Journal of cell science. 2009 May 1;122(Pt 9):1285–94. doi: 10.1242/jcs.033969. PubMed PMID: 19386895. Pubmed Central PMCID: 2721001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nature genetics. 2006 Mar;38(3):337–42. doi: 10.1038/ng1743. PubMed PMID: 16444271. [DOI] [PubMed] [Google Scholar]
- 37.Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nature genetics. 2007 May;39(5):650–4. doi: 10.1038/ng2020. PubMed PMID: 17417636. [DOI] [PubMed] [Google Scholar]
- 38.Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Hogh JK, et al. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010 Jul;65(7):911–8. doi: 10.1111/j.1398-9995.2010.02326.x. PubMed PMID: 20132155. [DOI] [PubMed] [Google Scholar]
- 39.Kezic S, O’Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. The Journal of allergy and clinical immunology. 2012 Apr;129(4):1031–9. e1. doi: 10.1016/j.jaci.2011.12.989. PubMed PMID: 22322004. Pubmed Central PMCID: 3627959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. The Journal of allergy and clinical immunology. 2010 Dec;126(6):1184–90. e3. doi: 10.1016/j.jaci.2010.09.015. PubMed PMID: 21036388. Pubmed Central PMCID: 3627960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. The New England journal of medicine. 2011 Oct 6;365(14):1315–27. doi: 10.1056/NEJMra1011040. PubMed PMID: 21991953. [DOI] [PubMed] [Google Scholar]
- 42.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. Journal of cell science. 2006 Mar 1;119(Pt 5):797–806. doi: 10.1242/jcs.02888. PubMed PMID: 16495480. [DOI] [PubMed] [Google Scholar]
- 43.Jonca N, Leclerc EA, Caubet C, Simon M, Guerrin M, Serre G. Corneodesmosomes and corneodesmosin: from the stratum corneum cohesion to the pathophysiology of genodermatoses. European journal of dermatology : EJD. 2011 May;21(Suppl 2):35–42. doi: 10.1684/ejd.2011.1264. PubMed PMID: 21628128. [DOI] [PubMed] [Google Scholar]
- 44.James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatologic clinics. 2011 Jul;29(3):405–12. viii. doi: 10.1016/j.det.2011.03.012. PubMed PMID: 21605805. Pubmed Central PMCID: 3108573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Molecular biology of the cell. 2007 Sep;18(9):3607–19. doi: 10.1091/mbc.E07-02-0124. PubMed PMID: 17596512. Pubmed Central PMCID: 1951746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasilopoulos Y, Cork MJ, Murphy R, Williams HC, Robinson DA, Duff GW, et al. Genetic association between an AACC insertion in the 3′UTR of the stratum corneum chymotryptic enzyme gene and atopic dermatitis. The Journal of investigative dermatology. 2004 Jul;123(1):62–6. doi: 10.1111/j.0022-202X.2004.22708.x. PubMed PMID: 15191543. [DOI] [PubMed] [Google Scholar]
- 47.Kuo IH, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2013 Feb;131(2):266–78. doi: 10.1016/j.jaci.2012.12.1563. PubMed PMID: 23374259. [DOI] [PubMed] [Google Scholar]
- 48.Niebuhr M, Langnickel J, Draing C, Renz H, Kapp A, Werfel T. Dysregulation of toll-like receptor-2 (TLR-2)-induced effects in monocytes from patients with atopic dermatitis: impact of the TLR-2 R753Q polymorphism. Allergy. 2008 Jun;63(6):728–34. doi: 10.1111/j.1398-9995.2008.01721.x. PubMed PMID: 18445187. [DOI] [PubMed] [Google Scholar]
- 49.Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. The Journal of allergy and clinical immunology. 2008 Apr;121(4):872–7. e9. doi: 10.1016/j.jaci.2008.01.026. PubMed PMID: 18325573. [DOI] [PubMed] [Google Scholar]
- 50.Dinwiddie DL, Kingsmore SF, Caracciolo S, Rossi G, Moratto D, Mazza C, et al. Combined DOCK8 and CLEC7A mutations causing immunodeficiency in 3 brothers with diarrhea, eczema, and infections. The Journal of allergy and clinical immunology. 2013 Feb;131(2):594–7. e1–3. doi: 10.1016/j.jaci.2012.10.062. PubMed PMID: 23374272. Pubmed Central PMCID: 3570814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. The Journal of allergy and clinical immunology. 2007 Jul;120(1):150–5. doi: 10.1016/j.jaci.2007.04.031. PubMed PMID: 17512043. Pubmed Central PMCID: 2669594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howell MD, Boguniewicz M, Pastore S, Novak N, Bieber T, Girolomoni G, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clinical immunology. 2006 Dec;121(3):332–8. doi: 10.1016/j.clim.2006.08.008. PubMed PMID: 17015038. [DOI] [PubMed] [Google Scholar]
- 53.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. The Journal of investigative dermatology. 2005 Oct;125(4):738–45. doi: 10.1111/j.0022-202X.2005.23776.x. PubMed PMID: 16185274. [DOI] [PubMed] [Google Scholar]
- 54.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999 Oct 29;286(5441):958–61. doi: 10.1126/science.286.5441.958. PubMed PMID: 10542151. [DOI] [PubMed] [Google Scholar]
- 55.Siberry GK, Leister E, Jacobson DL, Foster SB, Seage GR, 3rd, Lipshultz SE, et al. Increased risk of asthma and atopic dermatitis in perinatally HIV-infected children and adolescents. Clinical immunology. 2012 Feb;142(2):201–8. doi: 10.1016/j.clim.2011.10.005. PubMed PMID: 22094294. Pubmed Central PMCID: 3273595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. The Journal of allergy and clinical immunology. 2014 Apr;133(4):1092–8. doi: 10.1016/j.jaci.2013.09.044. PubMed PMID: 24290292. Pubmed Central PMCID: 3972266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. The Journal of allergy and clinical immunology. 2008 Dec;122(6):1082–6. doi: 10.1016/j.jaci.2008.09.037. PubMed PMID: 18992930. [DOI] [PubMed] [Google Scholar]
- 58.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, et al. Complete DiGeorge syndrome: development of rash, lymphadenopathy, and oligoclonal T cells in 5 cases. The Journal of allergy and clinical immunology. 2004 Apr;113(4):734–41. doi: 10.1016/j.jaci.2004.01.766. PubMed PMID: 15100681. [DOI] [PubMed] [Google Scholar]
- 59.Wada T, Schurman SH, Garabedian EK, Yachie A, Candotti F. Analysis of T-cell repertoire diversity in Wiskott-Aldrich syndrome. Blood. 2005 Dec 1;106(12):3895–7. doi: 10.1182/blood-2005-06-2336. PubMed PMID: 16091449. Pubmed Central PMCID: 1895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dasouki M, Okonkwo KC, Ray A, Folmsbeel CK, Gozales D, Keles S, et al. Deficient T Cell Receptor Excision Circles (TRECs) in autosomal recessive hyper IgE syndrome caused by DOCK8 mutation: implications for pathogenesis and potential detection by newborn screening. Clinical immunology. 2011 Nov;141(2):128–32. doi: 10.1016/j.clim.2011.06.003. PubMed PMID: 21763205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. The Journal of clinical investigation. 2007 Feb;117(2):407–18. doi: 10.1172/JCI29539. PubMed PMID: 17218989. Pubmed Central PMCID: 1764857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heyd J, Donnenberg AD, Burns WH, Saral R, Santos GW. Immunoglobulin E levels following allogeneic, autologous, and syngeneic bone marrow transplantation: an indirect association between hyperproduction and acute graft-v-host disease in allogeneic BMT. Blood. 1988 Aug;72(2):442–6. PubMed PMID: 3042039. [PubMed] [Google Scholar]
- 63.Dong S, Maiella S, Xhaard A, Pang Y, Wenandy L, Larghero J, et al. Multiparameter single-cell profiling of human CD4+FOXP3+ regulatory T-cell populations in homeostatic conditions and during graft-versus-host disease. Blood. 2013 Sep 5;122(10):1802–12. doi: 10.1182/blood-2013-02-482539. PubMed PMID: 23818545. [DOI] [PubMed] [Google Scholar]
- 64.Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009 Jun 18;113(25):6477–84. doi: 10.1182/blood-2008-09-176594. PubMed PMID: 19258596. [DOI] [PubMed] [Google Scholar]
- 65.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011 Apr 7;117(14):3921–8. doi: 10.1182/blood-2010-10-311894. PubMed PMID: 21292771. [DOI] [PubMed] [Google Scholar]
- 66.Darsow U, Pfab F, Valet M, Huss-Marp J, Behrendt H, Ring J, et al. Pruritus and atopic dermatitis. Clinical reviews in allergy & immunology. 2011 Dec;41(3):237–44. doi: 10.1007/s12016-010-8230-2. PubMed PMID: 21207193. [DOI] [PubMed] [Google Scholar]
- 67.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature immunology. 2004 Jul;5(7):752–60. doi: 10.1038/ni1084. PubMed PMID: 15184896. [DOI] [PubMed] [Google Scholar]
- 68.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. The Journal of allergy and clinical immunology. 2006 Feb;117(2):411–7. doi: 10.1016/j.jaci.2005.10.033. PubMed PMID: 16461142. [DOI] [PubMed] [Google Scholar]
- 69.Arita K, South AP, Hans-Filho G, Sakuma TH, Lai-Cheong J, Clements S, et al. Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis. American journal of human genetics. 2008 Jan;82(1):73–80. doi: 10.1016/j.ajhg.2007.09.002. PubMed PMID: 18179886. Pubmed Central PMCID: 2253984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin MW, Lee DD, Liu TT, Lin YF, Chen SY, Huang CC, et al. Novel IL31RA gene mutation and ancestral OSMR mutant allele in familial primary cutaneous amyloidosis. European journal of human genetics : EJHG. 2010 Jan;18(1):26–32. doi: 10.1038/ejhg.2009.135. PubMed PMID: 19690585. Pubmed Central PMCID: 2987153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. The Journal of allergy and clinical immunology. 2014 Feb;133(2):448–60. doi: 10.1016/j.jaci.2013.10.048. PubMed PMID: 24373353. Pubmed Central PMCID: 3960328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature neuroscience. 2011 May;14(5):595–602. doi: 10.1038/nn.2789. PubMed PMID: 21460831. Pubmed Central PMCID: 3181150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006 Mar 24;124(6):1269–82. doi: 10.1016/j.cell.2006.02.023. PubMed PMID: 16564016. [DOI] [PubMed] [Google Scholar]
- 74.Boguniewicz M, Nicol N, Kelsay K, Leung DY. A multidisciplinary approach to evaluation and treatment of atopic dermatitis. Seminars in cutaneous medicine and surgery. 2008 Jun;27(2):115–27. doi: 10.1016/j.sder.2008.05.001. PubMed PMID: 18620133. [DOI] [PubMed] [Google Scholar]
- 75.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? The Journal of investigative dermatology. 2012 Mar;132(3 Pt 2):949–63. doi: 10.1038/jid.2011.435. PubMed PMID: 22217737. Pubmed Central PMCID: 3279586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? The Journal of investigative dermatology. 1991 Apr;96(4):523–6. doi: 10.1111/1523-1747.ep12470233. PubMed PMID: 2007790. [DOI] [PubMed] [Google Scholar]
- 77.Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. The Journal of investigative dermatology. 2000 Sep;115(3):406–13. doi: 10.1046/j.1523-1747.2000.00072.x. PubMed PMID: 10951276. [DOI] [PubMed] [Google Scholar]
- 78.Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatric dermatology. 1997 Jul-Aug;14(4):321–4. doi: 10.1111/j.1525-1470.1997.tb00968.x. PubMed PMID: 9263319. [DOI] [PubMed] [Google Scholar]
- 79.Grimalt R, Mengeaud V, Cambazard F, Study Investigators G The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214(1):61–7. doi: 10.1159/000096915. PubMed PMID: 17191050. [DOI] [PubMed] [Google Scholar]
- 80.Cork MJ, Britton J, Butler L, Young S, Murphy R, Keohane SG. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. The British journal of dermatology. 2003 Sep;149(3):582–9. doi: 10.1046/j.1365-2133.2003.05595.x. PubMed PMID: 14510993. [DOI] [PubMed] [Google Scholar]
- 81.Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatric dermatology. 2009 May-Jun;26(3):273–8. doi: 10.1111/j.1525-1470.2009.00911.x. PubMed PMID: 19706087. Pubmed Central PMCID: 2762386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gutman AB, Kligman AM, Sciacca J, James WD. Soak and smear: a standard technique revisited. Archives of dermatology. 2005 Dec;141(12):1556–9. doi: 10.1001/archderm.141.12.1556. PubMed PMID: 16365257. [DOI] [PubMed] [Google Scholar]
- 83.Loden M. Effect of moisturizers on epidermal barrier function. Clinics in dermatology. 2012 May-Jun;30(3):286–96. doi: 10.1016/j.clindermatol.2011.08.015. PubMed PMID: 22507043. [DOI] [PubMed] [Google Scholar]
- 84.Lee CH, Chuang HY, Shih CC, Jong SB, Chang CH, Yu HS. Transepidermal water loss, serum IgE and beta-endorphin as important and independent biological markers for development of itch intensity in atopic dermatitis. The British journal of dermatology. 2006 Jun;154(6):1100–7. doi: 10.1111/j.1365-2133.2006.07191.x. PubMed PMID: 16704640. [DOI] [PubMed] [Google Scholar]
- 85.McClain RW, Yentzer BA, Feldman SR. Comparison of skin concentrations following topical versus oral corticosteroid treatment: reconsidering the treatment of common inflammatory dermatoses. Journal of drugs in dermatology : JDD. 2009 Dec;8(12):1076–9. PubMed PMID: 20027934. [PubMed] [Google Scholar]
- 86.Paller AS, Eichenfield LF, Kirsner RS, Shull T, Jaracz E, Simpson EL, et al. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics. 2008 Dec;122(6):e1210–8. doi: 10.1542/peds.2008-1343. PubMed PMID: 19015204. [DOI] [PubMed] [Google Scholar]
- 87.Fonacier L, Spergel J, Charlesworth EN, Weldon D, Beltrani V, Bernhisel-Broadbent J, et al. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. The Journal of allergy and clinical immunology. 2005 Jun;115(6):1249–53. doi: 10.1016/j.jaci.2005.04.006. PubMed PMID: 15940142. [DOI] [PubMed] [Google Scholar]
- 88.Eberting CL, Davis J, Puck JM, Holland SM, Turner ML. Dermatitis and the newborn rash of hyper-IgE syndrome. Archives of dermatology. 2004 Sep;140(9):1119–25. doi: 10.1001/archderm.140.9.1119. PubMed PMID: 15381553. [DOI] [PubMed] [Google Scholar]
- 89.Krakowski AC, Eichenfield LF, Dohil MA. Management of atopic dermatitis in the pediatric population. Pediatrics. 2008 Oct;122(4):812–24. doi: 10.1542/peds.2007-2232. PubMed PMID: 18829806. [DOI] [PubMed] [Google Scholar]
- 90.Birnie AJ, Bath-Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. The Cochrane database of systematic reviews. 2008;(3) doi: 10.1002/14651858.CD003871.pub2. CD003871. PubMed PMID: 18646096. [DOI] [PubMed] [Google Scholar]
- 91.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009 May;123(5):e808–14. doi: 10.1542/peds.2008-2217. PubMed PMID: 19403473. [DOI] [PubMed] [Google Scholar]
- 92.Wahlgren CF, Hagermark O, Bergstrom R. The antipruritic effect of a sedative and a non-sedative antihistamine in atopic dermatitis. The British journal of dermatology. 1990 Apr;122(4):545–51. doi: 10.1111/j.1365-2133.1990.tb14732.x. PubMed PMID: 2110817. [DOI] [PubMed] [Google Scholar]
- 93.Rukwied R, Lischetzki G, McGlone F, Heyer G, Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. The British journal of dermatology. 2000 Jun;142(6):1114–20. doi: 10.1046/j.1365-2133.2000.03535.x. PubMed PMID: 10848733. [DOI] [PubMed] [Google Scholar]
- 94.Kay GG. The effects of antihistamines on cognition and performance. The Journal of allergy and clinical immunology. 2000 Jun;105(6 Pt 2):S622–7. doi: 10.1067/mai.2000.106153. PubMed PMID: 10856168. [DOI] [PubMed] [Google Scholar]
- 95.Goodyear HM, Spowart K, Harper JI. ‘Wet-wrap’ dressings for the treatment of atopic eczema in children. The British journal of dermatology. 1991 Dec;125(6):604. doi: 10.1111/j.1365-2133.1991.tb14807.x. PubMed PMID: 1760370. [DOI] [PubMed] [Google Scholar]
- 96.Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. The British journal of dermatology. 2006 Apr;154(4):579–85. doi: 10.1111/j.1365-2133.2006.07157.x. PubMed PMID: 16536797. [DOI] [PubMed] [Google Scholar]
- 97.Lee JH, Lee SJ, Kim D, Bang D. The effect of wet-wrap dressing on epidermal barrier in patients with atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2007 Nov;21(10):1360–8. doi: 10.1111/j.1468-3083.2007.02277.x. PubMed PMID: 17958842. [DOI] [PubMed] [Google Scholar]
- 98.Schnopp C, Holtmann C, Stock S, Remling R, Folster-Holst R, Ring J, et al. Topical steroids under wet-wrap dressings in atopic dermatitis--a vehicle-controlled trial. Dermatology. 2002;204(1):56–9. doi: 10.1159/000051811. PubMed PMID: 11834851. [DOI] [PubMed] [Google Scholar]
- 99.Denby KS, Beck LA. Update on systemic therapies for atopic dermatitis. Current opinion in allergy and clinical immunology. 2012 Aug;12(4):421–6. doi: 10.1097/ACI.0b013e3283551da5. PubMed PMID: 22622476. [DOI] [PubMed] [Google Scholar]
- 100.Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A, et al. Management of difficult-to-treat atopic dermatitis. The journal of allergy and clinical immunology In practice. 2013 Mar;1(2):142–51. doi: 10.1016/j.jaip.2012.09.002. PubMed PMID: 24565453. [DOI] [PubMed] [Google Scholar]