Abstract
Objective
To assess the extent to which playing a musical instrument is associated with cortical thickness development among healthy youths.
Method
Participants were part of the National Institutes of Health (NIH) Magnetic Resonance Imaging (MRI) Study of Normal Brain Development. This study followed a longitudinal design such that participants underwent MRI scanning and behavioral testing on up to three separate visits, occurring at 2-year intervals. MRI, IQ, and music training data were available for 232 youths (334 scans), ranging from 6–18 years of age. Cortical thickness was regressed against the number of years each youth had played a musical instrument. Next, thickness was regressed against an “Age × Years of Playing” interaction term. Age, gender, total brain volume, and scanner were controlled for in analyses. Participant ID was entered as a random effect to account for within-person dependence. False discovery rate correction was applied (p ≤ 0.05).
Results
There was no association between thickness and years playing a musical instrument. The “Age × Years of Playing” interaction was associated with thickness in motor, premotor, and supplementary motor cortices, as well as prefrontal and parietal cortices. Follow-up analysis revealed that musical training was associated with an increased rate of thickness maturation. Results were largely unchanged when IQ and handedness were included as covariates.
Conclusion
Playing a musical instrument was associated with more rapid cortical thickness maturation within areas implicated in motor planning and coordination, visuospatial ability, and emotion and impulse regulation. However, given the quasi-experimental nature of this study, we cannot rule out the influence of confounding variables.
Keywords: music, MRI, cortical thickness
Introduction
In our programmatic research on quantitative traits of developmental psychopathology, we have argued that all children exhibit symptoms of inattention, aggression, anxiety and sadness, and emotional dysregulation, and that these symptoms are influenced by genes and environments (both negative and positive). We have hypothesized that purely categorical diagnostic conceptualizations belie the true nature of behavior, as well as its underlying biology. Following from this dimensional conceptualization of psychopathology, children with attention-deficit/hyperactivity disorder (ADHD) are not categorically different from children who do not meet criteria for ADHD; rather, they are quantitatively more severe in that they possess more symptoms than children who do not meet ADHD criteria.1, 2 In support of this dimensional conceptualization of psychopathology, we have published numerous behavioral genetic articles demonstrating that attention problems,3 aggressive behavior,4 anxiety/depression,5 and dysregulation6 are best conceptualized as existing on a continuum, and again are influenced in almost equal parts by genetic and environmental contributions. We have added to our behavioral genetic argument a series of investigations into the brain structural correlates of these same behaviors. Interestingly, we have found that normal, subclinical variance in psychopathological traits (e.g., inattention, anxious/depressed symptoms) largely map to the same neural networks posited to underpin clinically significant psychopathology (e.g., ADHD, major depressive disorder [MDD]). For example, we have reported that subclinical anxious/depressed symptoms among healthy youths are related to cortical thickness maturation within aspects of the medial prefrontal network—a network heavily implicated in the mediation of clinically significant mood and anxiety symptomatology.7, 8 Similarly, we have found that subclinical inattention and hyperactivity among healthy youths are associated with cortical thickness maturation in fronto-parietal areas—regions implicated in the pathophysiology of ADHD, as well as attentional control and behavioral inhibition.9 We have also revealed associations between normal variance in aggressive behavior among typically developing youths and cortical thickness within the anterior cingulate.10 Taken together, these findings in typically developing children added support to the idea that human emotions and behaviors exist on a continuum, rather than in categories, and further, that each type of behavior can be mapped to distinct networks in the human brain. In sum, the findings that genes and environment influence the degree to which a child expresses a trait combined with the findings that variation in cortical thickness and developmental effects correlate to the degree that a child expresses a behavior almost immediately leads to the desire to investigate environmental factors that might influence traits via influencing brain structure and function. To put it simply, are there environmental factors that might serve to influence aspects of brain maturation?
Years ago our group decided to pursue research in resilience and wellness. We aimed to determine how health-promoting activities might be associated with better outcomes in children and reported on the behavioral genetic architecture into the health benefits of exercise, music, and reading.2 Taking this same approach to structural neuroimaging, we aimed to look at a wellness activity reported by others to be health promoting and having an effect on brain structure and function, and to study that activity in the same dataset on which we published our behavioral findings.
One such wellness activity is learning to play a musical instrument. In the context of music training, structural MRI studies display strong evidence for an environmental training effect rather than a genetic predisposition. One study showed increased aptitude after 15 months of training for the experimental group versus the control group on finger motor tasks and melody/rhythmic tasks, but not on nonmusical tasks.11 Brain deformation changes were observed in motor areas, the corpus callosum, and the right primary auditory region, all areas important for music performance and auditory processing.11 In addition, unexpected areas increased in volume compared to those of the controls; these included various frontal areas, the left posterior peri-cingulate, and the left middle occipital region. There is evidence that musicians have brain architecture that is altered based on amount of practice and age at which music lessons are initiated.11, 12 Additionally, there is evidence that short-term musical training in early childhood correlates with musically relevant motor and auditory cortical changes.11 In their review, Bilhartz et al. note a significant association between early musical instruction and spatial-temporal reasoning abilities.13 One of many studies demonstrating this finding was set in a classroom and consisted of 62 kindergarten children assigned to group keyboard instruction for 20 minutes twice a week in groups of 10.14 While visual memory was unchanged compared to the control group, spatial-temporal task performance improved in the musical group increasingly over the year as compared to the control group.14 Childhood practice time correlated with increased fractional anisotropy (FA, a measurement the investigators used to infer increased microstructural properties of white matter) in bilateral posterior limbs of the internal capsule (the right side showing the only significant difference than for non-pianists),and two corpus callosum tracts (isthmus extending into the upper splenium and the callosal body and fiber tracts in the frontal lobe)15. These tracts continue to mature at least until age 17.15 Adolescent practice time correlated with increased FA in the splenium (interhemispheric fibers from the superior temporal and occipital cortical areas) and the body of the corpus callosum. Adult practicing time correlated with FA in the left anterior limb of the internal capsule and the fiber bundle in the right temporoparietal junction (the arcuate fasciculus). Bengtsson et al.15 noted that these findings are in line with corticocortical fibers having the most extended myelination cycle (they mature into an individual’s 30s).
Given these data as a starting point, we decided to investigate the relations between musical training and cortical structure in a naturalistic sample. Specifically, we examine the extent to which participation in music training is associated with the rate of cortical thickness development among healthy youths. In the National Institutes of Health (NIH) pediatric development data set, we have music training and Wechsler Abbreviated Scale of Intelligence (WASI) IQ data on 232 children aged 6–18 years. On 112 of those children, we have images at more than one time point yielding a total of 334 scans in which we test the relations between cortical thickness and music training. Our aim was to determine if music training had specific effects on cortical organization in this sample and if so, if these brain regions correlate with any of the prior findings we reported on structural correlates of behavior such as aggression, inattention, anxiety, sadness, or dysregulation. Such data might give us strategies for using health promoting activities in the prevention or treatment of common quantitative behavioral problems.
Method
Sampling and Recruitment
The NIH MRI Study of Normal Brain Development is a large, multi-site project that establishes a normative database to study relations between healthy brain maturation and behaviour.16 Participants were recruited throughout the United States utilizing a population-based sampling method aimed at minimizing selection bias.17 Using available US Census 2000 data, a representative, typically developing sample was recruited at 6 pediatric study centers. The 6 pediatric centers consisted of: Children's Hospital (Boston), Children's Hospital Medical Center (Cincinnati), University of Texas Houston Medical School (Houston), University of California, Los Angeles Neuropsychiatric Institute and Hospital (Los Angeles), Children's Hospital of Philadelphia (Philadelphia), and Washington University (St. Louis). Recruitment was monitored throughout the study, ensuring that enrollment across all pediatric centers was demographically representative with regard to age, gender, ethnicity, and socioeconomic status (full demographic features of participants are provided in16). The study was approved by an institutional review board, and informed consent was obtained from parents, as well as child assent. The Objective 1 database (release 4.0) used in this study included 431 healthy youths, and upon enrollment (i.e., first study visit), ages ranged from 4 years and 6 months to 18 years and 3 months. The study followed a longitudinal design such that participants underwent MRI brain scanning and behavioral testing on three separate visits, occurring at roughly 2-year intervals. Given that the aim of the NIH MRI Study of Normal Brain Development was to study healthy, typically developing children, stringent exclusion criteria were utilized, including: meeting criteria for a current or past Axis-I disorder on structured parent or child interview (Diagnostic Interview for Children and Adolescents)--exceptions, however, included simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency; family history of major Axis-I disorder; family history of inherited neurological disorder or intellectual disability due to non-traumatic events; abnormality on neurological examination; gestational age at birth less than 37 weeks or greater than 42 weeks; and intrauterine exposure to substances known or highly suspected to alter brain structure or function (for further information, see16). Structural MRI and behavioral data were stored and analyzed within a database at the Data Coordinating Center of the Montreal Neurological Institute (MNI), McGill University, Montreal, Quebec, Canada.
MRI protocol
In order to collect data that would permit automated morphometric analysis, as well as accommodate time constraints associated with the participant age range, 30–45 minutes of data acquisition were provided. Both General Electric (GE) and Siemens Medical Systems (Siemens) scanners were used in the NIH Normal Brain Development study. Slice thickness of ~1.5mm was allowed for GE scanners because of the scanners’ limit of 124 slices. A 3D T1-weighted spoiled gradient recalled (SPGR) echo sequence was selected. Inter-site reliability was monitored with the American College of Radiology phantom, as well as a living phantom, that were both scanned at regular intervals at each site.16 All MRI scanners used in the NIH Normal Brain Development study were 1.5 T systems. For further details regarding MRI sequence parameters, the reader is directed to16.
Automated Image Processing
Quality-controlled native MR images were processed through the CIVET automated pipeline (version 1.1.9, 2006), a fully automated structural image analysis system. Processing steps were implemented using the Canadian Brain Imaging Network protocol (http://www.cbrain.mcgill.ca). To account for gross volumetric differences between participants, native MR images were linearly registered to a standardized MNI-Talairach space based on the ICBM152 dataset.18–20 Intensity non-uniformity artifacts introduced by the scanner were corrected for using N3.21 Subsequent classification of white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) was performed using the Intensity Normalized Stereotaxic Environment for the Classification of Tissue (INSECT) algorithm.22 The pipeline includes the Constrained Laplacian Automated Segmentation with Proximities (CLASP) algorithm for generating high-resolution hemispheric surfaces with 40,962 vertices per hemisphere.23–26 Hemispheric surfaces were generated for both the WM/GM interface, as well as the GM/CSF (i.e., pial surface). Both surfaces for each hemisphere were non-linearly registered to an average surface created from the ICBM152 dataset to establish correspondence of vertices (i.e., cortical points) between participants.19, 24, 27 A reverse linear transformation was performed on each participant’s images, allowing for cortical thickness estimations to be made at each cortical point in the MR image’s native space.28 At each cortical point, cortical thickness was calculated using the tlink metric.29 As has been previously reported by members of our group, blurring along the cortical surface is a critical step in conducting cortical thickness analyses and serves to increase the sensitivity of cortical thickness analysis.29 To increase the signal-to-noise ratio, each participant’s cortical thickness map was blurred using a 20-millimeter full width at half maximum surface-based diffusion smoothing kernel.30 This kernel size closely approximates previously recommended values, affording optimal sensitivity for cortical thickness analysis.29 A visual quality control of the native cortical thickness images of each participant was carried out by members of our group to ensure that there were no significant aberrations in cortical thickness estimates for a given participant (inter-rater reliability was .93).31
Current Sample
In the present study, quality-controlled MR data and music training information were available for 232 youths, ranging from 6.1 to 18.2 years of age (with data available at up to three time points for each participant). A total of 334 MRI scans were analyzed for these participants. Of the 232 youths, 150 participants had data available at only one time point (64.7%), 62 had data available at two time points (26.7%), and 20 participants had data for all three time points (8.6%).
Data Analysis
Cortical thickness analyses were carried out using SurfStat, a toolbox created for MATLAB 7 (The MathWorks, Inc., Natick, Massachusetts) by Dr. Keith Worsley (http://www.math.mcgill.ca/keith/surfstat/). Cortical thickness trajectories across the age range in our total sample have been found to be best described by first-order linear functions in contrast to cubic or quadratic functions, and, as a result, the relation between cortical thickness and age was modeled as a first-order linear function. Cortical thickness analyses were conducted using mixed-effects models. Mixed-effects models provide a way in which to analyze unbalanced longitudinal data while maximizing statistical power (i.e., utilizing all available data).32–34
First, each participant's absolute native-space cortical thickness at each point on the cortical surface was linearly regressed against the number of years each participant had played a musical instrument. Age, total brain volume (TBV), gender, and scanner site were statistically controlled for in the model. All terms in the model were mean-centered.
Cortical Thickness = intercept + d1 + β1(age) + β2(TBV) + β3(Gender) + β4(Scanner Site) + β5(Years Playing) + e
In a second model, in order to test the degree to which musical training moderates cortical thickness development, thickness was regressed against an “Age × Years of Playing” interaction term.
Cortical Thickness = intercept + d1 + β1(age) + β2(TBV) + β3(Gender) + β4(Scanner Site) + β5(Years Playing) + β6(age ×Years Playing) + e
In each mixed-effects model, participant ID was entered as a random effect in order to account for within-individual dependence. To control for multiple comparisons, false discovery rate (FDR) correction was applied to the entire cortical surface (p ≤ 0.05).
Results
Table 1 shows descriptive statistics for the participants analyzed in the present study. Males and females did not differ with regard to years playing a musical instrument (t = −.38, p = .70), or WASI IQ score (t = 1.91, p = .06). Adjusted household income was available at 305 of the 334 time points that were analyzed. Adjusted household income was not significantly associated with years playing a musical instrument (r = .054, p = .35).
TABLE 1.
Demographics
| N = 232 (334 scans) | |
|---|---|
| Age (years) | 12.39 (SD = 3.07) |
| Years Playing Musical Instrument | 2.15 (SD = 2.50) |
| Gender | Females = 132 (192 scans; 57.5%) |
| Males= 102 (142 scans; 42.5%) | |
| Handedness | Right=303 (90.7%) |
| Left=31 (9.3%) | |
| IQ | 112.97 (SD = 12.30) |
Note: Data were available for 132 females (with a 192 scans collected on those 132 females; i.e., there were repeated scans on some participants). Similarly, data were available for 102 males (with 142 scans collected on those 102 males; i.e., there were repeated scans on some participants).
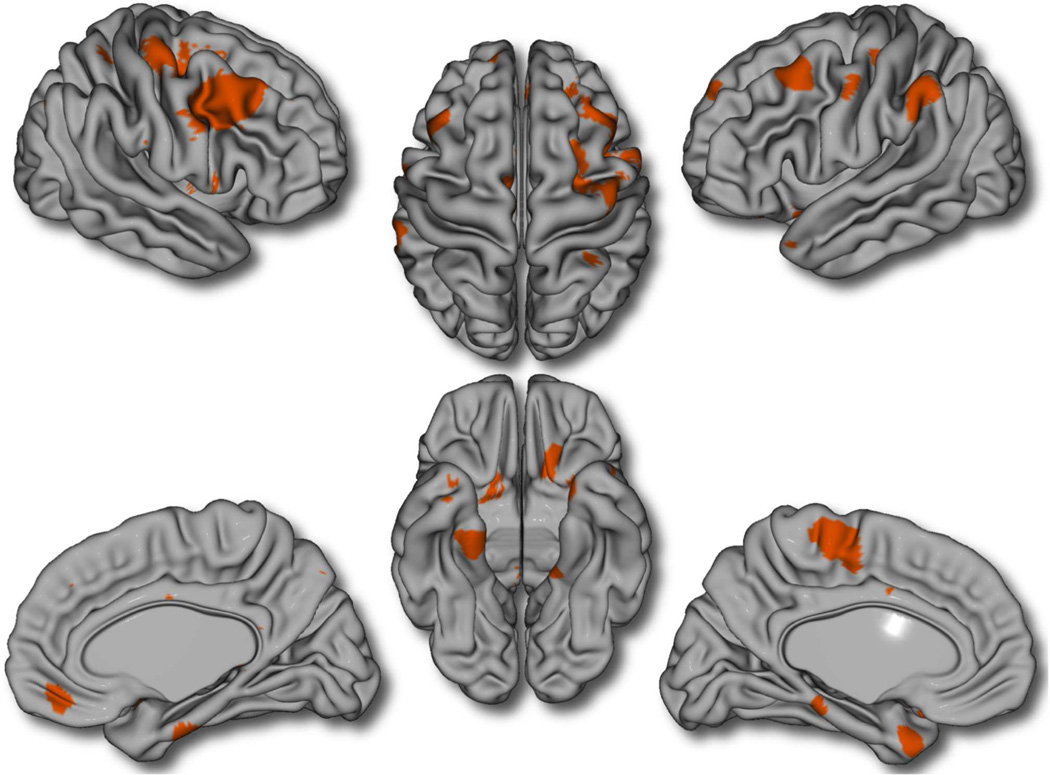
There was no first-order association between cerebral cortical thickness and years of playing a musical instrument. The “Age × Years Playing” interaction term was associated with thickness in a number of brain regions including right premotor and primary motor cortices, left primary and supplementary motor cortices, left angular gyrus, right superior parietal cortex, bilateral dorsolateral prefrontal cortex (DLPFC), left posterior orbitofrontal cortex (OFC), right medial prefrontal cortex (PFC, including part of the medial OFC), bilateral parahippocampal gyri, and left temporal pole (p ≤ 0.05, FDR-corrected) (Figure 1). Of note, these associations remained significant when not controlling for total brain volume (see Supplemental Figure S1, available online). Similar albeit less significant results were obtained when the analysis was rerun and “years playing an instrument” was dichotomized into “playing an instrument” versus “no history of playing an instrument.” There were no significant “Gender × Years Playing” or three-way “Gender × Age × Year Playing” interactions on cortical thickness, indicating the absence of gender-specific effects on this observed maturational pattern. Results were not meaningfully altered when IQ and handedness were included as covariates in the analyses.
FIGURE 1.
Brain areas where local cortical thickness is associated with the “Age × Years of Playing” interaction (N = 232; 334 time points). Note: The figure is shown at p ≤ 0.05 with a false discovery rate correction. Controlled for age, gender, total brain volume, and scanner.
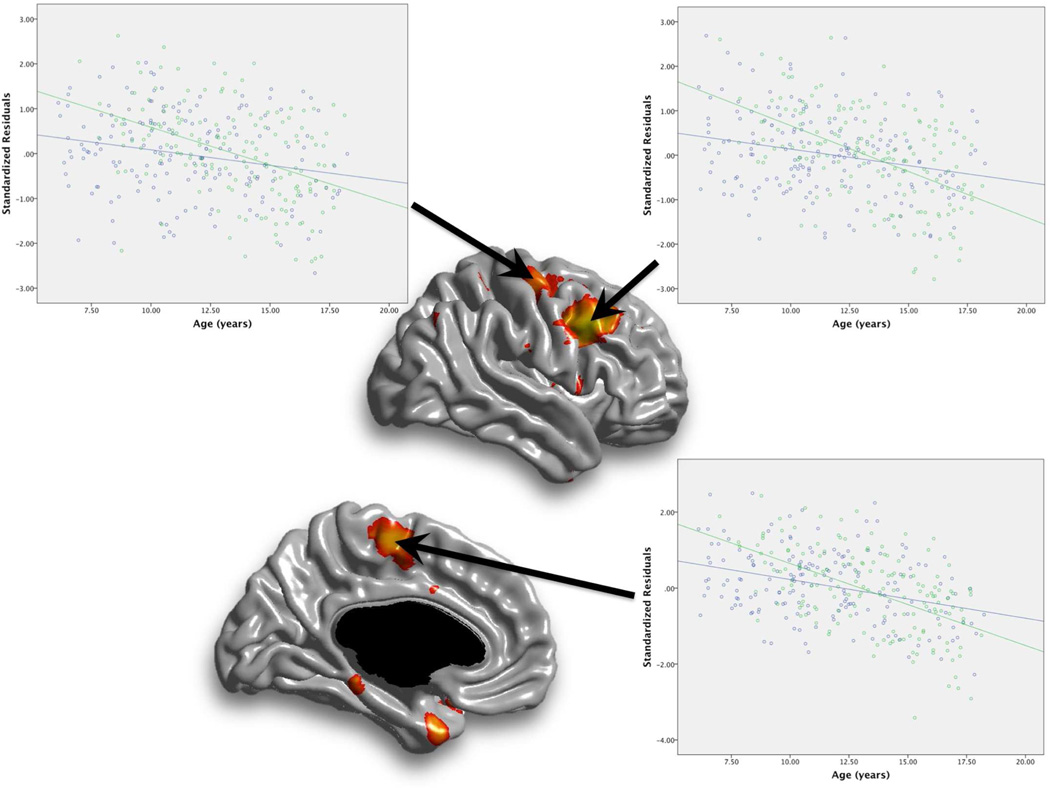
In order to decompose the “Age × Years Playing” interaction on cortical thickness, thickness values were obtained from peak regions in the right premotor cortex, right motor cortex, and left supplementary motor areas and analyzed within SPSS version 18.0 (SPSS Inc., Chicago, IL). Next, the age by thickness relationship was plotted for youths with years of playing a musical instrument partitioned into less than 2 years, and greater than or equal to 2 years. This follow-up analysis revealed that musical training was associated with an increased rate of age-related thinning (Figure 2). This same pattern was observed in other cortical regions associated with the “Age × Years Playing” interaction term.
FIGURE 2.
Plot of the “Age by Years of Playing” interaction on cortical thickness in the right premotor area, right motor cortex, and left supplementary motor area. Note: For illustrative purposes, years of playing a musical instrument has been partitioned into less than 2 years (blue data points), and greater than or equal to 2 years (green data points). Values on the y-axis are the standardized residuals of the linear regression between local cortical thickness and gender, total brain volume, and scanner site to account for these variables.
In a series of follow-up analyses, we investigated the relationship between years playing a musical instrument and cortical surface area, as well as the degree to which surface area development was moderated by years of playing a musical instrument. In both analyses, no associations survived FDR correction for multiple comparisons.
Discussion
Music training was associated with the rate of cortical thickness maturation in a number of brain areas distributed throughout the right premotor and primary cortices, the left primary and supplementary motor cortices, bilateral parietal cortices, bilateral orbitofrontal cortices, as well as bilateral parahippocampal gyri. Our finding that music training was associated with cortical thickness development in the premotor and primary motor cortices is not surprising given that both regions contribute to the control and execution of movement. It is posited that the premotor region plays a particularly important role in the preparation and sensory guidance of movement, both of which are key characteristics of music training. In the same way, the supplementary motor area is thought to play a role in the planning and coordination of movement, again key skills in music production. With regard to bilateral parietal associations, the posterior parietal cortex serves to integrate multisensory information that may be related to motoric activity. Taken together, it is reasonable that we found evidence of accelerated cortical development in these regions given the motoric and multimodal sensory integration associated with music training. Although speculative, increased cortical thickness maturation might reflect pruning processes associated with the prolonged playing of a musical instrument. Alternatively, the expedited thinning observed in this study may stem from the myelination of lower cortical layers associated with music-related fiber pathways—the “thinning” may reflect an altered boundary between cortical gray matter and white matter and, thus, the “thinning” is merely an artifact of MRI. Our findings further suggest that music training is associated with cortical thickness development, but not cortical surface area development. This latter finding is unsurprising given evidence indicating that cortical thickness and surface area represent independent properties of the primate cortex, each underpinned by largely unique genetic factors.35
Music training was also found to influence cortical thickness maturation within aspects of the DLPFC. Myriad imaging and neuropsychological studies have implicated the DLPFC in aspects of executive functioning including working memory, attentional control, as well as organization and planning for the future. Interestingly, developmental structural neuroimaging studies have shown that participants with quantitatively higher scores on attention problems exhibit delayed cortical thickness maturation in portions of the DLPFC as well as other cortical regions (See Supplemental Figure S2, available online, taken from Ducharme et al.9). Future research may benefit from examining the extent to which music training affects cortical development among youths with clinically significant attention problems. Although entirely speculative, it is possible that music training’s influence on cortical maturation, particularly in prefrontal regions, may serve to mitigate aspects of ADHD symptomatology. Music training was also associated with the rate of cortical thickness development in both orbitofrontal and ventromedial prefrontal cortices, brain areas that play a critical role in inhibitory control, as well as aspects of emotion processing. Indeed, portions of the OFC have been implicated in emotion regulatory processes via top-down modulation of the amygdalae. Future research would benefit from looking at the degree to which music training affects brain development as well as measures of behavioral and affective regulation.
Decomposition of the “Age × Years Playing a Musical Instrument” interaction on cortical thickness revealed evidence of music training being associated with more rapid cortical thickness development in a number of cortical regions. It is noteworthy that, when looking at youths under 10 years of age, time playing a musical instrument was positively associated with cortical thickness in regions such as the right DLPFC. Thus, it is possible that early exposure to music training may be associated with long-term effects on cortical development. However, given that youths in the present study were not randomly selected to take part in music training, we cannot rule out possible confounding factors.
Sadly, the National Educational Longitudinal Survey (managed by the National Center for Education Statistics at the Office for Educational Research and Improvement, United States Department of Education) has reported that 74.2% of 10th graders “rarely or never” participate in out-of-school music, art, or dance lessons. This same study also found that 85.9% of 12th graders “rarely or never” participate in out-of-school music, art, or dance lessons. Such statistics, when taken in the context of our present neuroimaging results, underscore the vital importance of finding new and innovative ways in which to make music training more widely available to youths beginning in childhood.
Developing a strategy to bring music training to more children may well result in improved brain-behavior health. However, like many health promoting activities, it appears that music training in childhood is an activity of those with wealth. While the most potent changes in neuroarchitecture correlate with number of hours of practice, the work of Bilhartz et al.13 showed that, despite being assigned to experimental groups receiving different levels of intervention, household income influenced the actual training the children received; higher income household children ultimately received greater exposure to music training, despite random group assignment.13
Others outside of the US have been more aggressive about the possibility of delivering music training to the disadvantaged. In Caracas, Venezula, Masetro Abreu developed ‘El Sistema’ (http://elsistemausa.org/), a music education program implemented throughout Venezuela that has served over 2 million of America’s most at-risk children and currently serves over 500,000 Venezulean children. In addition to demonstrating that musical training can be delivered in a school setting nationwide to at-risk children, the program has resulted in a 20% reduction in school drop-out, a 22% increase in participation in community activities, and a 28% increase in employment for those children who participate in the program.36 Champions of the El Sistema approach exist in many cities around the US, and perhaps someday more children in the US will have access to the benefits of the health promoting, brain building activity of musical training.
The present study has several limitations that must be considered. First, participants were not randomly assigned to study conditions. Given the quasi-experimental nature of this study, we cannot rule out the possibility that confounding factors might have influenced our results. As reported above, in this sample, adjusted household income was not associated with years spent playing a musical instrument. However, children who are afforded the opportunity to play music instruments are undoubtedly exposed to other health and wellness-promoting activities that may affect brain development. Randomized controlled studies may help to more definitively identify brain maturational patterns associated with music training. Second, only structural neuroimaging data were acquired as apart of the NIH Normal Brain Development study, so we cannot speak to the functional implications of more rapid cortical thickness maturation in fronto-parietal regions. Future studies incorporating multi-modal neuroimaging techniques may help to link structural and functional findings. Despite these limitations, this study represents the largest investigation of the association between playing a musical instrument and brain development.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Institute of Child Health and Human Development (NICHD), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS; contract numbers N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, N01-NS-9-2315, N01-NS-9-2316, N01-NS-9-2317, N01-NS-9-2319, and N01-NS-9-2320). Dr. Albaugh is funded by a grant from the Child and Adolescent Psychology Training and Research Foundation. Dr. Ducharme receives financial support from the Canadian Institutes of Health Research with a Frederick Banting and Charles Best Canada Graduate Scholarships-Master’s Awards. Dr. Karama is supported by the Fonds de Recherche en Santé du Québec.
Dr. Hudziak has received grant or research funding from NIH and the National Institute of Diabetes and Digestive and Kidney Disease. His primary appointment is with the University of Vermont. He has additional appointments with Erasmus University in Rotterdam, Netherlands, Washington University School of Medicine in St. Louis, Dartmouth School of Medicine in Hanover, New Hampshire, and Avera Institute of Human Behavioral Genetics in Sioux Falls, South Dakota. Dr. Botteron has received grant or research support from NICHD, the National Institute of Biomedical Imaging and Bioengineering, NIMH, and Autism Speaks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
This article was reviewed under and accepted by ad hoc editor Guido K.W. Frank, MD.
Disclosure: Drs. Albaugh, Ducharme, Karama, Spottswood, Evans, and Ms. Crehan report no biomedical financial interests or potential conflicts of interest.
Information on the Brain Development Cooperative Group and key personnel from the six pediatric study centers may be found online at www.nih-pediatricmri.org. The views in this paper do not necessarily represent the official views of NICHD, NIDA, NIMH, NINDS, NIH, the US Department of Health and Human Services, or any other agency of the United States Government.
Contributor Information
James J. Hudziak, Vermont Center for Children, Youth, and Families, University of Vermont College of Medicine, Burlington, VT..
Matthew D. Albaugh, Vermont Center for Children, Youth, and Families, University of Vermont College of Medicine, Burlington, VT..
Simon Ducharme, Massachusetts General Hospital, Boston, and McLean Hospital, Harvard University, Belmont, MA..
Sherif Karama, McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada..
Margaret Spottswood, Cambridge Health Alliance, Harvard University, Cambridge, MA..
Eileen Crehan, Vermont Center for Children, Youth, and Families, University of Vermont College of Medicine, Burlington, VT..
Alan C. Evans, McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada..
Kelly N. Botteron, Mallinckrodt Institute of Radiology, Washington University in St. Louis, School of Medicine, St. Louis..
REFERENCES
- 1.Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International journal of methods in psychiatric research. 2007;16(Suppl 1):S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudziak JJ. Developmental psychopathology and wellness : genetic and environmental influences. 1st ed. Washington, DC: American Psychiatric Publishing; 2008. [Google Scholar]
- 3.Lubke GH, Hudziak JJ, Derks EM, van Bijsterveldt TC, Boomsma DI. Maternal ratings of attention problems in ADHD: evidence for the existence of a continuum. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1085–1093. doi: 10.1097/CHI.0b013e3181ba3dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudziak JJ, van Beijsterveldt CE, Bartels M, et al. Individual differences in aggression: genetic analyses by age, gender, and informant in 3-, 7-, and 10-year-old Dutch twins. Behavior genetics. 2003;33(5):575–589. doi: 10.1023/a:1025782918793. [DOI] [PubMed] [Google Scholar]
- 5.Boomsma DI, van Beijsterveldt CE, Hudziak JJ. Genetic and environmental influences on Anxious/Depression during childhood: a study from the Netherlands Twin Register. Genes, brain, and behavior. 2005;4(8):466–481. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 6.Hudziak JJ, Althoff RR, Derks EM, Faraone SV, Boomsma DI. Prevalence and genetic architecture of Child Behavior Checklist-juvenile bipolar disorder. Biol Psychiatry. 2005;58(7):562–568. doi: 10.1016/j.biopsych.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Ducharme S, Albaugh MD, Hudziak JJ, et al. Anxious/Depressed Symptoms are Linked to Right Ventromedial Prefrontal Cortical Thickness Maturation in Healthy Children and Young Adults. Cereb Cortex. 2013 Jun 7; doi: 10.1093/cercor/bht151. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducharme S, Hudziak JJ, Botteron KN, et al. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry. 2012;51(1):18–27. e12. doi: 10.1016/j.jaac.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducharme S, Hudziak JJ, Botteron KN, et al. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol Psychiatry. 2011;70(3):283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyde KL, Lerch J, Norton A, et al. The effects of musical training on structural brain development: a longitudinal study. Ann N Y Acad Sci. 2009;1169:182–186. doi: 10.1111/j.1749-6632.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- 12.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. Journal of Neuroscience. 2003;23(27):9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilhartz TD, Bruhn RA, Olson JE. The effect of early musical training on child cognitive development. Journal of Applied Developmental Psychology. 2000;20(4):615–636. [Google Scholar]
- 14.Rauscher FH, Zupan MA. Classroom keyboard instruction improves kindergarten children's spatial-temporal performance: A field experiment. Early Child Res Q. 2000;15(2):215–228. [Google Scholar]
- 15.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 16.Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 17.Waber DP, De Moor C, Forbes PW, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13(5):729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 18.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 19.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imaging. Stuttgart, NY: Georg Thieme; 1988. [Google Scholar]
- 21.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 22.Zijdenbos AP, Forghani R, Evans AC. Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21(10):1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Singh V, MacDonald D, Lee J, Kim S, Evans A. Automated 3D extraction and evaluation of the outer cortical surface using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007;34(4):1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald D, Kabani N, Avis D, Evans A. Automated 3D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;13:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 26.Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13(2):375–380. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- 27.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9(Pt 2):58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 28.Ad-Dab'bagh Y, Singh V, Robbins S, et al. Native space cortical thickness measurement and the absence of correlation to cerebral volume. In: Zilles K, editor. 11th Annual Meeting of the Organization for Human Brain Mapping. Toronto: Organization for Human Brain Mapping; 2005. [Google Scholar]
- 29.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 30.Chung MK, Worsley KJ, Taylor J, Ramsay J, Robbins S, Evans AC. Diffusion smoothing on the cortical surface. Neuroimage. 2001;13(6):S95–S95. [Google Scholar]
- 31.Karama S, Ad-Dab'bagh Y, Haier RJ, et al. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds (vol 37, pg 145, 2009) Intelligence. 2009;37(4):431–442. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diggle P. Analysis of longitudinal data. 2nd ed. Oxford: Oxford University Press; 2002. [Google Scholar]
- 33.Shaw P, Gilliam M, Liverpool M, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168(2):143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer JD, Willett JB. Applied longitudinal data analysis : modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 35.Chen CH, Fiecas M, Gutiérrez ED, et al. Genetic topography of brain morphology. Proc Natl Acad Sci U S A. 2013;110(42):17089–17094. doi: 10.1073/pnas.1308091110. Epub 2013 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuesta J, Antola LC, Castillo G, et al. Proposal for a loan for a program to support the Centro de Acción Social por la Música, Phase II. Venezuela: Inter-American Development Bank; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.