Abstract
Objective
To investigate how statin use is associated with the probability of having an abnormal screening prostate-specific antigen (PSA) result according to common PSA thresholds for biopsy (>2.5, >4.0, and >6.5 ng/mL).
Methods
We conducted a cross-sectional study of 323,426 men aged 65+ who had a screening PSA test in 2003 at a VA facility. The primary predictor was the use of statin medications at the time of index screening PSA test. The main outcome was the screening PSA value. Poisson regressions were performed to calculate adjusted relative risks for having an abnormal screening PSA result according to statin usage.
Results
Percentages of men with PSA results exceeding commonly used thresholds >2.5, >4.0, and >6.5 ng/mL were 21.0%, 7.6%, and 1.6% respectively. These percentages decreased with statin use, increasing statin dose, duration of statin use, and potency of statin. For example, after adjusting for age, the percentage of men having a PSA exceeding 4.0 ng/mL ranged from 8.2% in non-statin users to 6.2% in men prescribed >40mg simvastatin dose. Adjusted relative risks of having a PSA >4.0 ng/mL were 0.89 (95% CI=0.86–0.93), 0.87 (95% CI=0.84–0.91), and 0.83 (95% CI=0.80–0.87) respectively for men on simvastatin dose of 5–20mg, >20–40mg, and >40mg versus non-statin users.
Conclusion
Statin use is associated with a reduction in the probability an older man will have an abnormal screening PSA result, regardless of PSA threshold. This reduction is more pronounced with higher statin dose, longer statin duration, and higher statin potency.
Keywords: Statin, Prostate-Specific Antigen, Screening, Elderly
INTRODUCTION
Prostate-specific antigen (PSA), a serine protease produced in the prostate, can be quantified in human serum by assays using several monoclonal antibodies. While controversial, PSA is the most commonly used laboratory blood test to screen men for prostate cancer. In 2000, Medicare began coverage of screening PSA and it has now become common among older men.1 This is despite the US Preventive Services Task Force (USPSTF) issuing a grade D recommendation against PSA screening in men aged 75 or older in 2008.2 For example, 44% of men aged 75+ reported a recent PSA test in the 2010 National Health Interview Survey.3 In addition, the majority of prostate cancer cases in the US are diagnosed following an elevated PSA.4 Therefore, factors that change PSA levels may affect which men are referred for prostate biopsy and ultimately who is diagnosed with prostate cancer.
A handful of small studies have shown that HMG-CoA reductase inhibitors, also known as statins, are associated with a statistically significant decline in PSA levels.5,6,7,8 Statins were approved for cholesterol reduction by the FDA in 1987 and are now one of the most widely used class of medications in the US among men aged 65+. However, prior studies of the relationship between statin use and PSA levels have included less than 5,000 men, which limited them from comprehensively controlling for potential confounding factors, and prior studies generally have not excluded men who received a PSA test for non-screening reasons, such as men with symptoms of prostate cancer. In addition, none of the studies evaluated how statin use changes the probability of having an abnormal screening PSA result according to common PSA thresholds for biopsy (>2.5, >4.0, and >6.5 ng/mL).
Our study, with the largest data set to date of >300,000 men, sought to clarify whether statin use is associated with PSA levels among screen-eligible men and thereby potentially changes which older men are referred for prostate biopsy. Statin use is most likely to impact biopsy decisions by changing screening PSA results to fall above or below the PSA threshold that is being used by a patient’s clinician to determine when to send a man for biopsy. Therefore in our study, we analyzed whether statin use, in terms of dose, duration and statin potency, changes the probability of having an abnormal screening PSA result according to three commonly used PSA thresholds for biopsy (>2.5, >4.0, and >6.5 ng/mL) while also accounting for other relevant patient characteristics.
MATERIAL AND METHODS
Study Design and Population
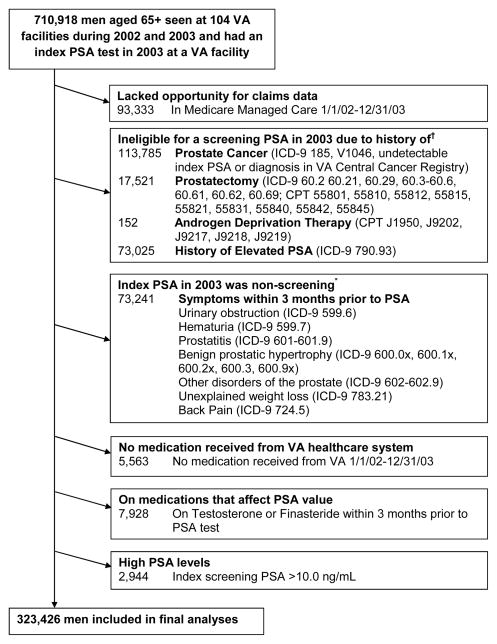
We conducted a cross-sectional cohort study of 323,426 men aged 65 or older who underwent PSA screening in the VA healthcare system in 2003 to determine the association between the use of statin medications and screening PSA levels. We established a cohort using the VA National Patient Care Database to identify 710,918 men aged 65 or older who had at least 1 outpatient visit in both 2002 and 2003 and had an index PSA test in 2003 at a VA.9 An index PSA test was defined as the first outpatient PSA test in the 2003 VA Decision Support System (DSS) National Data Extracts (NDEs) Laboratory Results dataset that includes results of selected tests, such as PSA, which was available for 104 of the127 VA facilities across the US.10 In addition, we used Medicare claims from the VA Information Resource Center to capture services provided to our cohort outside the VA but covered by Medicare.11 We excluded men enrolled in Medicare managed care from 1/1/02 to 12/31/03 because they lacked Medicare claims which were used to determine patients’ medical histories. Also, to create a cohort of screen-eligible men, we used VA and Medicare inpatient and outpatient claims and VA Central Cancer Registry to exclude men with a history of prostate cancer, prostatectomy, androgen deprivation therapy, or elevated PSA level between 1/1/99 and the date of the index PSA in 2003. We also excluded men who experienced specific symptoms (e.g., benign prostatic hypertrophy (BPH), urinary obstruction, hematuria, other disorders of the prostate, unexplained weight loss, and back pain) within 3 months before their index PSA tests were performed (Figure 1). In addition, we limited the study to men who received medications from the VA healthcare system in order to capture use of statin medications. Therefore, we used national VA Pharmacy Benefit Management (PBM) data to exclude men who did not receive any VA medications between 1/1/02 and 12/31/03. We also excluded men on finasteride or testosterone medications within 3 months before the index PSA tests because these medications affect PSA values. Lastly, men with an index PSA value >10.0 ng/mL were also excluded because the risk of prostate cancer in these men is high. This resulted in a final cross-sectional cohort of 323,426 men.
Figure 1.
Exclusion criteria used to define the final cohort of older men who underwent a screening PSA test in 2003 at a VA facility.
†History was defined by searching VA and Medicare inpatient and outpatient claims and the VA Central Cancer Registry between 1/1/99 and the date of the index PSA test in 2003. Abbreviations: ICD, International Classification of Diseases; CPT, Current Procedural Terminology.
*VA and Medicare claims were used to exclude men with prostate symptoms during the 3 months before their index PSA, because this PSA was considered a diagnostic test rather than a screening test.
Predictor Variables
The primary predictor variable for this study was the use of statin medications at the time of the index PSA screening test in 2003. The use of statin medication for each man was determined from the VA PBM records. Statin medications were those with standard ingredient names including: simvastatin, atorvastatin, pravastatin, fluvastatin, rosuvastatin, and lovastatin.8 We noted the date prescriptions were dispensed, the medication name, the number of days supplied, and the dosage of each medication to calculate three domains of statin use: 1) The first domain was to define if a man had a prescription for statin medications on the day of his index PSA test, and if so we determined the dose. Men were considered a statin user if they filled a statin prescription that overlapped with the date of their index PSA test. To calculate the statin dosage, each statin medication was translated into a “simvastatin equivalents” dose (in mg) following standard algorithms. For example, the conversion ratio from atorvastatin to simvastatin is 1:2, from pravastatin or lovastatin to simvastatin are both 2:1, from fluvastatin to simvastatin is 4:1, and from rosuvastatin to simvastatin is 1:8.12 After the conversion to a simvastatin equivalent dose, men were classified into approximate tertiles of statin dose: 5–20mg, >20–40mg, and >40mg. 2) The second domain was to define how long a statin user had been using a statin during the 1 year before the index PSA. This was calculated as the total number of months on any statin prescription over the one year period. In addition, as a common method for assessing adherence to statin medications, fixed medication possession ratio (MPR) was calculated by summing days of supply within 90 days prior to the index PSA date divided by 90 days of specified period. Then patients with MPR at least 0.8 were defined as adherent.13 3) The third domain was to define the type of statin prescribed which included low (e.g., pravastatin, lovastatin, fluvastatin), medium (e.g., simvastatin) and high (e.g., atorvastatin, rosuvastatin) potency statins.14
Other baseline characteristics known to influence the use and results of PSA screening, listed in Table 1, were also obtained from VA and Medicare data and from the 2000 US census.15,16 Age on the date of index PSA screening was categorized into five groups: 65 to 69 years, 70 to 74 years, 75 to 79 years, 80 to 84 years, and 85 years and older. Race/Ethnicity was determined primarily from Medicare administrative data, and VA race data was used to fill in missing data (<1%). Health status was defined by the Deyo adaption of the Charlson Comorbidity Index, which is a summary measure of 19 chronic diseases selected and weighted according to their association with mortality.17 The low-density lipoprotein (LDL) cholesterol level within one year and closest to the index PSA screening date was also extracted from the VA DSS NDEs Laboratory Results.
Table 1.
Baseline characteristics of study participants (N=323,426)
| Characteristic | N (%)a
|
|
|---|---|---|
| Non-Statin User N = 195,977 |
Statin User N = 127,449 |
|
| Age, years | ||
| 65–69 | 70,759 (36.1) | 38,261 (30.0) |
| 70–74 | 58,800 (30.0) | 45,647 (35.8) |
| 75–79 | 40,785 (20.8) | 29,791 (23.4) |
| 80–84 | 20,203 (10.3) | 11,714 (9.2) |
| 85+ | 5,430 (2.8) | 2,036 (1.6) |
|
| ||
| Race | ||
| White | 171,991 (87.8) | 117,013 (91.8) |
| Black | 16,863 (8.6) | 7,326 (5.7) |
| Hispanic | 4,046 (2.1) | 1,609 (1.3) |
| Other | 2,515 (1.3) | 1,236 (1.0) |
| Unknown | 562 (0.3) | 265 (0.2) |
|
| ||
| Charlson Scoreb | ||
| 0 (best health) | 127,608 (65.1) | 74,799 (58.7) |
| 1–2 (average health) | 51,230 (26.1) | 38,874 (30.5) |
| ≥ 3 (worst health) | 17,139 (8.8) | 13,776 (10.8) |
|
| ||
| Marriedc | ||
| Yes | 135,253 (69.4) | 95,840 (75.5) |
| No | 59,541 (30.6) | 31,041 (24.5) |
| Lived in ZCTA in which ≥ 25% of adults had a college educationd | ||
| Yes | 51,790 (27.3) | 35,177 (28.4) |
| No | 137,890 (72.7) | 88,779 (71.6) |
|
| ||
| ZCTA Median Income in Tertiles | ||
| Highest tertile (>=$41,128) | 60,882 (32.1) | 42,665 (34.4) |
| Middle tertile ($32,382–$41,128) | 63,467 (33.5) | 43,187 (34.8) |
| Lowest tertile (<$32,382) | 65,313 (34.4) | 38,101 (30.7) |
|
| ||
| LDL Cholesterol Levels | ||
| >130 | 42,186 (21.5) | 12,239 (9.6) |
| >100–130 | 55,326 (28.2) | 32,871 (25.8) |
| 70–100 | 42,580 (21.7) | 47,449 (37.2) |
| <70 | 13,606 (6.9) | 15,547 (12.2) |
| No cholesterol test available | 42,279 (21.6) | 19,343 (15.2) |
All P-values are <0.0001.
The percent values are presented as column percents.
Charlson-Deyo comorbidity scores were calculated from VA and Medicare inpatient and outpatient claims during the 12 months before the index PSA date. Men were categorized as being in best health if they had a Charlson score of 0, average health if they had a Charlson score of 1–2, and worst health if they had a Charlson score of ≥3.
Marital status is abstracted from the Veterans Affairs National Patient Care Database. Data were missing for 0.5% of men in the cohort.
ZCTA=Zip Code Tabulation Area. Through linkage to the 2000 US Census, we determined the percentage of adults with a college degree who lived with a veteran’s ZCTA and the median income for adults aged 65 years and older who lived within that ZCTA. Education and Income data, 3.0% and 3.0% respectively, were missing for men in the cohort.
Outcome Variable
The main outcome variable was the index PSA value (ng/mL) from the VA DSS NDEs Laboratory Results, which records PSA results obtained in the course of clinical practice at each VA facility. PSA values were examined according to several published common thresholds for defining an abnormal PSA level (>2.5, >4.0, and >6.5 ng/mL).18,19
Statistical Analysis
We computed frequencies and proportions of demographic and other factors according to statin use, and compared the associations using Chi-squared tests. For men who were statin users on the date of their screening PSA, we reported their simvastatin equivalent dosage, number of months of statin use within the year before their index PSA test, and the statin medication potency. Because there are different PSA thresholds, we performed logistic regressions to calculate age-adjusted percentages of men with PSA values that exceeded three commonly used thresholds (>2.5, >4.0, and >6.5 ng/mL) to provide men with the probability of having an abnormal screening PSA result according to their simvastatin equivalent dose, and also tested for linear trend across statin dose. In addition, we used Poisson regression with robust error variance to calculate relative risks and 95% confidence intervals of having an index screening PSA >4.0 ng/mL (the most commonly used threshold) according to the three domains of statin usage, adjusting for demographic factors as well as according to other baseline characteristics listed in Table 1. All analyses were conducted using software SAS 9.3 (SAS Institute, Cary, North Carolina) and STATA 12.1 (College Station, TX), and all tests of statistical significance were two-sided. The Committee on Human Research at the University of California, San Francisco, and the Committee for Research and Development at the San Francisco VA Medical Center approved the study.
RESULTS
Participant Characteristics
Our cohort included 323,426 men who had a screening PSA test in 2003 at 104 VA facilities. Their mean age was 73 years (age range, 65–107 years), 89.4% were white, and 9.6% had a Charlson-Deyo comorbidity index of 3 or higher, while 127,449 (39.4%) were statin users at the time of their index PSA tests. Baseline characteristics differed between statin users and non-statin users for all variables in Table 1 (all P <0.0001). For example, statin users were less likely to be over age 80 years, or live in low education or low income zip code tabulation area (ZCTA), or have a LDL cholesterol level greater than 100 mg/dL. Statin users were more likely to be white, married, and be in average or worst health categories.
Statin Use and Screening PSA Results
For the 127,449 statin users, the approximate tertile distribution of their simvastatin equivalents dose was as follows: 46,628 (36.6%) men were prescribed a low statin dose (5–20mg), 42,216 (33.1%) men were prescribed a medium statin dose (>20–40mg), and 38,605 (30.3%) men were prescribed a high statin dose (>40mg). The majority (85.4%) of statin users were prescribed statins for >6–12 months before their index PSA tests, while only 5.9% of statin users were prescribed statins for 0–3 months, and 8.7% of men were prescribed statins for more than 3 months but less than 6 months. Moreover, the majority (84.0%) of statin users were prescribed simvastatin, while 12.9% of men were on a low potency statin (among which, lovastatin comprised 95% of prescriptions compared to pravastatin and fluvastatin), and only 3.1% of men were on a high potency statin (among which, atorvastatin accounted for >99% of prescriptions). In addition, among all statin users, 78.9% were statin adherent in the past 90-day time interval before their index PSA dates based on the calculation of MPR.
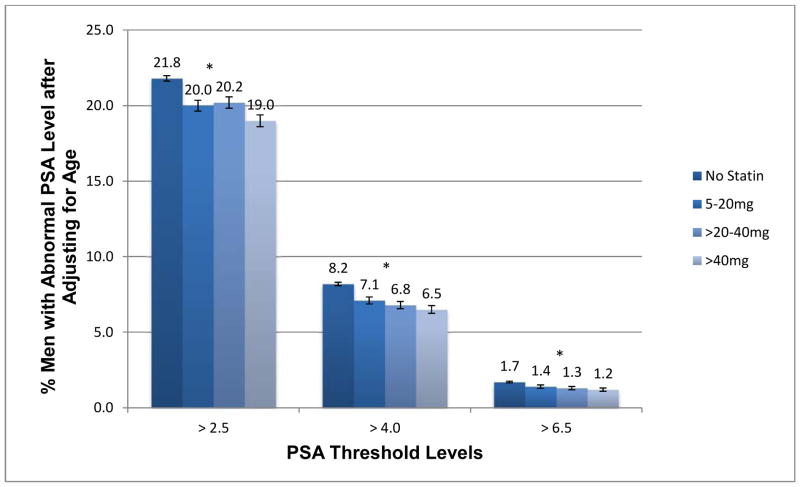
Among the 323,426 men in this cohort, 67,842 (21.0%) had an index screening PSA level exceeding 2.5 ng/mL, 24,670 men (7.6%) had a level exceeding 4.0 ng/mL, and 5,062 men (1.6%) had a level exceeding 6.5 ng/mL. The percentage of men who had a PSA level exceeding these three commonly used thresholds varied according to their statin dose. After adjusting for age, the percentage of men having a PSA >2.5 ng/mL ranged from 21.8% in non-statin users to 19.0% in men prescribed the highest statin dose, which resulted in a relative risk reduction of 13%. Findings were consistent but with stronger relative risk reductions of 21% and 29% for the PSA cut-points of 4.0 and 6.5 respectively (see Figure 2). Trend tests across statin dose were significant for all three PSA thresholds (P <0.0001).
Figure 2.
Age-adjusted percentage of men with an index screening PSA value above three commonly used thresholds, according to simvastatin equivalent dose used at the time of index PSA (N=323,426).
*P-values for trend tests are <0.0001. Error bars represent 95% confidence intervals.
Because PSA >4.0 ng/mL is the most commonly used threshold in clinical practice, we further examined the association between several domains of statin use (e.g., duration, potency) and having a screening PSA >4.0 ng/mL. Similar to what was seen for increasing statin dose, the proportions of men having a PSA >4.0 ng/mL decreased as the duration of statin use increased, and as the potency of statin increased (Table 2). The proportion of men having an index PSA level exceeding 4.0 ng/mL was lower among men in worse health. In contrast, the proportions of men having a PSA >4.0 ng/mL were higher for older or non-married men (Table 2).
Table 2.
Associations of baseline characteristics and having an index screening PSA >4.0 ng/mL (N=323,426)
| Characteristica | N (% who had PSA >4.0 ng/mL) | Relative Risk (95% CI) | Adjusted Relative Risk (95% CI)b |
|---|---|---|---|
|
| |||
| Statin Dosage | |||
| None | 15,962 (8.1) | 1.00 (Ref.) | 1.00 (Ref.) |
| 5–20mg | 3,385 (7.3) | 0.89 (0.86, 0.92) | 0.89 (0.86, 0.93) |
| >20mg–40mg | 2,892 (6.9) | 0.84 (0.81, 0.87) | 0.87 (0.84, 0.91) |
| >40mg | 2,431 (6.3) | 0.77 (0.74, 0.81) | 0.83 (0.80, 0.87) |
|
| |||
| Number of Months on Statin within the Year Prior to PSA | |||
| 0–3 months | 14,369 (8.4) | 1.00 (Ref.) | 1.00 (Ref.) |
| >3–6 months | 1,560 (7.2) | 0.86 (0.81, 0.90) | 0.93 (0.88, 0.98) |
| >6 months | 8,741 (6.7) | 0.79 (0.77, 0.81) | 0.84 (0.82, 0.86) |
|
| |||
| Statin Potency | |||
| None | 15,962 (8.1) | 1.00 (Ref.) | 1.00 (Ref.) |
| Low Potency (Lovastatin, Pravastatin, Fluvastatin) | 1,180 (7.2) | 0.88 (0.84, 0.94) | 0.90 (0.85, 0.95) |
| Medium Potency (Simvastatin) | 7,264 (6.8) | 0.83 (0.81, 0.86) | 0.86 (0.84, 0.89) |
| High Potency (Atorvastatin, Rosuvastatin) | 264 (6.6) | 0.81 (0.72, 0.92) | 0.89 (0.79, 1.00) |
|
| |||
| Age, years | |||
| 65–69 | 5,917 (5.4) | 1.00 (Ref.) | 1.00 (Ref.) |
| 70–74 | 7,266 (7.0) | 1.28 (1.24, 1.33) | 1.30 (1.25, 1.34) |
| 75–79 | 6,577 (9.3) | 1.72 (1.66, 1.78) | 1.79 (1.72, 1.85) |
| 80–84 | 3,814 (12.0) | 2.20 (2.12, 2.29) | 2.30 (2.21, 2.39) |
| 85+ | 1,096 (14.7) | 2.70 (2.55, 2.87) | 2.78 (2.62, 2.96) |
|
| |||
| Race | |||
| White | 20,968 (7.3) | 1.00 (Ref.) | 1.00 (Ref.) |
| Black | 2,794 (11.6) | 1.59 (1.53, 1.65) | 1.60 (1.54, 1.67) |
| Hispanic | 515 (9.1) | 1.26 (1.15, 1.36) | 1.10 (1.01, 1.21) |
| Other | 303 (8.1) | 1.11 (1.00, 1.24) | 1.12 (1.01, 1.25) |
| Unknown | 90 (10.9) | 1.50 (1.23, 1.82) | 1.33 (1.08, 1.63) |
|
| |||
| Charlson Score | |||
| 0 (best health) | 16,241 (8.0) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1–2 (average health) | 6,276 (7.0) | 0.87 (0.84, 0.89) | 0.84 (0.82, 0.86) |
| ≥ 3 (worst health) | 2,153 (7.0) | 0.87 (0.83, 0.91) | 0.80 (0.77, 0.84) |
|
| |||
| Married | |||
| Yes | 16,683 (7.2) | 1.00 (Ref.) | 1.00 (Ref.) |
| No | 7,833 (8.7) | 1.20 (1.17, 1.23) | 1.15 (1.12, 1.18) |
|
| |||
| Lived in ZCTA in which ≥ 25% of adults had a college education | |||
| Yes | 6,727 (7.7) | 1.00 (Ref.) | 1.00 (Ref.) |
| No | 17,179 (7.6) | 0.98 (0.95, 1.01) | 1.00 (0.97, 1.03) |
|
| |||
| ZCTA Median Income in Tertiles | |||
| Highest tertile (>=$41,128) | 7,891 (7.6) | 1.00 (Ref.) | 1.00 (Ref.) |
| Middle tertile ($32,382–$41,128) | 7,863 (7.4) | 0.97 (0.94, 1.00) | 0.97 (0.93, 1.00) |
| Lowest tertile (<$32,382) | 8,152 (7.9) | 1.03 (1.00, 1.07) | 0.98 (0.95, 1.02) |
Associations between baseline characteristics and having a screening PSA >4.0 ng/mL were significant (P <0.0001) for all relationships in bold.
Adjusted for age, race, Charlson score, marital status, education and income.
In the multivariable model, after adjusting for demographics factors, relative risks and 95% confidence intervals of having an index screening PSA >4.0 ng/mL were similar to unadjusted ones (Table 2). Furthermore, after adjusting for LDL cholesterol levels as well as the demographic factors, relative risks remained significant but were slightly attenuated.
COMMENT
This is the largest study to investigate the association between statin use and the percentage of men who had a screening PSA level exceeding three commonly used thresholds for biopsy (>2.5, >4.0, and >6.5 ng/mL). We found the age-adjusted proportions of men with an abnormal PSA result decreased as the statin dose increased regardless of the PSA threshold used. These findings provide useful information to patients and clinicians who are considering PSA screening, particularly given the recent 2013 ACC/AHA Blood Cholesterol Guidelines which recommended high-intensity or the maximum tolerated statin dose for treatment of blood cholesterol.20
Our finding that statin use is associated with a reduction in the percentage of men aged 65+ who have an abnormal PSA result adds to the literature of smaller studies that have evaluated the effect of statins on PSA levels.5,6,7,8 A retrospective study found statins reduced PSA in patients with BPH and inferred that statin medications could improve lower urinary tract symptoms.21 A recent study showed an independent and significant association of statin use with decreased prostate cancer recurrence in men treated with brachytherapy. 22 Our study, which involves >300,000 men, found ~2% absolute reduction in having a screening PSA >4.0 ng/mL if a man was taking a statin medication. And, the relative risk reduction of having an abnormal screening PSA level in men on the highest statin dose compared to men not on statins was greater for higher PSA cut-points than for lower cut-points. For example, men on a statin dose >40mg versus non-statin users had a relative risk reduction of 13% for having a PSA exceeding 2.5 ng/mL and a relative risk reduction of 29% for having a PSA >6.5ng/ml. These results are consistent with a prior smaller longitudinal study.6 In addition, we noted that increasing statin dose, longer duration of statin use, and higher statin potency were all significantly associated with a reduction in the probability of having a screening PSA >4.0 ng/mL after adjusting for demographics factors. Collectively these findings provide strong evidence that statins are associated with decreased PSA levels.
Even after controlling for LDL cholesterol levels, the inverse associations between statin use and having an abnormal screening PSA result remained but were slightly attenuated, suggesting there may be both cholesterol and non-cholesterol mechanisms through which statins lower PSA levels. This finding was consistent with two prior observational studies which suggested additional non-cholesterol mediated mechanisms of statins on prostate biology.6,23 Possible mechanisms include inhibiting inflammation,24 angiogenesis,25 cell proliferation,26,27 migration and adhesion,28 and invasion.29
Our study had several limitations. First, laboratory data did not indicate the reasons for ordering PSA tests. As a result, some of the tests may have been performed for non-screening reasons. However, we conducted chart reviews which showed most PSA tests in our cohort were screening tests.16 Second, statin use in our study was based on prescription data, rather than actual compliance, which may overestimate statin use among patients. Third, our cohort consists of men who received care in the VA healthcare system, so the generalizability to non-veterans is uncertain. Regardless, the VA is the largest health care system for men in the nation and has the largest number of PSA results across the US compared to any other data source.30 Fourth, while our data were from 2003, given the ongoing controversy surrounding PSA screening these data remain timely and relevant. Fifth, we did not determine who was ultimately diagnosed with prostate cancer. Our main study goal was to present probabilities of having an abnormal PSA result according to different measures of statin usage and different PSA thresholds for biopsy. As such, we are unable to comment on the mechanism explaining why statin users were less likely to have abnormal PSA values. Lastly, details about PSA assays were not available so whether it plays a role in the association between statins and PSA levels is unknown and could be a future area of research. Despite these limitations, our study has many strengths including a sample size exceeding 300,000 men which allowed us to study varying measures of statin use and different PSA thresholds and to perform adjusted analyses to take into account many potential confounding factors.
CONCLUSION
This study shows that statins have a small but consistent effect on reducing the probability an older man will have an abnormal screening PSA result regardless of the PSA thresholds for biopsy used (>2.5, >4.0, and >6.5 ng/mL). This reduction becomes more pronounced with higher statin dose, longer statin duration and higher statin potency. These findings raise questions about the mechanism through which statins affect PSA as well as how statins affect outcomes of PSA screening in older men. This study informs clinicians who order PSA screening tests that men on statins have a slightly lower risk for having an abnormal PSA result and therefore a lower risk for having to making decisions about whether to pursue a biopsy after PSA screening. Whether the lower risk of having an abnormal screening PSA result reduces the risk of prostate cancer or decreases the sensitivity of PSA screening to detect prostate cancer is uncertain at this time and deserves further evaluation.
Acknowledgments
This work was supported by grant R01 CA134425 from the National Cancer Institute at the National Institutes of Health (Drs Walter, Freedland, and Hoffman), by grant K24AG041180 from the National Institute on Aging at the National Institutes of Health (Dr Walter), by grant K24CA160653 from the National Cancer Institute at the National Institutes of Health (Dr Freedland), and by the New Mexico Veterans Affairs Health Care System (Dr Hoffman).
Footnotes
The authors report no conflicts of interest related to the work described in this manuscript.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of any of these funding agencies.
The funding sources had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walter LC, Bertenthal D, Lindquist K, et al. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296:2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening for prostate cancer. US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Prasad SM, Drazer MW, Huo D, et al. 2008 US Preventive Services Task Force recommendations and prostate cancer screening rates. JAMA. 2012;307:1692–1694. doi: 10.1001/jama.2012.534. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 5.Cyrus-David MS, Weinberg A, Thompson T, et al. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. J Urol. 2005;173:1923–1925. doi: 10.1097/01.ju.0000158044.94188.88. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton RJ, Goldberg KC, Platz EA, et al. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511–1518. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 7.Chang SL, Harshman LC, Presti JC., Jr Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28:3951–3957. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mener DJ, Cambio A, Stoddard DG, et al. The impact of HMG-CoA reductase therapy on serum PSA. Prostate. 2010;70:608–615. doi: 10.1002/pros.21095. [DOI] [PubMed] [Google Scholar]
- 9.Dataset FY2009: VIReC Research User Guide. U.S. Dept. of Veterans Affairs HSRaDS, VA Information Resource Center; Hines, IL: VA Information Resource Center; 2011. VHA Medical SAS® Outpatient Datasets and Inpatient Encounters. Available at http://vaww.virec.research.va.gov/RUGs/MedSAS/RUG-MedSAS-OP-FY09-RA.pdf. [Google Scholar]
- 10.VHA Decision Support System Clinical National Data Extracts. 2. U.S. Dept. of Veterans Affairs HSRaDS; Hines, IL: VA Information Resource Center; 2009. VIReC Research User Guide. Available at http://vaww.virec.research.va.gov/RUGs/DSS-NDEs/RUG-DSS-NDE-2nd-Ed-CY09-RA.pdf. [Google Scholar]
- 11.Hynes DM, Koelling K, Stroupe K, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45:214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 12.PL Detail-Document, Statin Dose Comparison. Pharmacist’s Letter/Prescriber’s Letter. August 2009 (full update June 2013)
- 13.Kozma CM, Dickson M, Phillips AL, et al. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013;7:509–516. doi: 10.2147/PPA.S40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steen DL, Bhatt DL. Statin potency associated with incident diabetes in a real-world evaluation. Evid Based Med. 2014;19:68–68. doi: 10.1136/eb-2013-101445. [DOI] [PubMed] [Google Scholar]
- 15.US Census Bureau. Summary File 3. 2000 census of population and housing. 2002 Available at http://www.census.gov/prod/cen2000/doc/sf3.pdf.
- 16.Walter LC, Fung KZ, Kirby KA, et al. Five-year downstream outcomes following prostate-specific antigen screening in older men. JAMA Intern Med. 2013;173:866–873. doi: 10.1001/jamainternmed.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–864. [PubMed] [Google Scholar]
- 19.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst. 2005;97:1132–1137. doi: 10.1093/jnci/dji205. [DOI] [PubMed] [Google Scholar]
- 20.Stone NJ, Robinson J, Lichtenstein Ah, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Epub ahead of print November 12 Available at http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a.citation.
- 21.Lee SH, Park TJ, Bae MH, et al. Impact of treatment with statins on prostate-specific antigen and prostate volume in patients with benign prostatic hyperplasia. Korean J Urol. 2013;54:750–755. doi: 10.4111/kju.2013.54.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh DS, Koontz B, Freedland SJ, et al. Statin use is associated with decreased prostate cancer recurrence in men treated with brachytherapy. World J Urol. 2014 doi: 10.1007/s00345-014-1281-x. Epub ahead of print March 27 Available at http://link.springer.com.ucsf.idm.oclc.org/article/10.1007%2Fs00345-014-1281-x. [DOI] [PubMed]
- 23.Mondul AM, Selvin E, De Marzo AM, et al. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001–2004. Cancer Causes Control. 2010;21:671–678. doi: 10.1007/s10552-009-9494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 25.Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Porter DC, Chen X, et al. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci U S A. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on prostate cancer cell death. Carcinogenesis. 2001;22:1727–1731. doi: 10.1093/carcin/22.10.1727. [DOI] [PubMed] [Google Scholar]
- 28.Nubel T, Dippold W, Kleinert H, et al. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18:140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 29.Kusama T, Mukai M, Iwasaki T, et al. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Cancer Res. 2001;61:4885–4891. [PubMed] [Google Scholar]
- 30.Kizer KW, Demakis JG, Feussner JR. Reinventing VA health care: systematizing quality improvement and quality innovation. Med Care. 2000;38:I7–16. [PubMed] [Google Scholar]