Abstract
Background
Prolonged heart rate-corrected QT interval on the electrocardiogram (ECG) is associated with increased risk of myocardial infarction and cardiovascular disease (CVD)-related death in patients with prevalent coronary heart disease (CHD).
Objectives
We sought to examine the prognostic association between the baseline QT interval and incident cardiovascular events in individuals without known prior CVD.
Methods
The corrected baseline 12-lead ECG QT interval duration (QTcorr) was determined by adjustment for age, sex, race/ethnicity, and RR interval duration in 6,273 participants in the Multi-Ethnic Study of Atherosclerosis (MESA). Cox proportional hazards models adjusting for demographic and clinical risk factors were used to examine the association of baseline QTcorr with incident cardiovascular events.
Results
The mean age at enrollment was 61.7 ± 10 years, and 53.4% of participants were women. Cardiovascular events occurred in 291 participants over a mean follow-up of 8.0 ± 1.7 years. Each 10 ms increase in the baseline QTcorr was associated with incident heart failure (HR, 95% confidence interval (CI): 1.25 [1.14 to 1.37]), CVD events (HR, 95% CI: 1.12 [1.05 to 1.20]), and stroke (HR, 95% CI: 1.19 [1.07 to 1.32]) after adjustment for CVD risk factors and potential confounders. There was no evidence of interaction with sex or ethnicity.
Conclusion
The QT interval is associated with incident cardiovascular events in middle-aged and older adults without prior cardiovascular disease.
Keywords: Cardiovascular Disease, Coronary Heart Disease, Heart failure, Myocardial Infarction, QT interval, Stroke
Prolongation of the QT interval is associated with functional re-entry, torsade de pointes, and sudden death (1). Aside from its direct association with arrhythmia and sudden death, QT interval prolongation, perhaps through other mediating factors, is associated with mortality in high-risk individuals (2–5) and in the general population (6–11). Prolongation of the QT interval is also associated with incident stroke (7). However, the association of QT interval duration with incident cardiovascular events in healthy individuals has not been investigated. Increased catecholamine levels prolong the QT interval in healthy individuals and are associated with the development of atherosclerosis (12–14), and may, therefore, be mediators of any association between the QT interval and incident cardiovascular events. We sought to examine the association between baseline QT interval and incident cardiovascular events in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study participants
Inclusion criteria and methods of the MESA study were previously described (15). In brief, between July 2000 and August 2002, a total of 6,814 individuals aged 45 to 84 years and free of clinically apparent cardiovascular disease (CVD) were recruited from 6 U.S. areas: Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan and the Bronx, NY; and St. Paul, MN. A full list of participating MESA investigators and institutions can be found at the MESA study website (16). We excluded participants who were taking antiarrhythmic medications or medications that might prolong the QT interval (n = 205), and participants with QRS duration ≥120 ms (n = 336). The final sample size was 6,273.
The study complies with the Declaration of Helsinki, and the institutional review boards at all participating centers approved the study. All participants gave written informed consent.
Risk factor measures
Standardized questionnaires were used to obtain information about self-reported race/ethnicity, smoking history, and medication use for high blood pressure, high cholesterol, and diabetes. Participant height and weight were measured and body mass index (BMI; kg/m2) was calculated. Resting blood pressure was measured 3 times with participants in the seated position with an automated oscillometric sphygmomanometer (Critikon, GE Healthcare, Waukesha, WI, USA). The average of the last 2 measurements was used for analysis. Total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, and glucose levels were measured from blood samples obtained after a 12 h fast. Low-density lipoprotein (LDL) cholesterol was calculated with the Friedewald equation (17). Diabetes was defined as fasting glucose ≥126 mg/dL or use of hypoglycemic medications.
Electrocardiogram analysis of QT interval duration
Standard 12-lead electrocardiograms (ECGs) were digitally acquired using a GE MAC 1200 electrocardiograph [GE, Milwaukee, WI] at 10 mm/mV calibration and speed of 25 mm/s. ECG reading was performed centrally at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, Winston Salem, NC. All ECGs were initially inspected visually for technical errors and inadequate quality, and then automatically processed with the GE Marquette 12-SL program 2001 version (GE Marquette, Milwaukee, WI]. A global single measure of QT interval was defined as the time duration between the earliest QRS onset to the latest T wave offset (end) in the 12 ECG leads. We corrected the QT interval according to the American Heart Association, American College of Cardiology, and Heart Rhythm Society recommendations for the standardization and interpretation of the electrocardiogram using a linear regression function for adjustment for covariates (method fully described in the Statistical Methods section) (18).
Adjudication of events
The mean (SD) follow-up was 8.0 (1.7) years. In addition to 4 follow-up MESA study examinations, telephone updates regarding interim hospital admissions, cardiovascular outpatient diagnoses, and deaths were performed every 12 months. Self-reported diagnoses were confirmed by review of death certificates and medical records for hospitalizations and outpatient cardiovascular diagnoses. A detailed description of events and the process of adjudication can be found at the MESA website (16). Two physicians from the MESA study events committee independently reviewed all medical records for endpoint classification using pre-specified criteria. An incident cardiovascular event was defined as a composite of adjudicated myocardial infarction (MI), resuscitated cardiac arrest, coronary heart disease-related death, stroke, or stroke-related death. The specific incidences of stroke, MI, peripheral vascular disease (PVD), and heart failure (HF) were also determined.
Statistical methods
We utilized a linear regression method to adjust the QT interval for age, ethnicity, sex, and the RR interval using model residuals (19). Specifically, we set up a linear regression model with QT interval as the dependent variable and age (continuous), ethnicity (Caucasian, African American, Chinese American, and Hispanic), sex (female or male), and RR interval as independent variables. The RR interval was analyzed using restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles to allow a more flexible and nonlinear relationship between the QT and RR intervals. Model residuals represent the component of QT interval duration that is not explained by the independent variables. Because the average of the residuals is 0, we rescaled the residuals by adding the mean QT interval of the overall study population to calculate the final corrected QT interval (QTcorr). Sensitivity analyses were also performed with the Bazett heart rate–corrected QT interval (QTb) (20), and the Framingham QT correction formula (QTfr) (21).
Baseline characteristics were compared according to the QTcorr distribution (categorized at 5th and below, 5th to 50th, 50th to 95th, and 95th and above percentiles), and by clinically utilized thresholds for short QTb (≤ 390 ms), or QTb prolongation (≥ 460 ms)(18), using analysis of variance for continuous variables or Student t test, and chi-square tests for categorical variables. Kaplan–Meier survival curves were plotted after stratification of participants by QTcorr percentiles and compared by means of the log-rank test. Cox proportional hazards models were utilized to evaluate the association between baseline QTcorr and incident cardiovascular events. The following models were utilized: Model 1, unadjusted; Model 2, adjusted for age (continuous), ethnicity (Caucasian, African American, Chinese American, and Hispanic), and sex (female or male); and Model 3, further adjusted for: antihypertensive medication use (yes or no); systolic blood pressure (continuous); cigarette smoking (never, former, current); diabetes (yes or no); family history of ischemic heart disease (yes or no); LDL (continuous); HDL (continuous); QRS duration (continuous); aspirin use (yes or no); statin use (yes or no); body mass index (continuous); and education (<12 years, completed high school to bachelor’s degree, or graduate education). Models including MRI-derived left ventricular mass (antihypertensive medication use and systolic blood pressure omitted due to strong colinearity) were utilized in the subset of participants with MRI data to examine a potential mediating effect by left ventricular mass for the association of QTcorr with cardiovascular outcomes. To provide detailed analyses of the dose-response relationship of the QTcorr with incident cardiovascular events, we modeled QTcorr with restricted quadratic splines with knots at the 1st, 10th, and 99th percentiles of the QTcorr distribution to provide a description of the dose-response relationship. In spline analyses, we used the 50th percentile of the QTcorr distribution as the reference value (median). Finally, we examined the interaction of QTcorr with ethnicity and sex in its association with outcome using multiplicative interaction terms as well as using stratified analyses by sex and ethnicity. Comparisons of C statistics between Framingham Heart Study (FHS) risk scores (22–24) and FHS risk scores modified by the addition of 1 point for QTcorr ≥ 95th percentile (440.3 ms) for their association with incident CVD, HF, and stroke were performed. A 2-tailed p value <0.05 was considered statistically significant. Statistical analyses were performed using STATA statistical software v. 12.0 (STATACorp, LP, College Station, TX).
Results
The average (SD) age of study participants was 61.7 (10.1) years and 53.4% of study participants were female. The average (SD) QTcorr, QTb, and QTfr were 410.7 (27.8), 417.2 (20.6), and 414.8 (18.9), respectively. Table 1 summarizes the baseline characteristics by the QTcorr distribution (stratified at 5th and below, 5th to 50th, 50th to 95th, and 95th and above percentiles). A summary of baseline characteristics by clinically utilized thresholds for short QTb at ≤ 390 ms, or QTb prolongation at ≥460 ms can be found in Online Table 1.
Table 1.
Baseline Characteristics for All Patients and After Stratification by QTcorr Percentiles
| Total | QTcorr <5th (300–383 ms) | QTcorr 20th to <50th (383–409 ms) | QTcorr 50th to <95th (410–440 ms) | QTcorr ≥ 95th (441–503 ms) | p value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age, years | 61.7 ± 10.14 | 61.8 ± 10.8 | 61.7 ± 10.2 | 61.8 ± 10.0 | 61.3 ± 9.9 | 0.877 | |
|
| |||||||
| Sex, % Female | 53.4 | 42.9 | 56.1 | 52.0 | 52.7 | <0.001 | |
|
| |||||||
| Race, % | |||||||
| Caucasian | 38.3 | 32.1 | 39.0 | 38.5 | 36.2 | ||
| African American | 27.5 | 34.5 | 26.5 | 27.3 | 30.0 | 0.241 | |
| Chinese American | 12.0 | 11.8 | 12.0 | 12.2 | 10.8 | ||
| Hispanic | 22.2 | 21.6 | 22.5 | 22.0 | 23.0 | ||
|
| |||||||
| Hypertension, % | 43.8 | 32.4 | 38.5 | 47.3 | 56.5 | <0.001 | |
|
| |||||||
| Participants on antihypertensive medications, % | 36.0 | 32.4 | 31.7 | 38.1 | 46.5 | <0.001 | |
|
| |||||||
| Systolic blood pressure, mm Hg | 126.3 ± 21.45 | 122.4 ± 21.17 | 123.8 ± 20.40 | 127.9 ± 21.43 | 132.1 ± 23.91 | <0.001 | |
|
| |||||||
| Heart rate, beats/min | 63.1 ± 9.59 | 59.4 ± 9.93 | 63.7 ± 9.19 | 62.7 ± 9.58 | 63.6 ± 10.62 | <0.001 | |
|
| |||||||
| Diabetes, % | 11.1 | 10.5 | 10.5 | 10.6 | 15.5 | 0.007 | |
|
| |||||||
| Family history of heart disease, % | 42.6 | 40.9 | 43.2 | 42.4 | 41.7 | 0.811 | |
|
| |||||||
| Current Smoking, % | 13.1 | 12.2 | 12.4 | 12.5 | 18.7 | 0.001 | |
|
| |||||||
| QRS duration, ms | 91.4 ± 9.66 | 88.4 ± 9.54 | 90.1 ± 9.49 | 92.7 ± 9.43 | 92.9 ± 10.25 | <0.001 | |
|
| |||||||
| QT duration, ms | 410.7 ± 30.25 | 383.3 ± 24.55 | 397.8 ± 23.87 | 422.1 ± 26.53 | 429.2 ± 38.07 | <0.001 | |
|
| |||||||
| QTb duration, ms | 417.2 ± 20.61 | 377.5 ± 13.95 | 406.5 ± 11.33 | 427.5 ± 13.24 | 437.2 ± 27.35 | <0.001 | |
|
| |||||||
| QTcorr duration, ms | 410.7 ± 17.79 | 374.1 ± 9.38 | 399.6 ± 6.89 | 421.3 ± 8.08 | 451.2 ± 10.93 | <0.001 | |
|
| |||||||
| QTfr duration, ms | 414.8 ± 18.9 | 377.4 ± 11.2 | 403.9 ± 9.1 | 425.3 ± 10.4 | 433.9 ± 25.7 | <0.001 | |
|
| |||||||
| Height, cm | 166.2 ± 10.04 | 166.7 ± 10.13 | 165.9 ± 10.0 | 166.7 ± 10.0 | 165.2 ± 10.31 | <0.001 | |
|
| |||||||
| Weight, lbs | 172.8 ± 38.16 | 163.8 ± 35.16 | 169.9 ± 37.31 | 176.0 ± 38.14 | 176.2 ± 41.28 | <0.001 | |
|
| |||||||
| Body mass index, kg/m2 | 28.3 ± 5.47 | 26.63 ± 4.75 | 27.9 ± 5.37 | 28.7 ± 5.43 | 29.2 ± 6.07 | <0.001 | |
|
| |||||||
| Low-density lipoprotein cholesterol, mg/dL | 117.6 ± 31.43 | 115.23 ± 33.05 | 117.8 ± 31.28 | 117.7 ± 31.26 | 116.9 ± 31.96 | 0.532 | |
|
| |||||||
| High-density lipoprotein cholesterol, mg/dL | 51.1 ± 14.81 | 52.4 ± 16.38 | 51.4 ± 14.96 | 50.5 ± 14.46 | 51.1 ± 14.79 | 0.054 | |
|
| |||||||
| Aspirin, % | 31.0 | 31.1 | 30.9 | 31.2 | 30.0 | 0.932 | |
|
| |||||||
| Statins, % | 14.4 | 15.2 | 14.8 | 14.1 | 14.1 | 0.866 | |
|
| |||||||
| Education (Graduate) % | 17.8 | 21.3 | 18.5 | 17.7 | 13.5 | <0.001 | |
QTcorr = QT interval corrected for R-R interval, race, age and sex; QTb = QT corrected using Bazett formula; QTfr = QT interval corrected using the Framingham formula.
Table 2 presents the unadjusted and adjusted associations of baseline QTcorr with incident CVD events over the follow-up period. In fully adjusted models, each 10 ms increase in the baseline QTcorr was positively associated with incident HF (HR 1.25, 95% CI 1.14 to1.37, p <0.001), CVD (HR 1.12, 95% CI 1.05 to 1.20, p <0.001), and stroke (HR 1.19, 95% CI 1.07 to 1.32, p <0.001). Positive trends for association were also noted between baseline QTcorr and MI (HR 1.10, 95% CI 0.99 to 1.21, p = 0.055), PVD (HR 1.16, 95% CI 0.99 to 1.35, p = 0.054), and coronary heart disease (CHD) (HR 1.08, 95% CI 1.00 to 1.18, p = 0.065). Kaplan-Meier analyses revealed that participants with QTcorr intervals in the highest 5th percentile had increased risks of HF, CVD, and stroke (Online Figures 1–3). Consistent results were observed using the spline regression models, where the risk of HF, CVD and stroke events progressively increased with increasing QTcorr intervals (Figures 1–3).
Table 2.
The Association of Baseline Qtcorr Interval With Incident CVD Events
| Model 1* | Model 2† | Model 3‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Number Events/Number at risk§ | Hazard Ratio (per 10 ms) | 95% CI | p value | Number Events/Number. at Risk|| | Hazard Ratio (per 10 ms) | 95% CI | p value | Number Events/Number at Risk§ | Hazard Ratio (per 10 ms) | 95% CI | p value |
| Stroke | 109/5,913 | 1.22 | 1.11–1.35 | <0.001 | 109/5,913 | 1.21 | 1.10–1.34 | <0.001 | 102/5,441 | 1.19 | 1.07–1.32 | 0.001 |
| MI | 145/5,913 | 1.11 | 1.01–1.21 | 0.022 | 145/5,913 | 1.10 | 1.01–1.20 | 0.031 | 129/5,441 | 1.10 | 0.99–1.21 | 0.055 |
| HF | 146/5,913 | 1.30 | 1.19–1.41 | <0.001 | 146/5,913 | 1.29 | 1.19–1.39 | <0.001 | 128/5,441 | 1.25 | 1.14–1.37 | <0.001 |
| PVD | 63/5,913 | 1.19 | 1.04–1.35 | 0.01 | 63/5,913 | 1.18 | 1.04–1.34 | 0.012 | 52/5,441 | 1.16 | 0.99–1.35 | 0.054 |
| CHD | 187/5,913 | 1.08 | 1.00–1.17 | 0.044 | 187/5,913 | 1.08 | 1.00–1.16 | 0.064 | 167/5,441 | 1.08 | 1.00–1.18 | 0.065 |
| CVD | 291/5,913 | 1.13 | 1.06–1.20 | <0.001 | 291/5,913 | 1.12 | 1.06–1.20 | <0.001 | 264/5,441 | 1.12 | 1.05–1.20 | 0.001 |
Model 1, unadjusted.
Model 2, adjusted for age, ethnicity and sex.
Model 3, adjusted for Model 2 plus traditional risk factors (antihypertensive medication use, systolic blood pressure, current smoking, diabetes, family history of ischemic heart disease, LDL, HDL) and QRS duration, aspirin use, statin use, BMI, and education.
The number at risk varies for each model, depending upon participant follow-up and missing data.
Cardiovascular disease: including MI, resuscitated cardiac arrest, coronary heart disease death, stroke or stroke death.
CI = confidence interval; CVD = cardiovascular disease; HF = heart failure; MI = myocardial infarction; PVD = peripheral vascular disease; QTcorr = QT interval corrected for R-R interval, race, age, and sex.
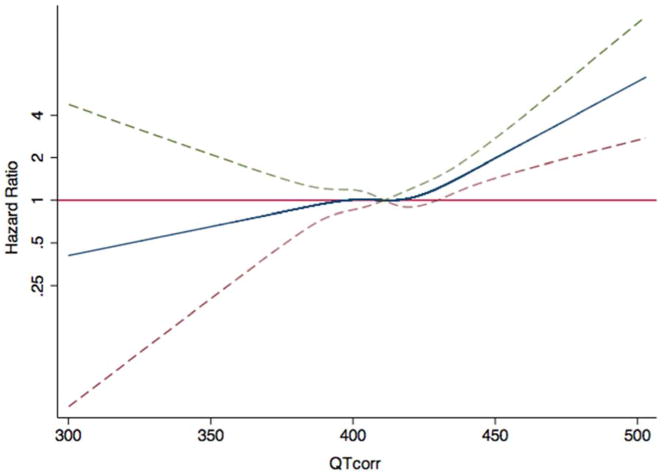
Figure 1. Hazard Ratios for HF as a Function of the QT Interval.
The blue line indicates multivariable-adjusted hazard ratios for heart failure (HF) as a function of QTcorr using restricted quadratic splines. The dashed lines delineate the upper and lower 95% confidence intervals. The horizontal red line indicates a hazard ratio of 1. The model was adjusted for age, ethnicity, sex, antihypertensive medication use, systolic blood pressure, cigarette smoking, diabetes, family history of ischemic heart disease, LDL, HDL, QRS duration, aspirin use, statin use, body mass index, and education. HDL = high-density lipoprotein; LDL = low-density lipoprotein.
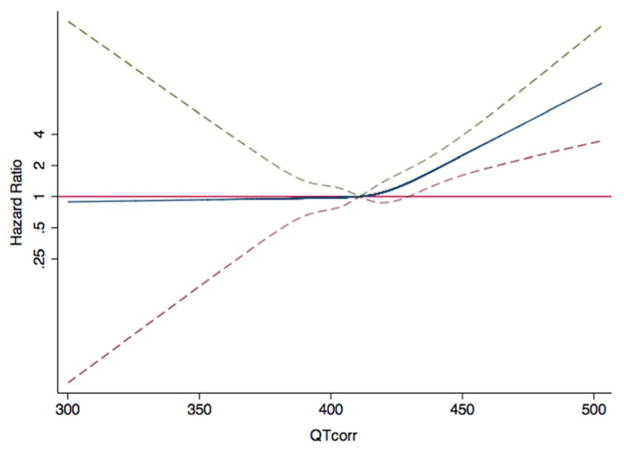
Figure 3. Hazard Ratios for Stroke as a Function of the QT Interval.
The blue line indicates multivariable-adjusted hazard ratios for stroke as a function of QTcorr using restricted quadratic splines. The dashed lines delineate the upper and lower 95% confidence intervals. The horizontal red line indicates a hazard ratio of 1. Adjustment factors are described in Figure 1.
In sensitivity analyses, each 10 ms increase in the QTb was also positively associated with incident HF (HR 1.27, 95% CI 1.18 to 1.38, p <0.001), CVD (HR 1.15, 95% CI 1.08 to 1.22, p <0.001), and stroke (HR 1.20, 95% CI 1.10 to 1.33, p <0.001) in fully adjusted models. Similarly, each 10 ms increase in the QTfr was also positively associated with incident HF (HR 1.23, 95% CI 1.12 to 1.34, p <0.001), CVD (HR 1.11, 95% CI 1.04 to 1.19, p = 0.002), and stroke (HR 1.17, 95% CI 1.06 to 1.30, p = 0.003) in fully adjusted models.
The baseline QTcorr had a weak positive association with left ventricular mass (Spearman’s rho 0.072, p <0.001). Despite reduced power, the baseline QTcorr interval remained associated with incident HF (HR 1.25, 95% CI 1.00 to 1.55, p = 0.051) and CVD (HR 1.20, 95% CI 1.04 to 1.37, p = 0.010) after adjustment for left ventricular mass and other potential confounders in the subset of participants with MRI data (Online Table 2). After adjustment for left ventricular mass in this small subset of participants, positive trends for association were also noted between baseline QTcorr and stroke (HR 1.21, 95% CI 0.99 to 1.48, p = 0.061), MI (HR 1.17, 95% CI 0.98 to 1.40, p = 0.087), PVD (HR 1.23, 95% CI 0.95 to 1.59, p = 0.114), and CHD (HR 1.17, 95% CI 0.98 to 1.40, p = 0.087).
C statistics for FHS risk scores modified with QTcorr were higher than FHS risk scores for their association with HF (0.735 versus 0.724, p <0.001), CVD (0.732 versus 0.730, p = 0.119) and stroke (0.712 versus 0.708, p = 0.024).
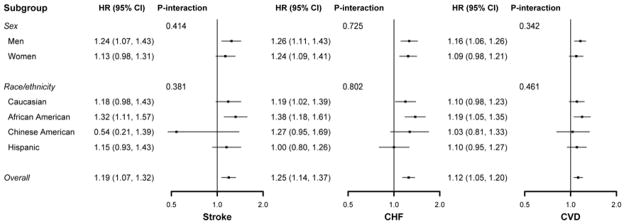
There was no evidence of multiplicative interaction with sex in the association of QTcorr with HF (HR for interaction 0.97, 95% CI 0.82 to 1.15, p = 0.725), CVD (HR for interaction 1.07, 95% CI 0.93 to 1.21, p = 0.342), or stroke (HR for interaction 1.09, 95% CI 0.89 to 1.33, p = 0.414). There was also no evidence of multiplicative interaction with ethnicity in the association of QTcorr with HF (HR for interaction 1.00, 95% CI 0.99 to 1.01, p = 0.834), CVD (HR for interaction 1.00, 95% CI 0.99 to 1.00, p = 0.388), or stroke (HR for interaction 0.99, 95% CI 0.99 to 1.01, p = 0.934). When stratified by sex and ethnicity, the association between QTcorr and HF, CVD, and stroke was similar among the subgroups (Figure 4).
Figure 4. Forest Plot of Hazard Ratios for HF, CVD, and Stroke after Stratification by Sex and Ethnicity.
The forest plot summarizes multivariable-adjusted hazard ratios (HRs) for heart failure (HF), cardiovascular disease (CVD), and stroke. Models were adjusted for age, ethnicity (if not stratified by ethnicity), sex (if not stratified by sex), antihypertensive medication use, systolic blood pressure, cigarette smoking, diabetes, family history of ischemic heart disease, LDL, HDL, QRS duration, aspirin use, statin use, body mass index, and education. There were no significant differences among the subgroups and no evidence for interaction. CI indicates confidence interval. Other abbreviations as in Figure 1.
Discussion
In this study, we report an independent positive association between the baseline corrected QT interval and cardiac and vascular events in middle-aged participants without prior cardiovascular disease. Importantly, the association is not limited to a specific sex or ethnicity. Individuals with prolonged ventricular repolarization, reflected on the surface ECG by prolongation of the QT interval, are predisposed to ventricular fibrillation and sudden death. Such an association is most evident in the setting of genetic abnormalities of potassium and sodium channels within cell membranes (25), as well as with comorbidities such as severe electrolyte imbalance, central nervous system injury, and myocardial infarction (26,27). Acquired prolongation of the corrected QT interval due to antiarrhythmic, antibiotic, antihistaminic, or psychotropic drugs is also associated with ventricular arrhythmia and sudden death when the QT interval exceeds 500 ms (28). In addition, several studies of participants with and without prior CVD have found a positive association between the baseline QT interval and incident cardiovascular mortality (8,10,19,29–31) and cardiovascular morbidity, including a composite of atherosclerotic disease, angina, and cardiomyopathy in a general population sample (32) and coronary events in hypertensive participants (5). However, the results have been inconsistent and negative studies of the association between the baseline QT interval and cardiovascular mortality (33) and coronary events (34) have also been published. A systematic review of 7 large prospective cohort studies reported that prolongation of the QT interval in patients with established CVD was associated with increased risk for total mortality, cardiovascular mortality and sudden death; however, among individuals without baseline CVD the QT interval was not associated with these outcomes (11). In contrast, another meta-analysis of 6 studies, with participants free of CVD at baseline, found a positive association between the QT interval and mortality (35).
Recently, Soliman and colleagues reported a strong association between the QT interval and incident stroke among 27,411 participants in the REGARDS study (7). The results of REGARDS are in full agreement with our findings. Our study differs from the REGARDS study in that the baseline prevalence of CVD in the REGARDS study was 17% among all participants, and 43% among those with prolonged QT. Additionally, the REGARDS study was designed to investigate the causes of regional and racial disparities in stroke mortality, oversampling African-Americans and residents of the southeastern belt United States region.
Several studies support an association between the QT interval and cardiovascular events in healthy individuals. Dekker and colleagues reported an association between prolonged QTb (>420 ms) and the combined end point of myocardial infarction (MI) and CHD death compared with men with QTb <385 ms in the Zutphen study cohort that included 851 middle-aged men without previous MI (6). In another study, Dekker and colleagues noted an association between corrected QT and CHD, CVD mortality, and total mortality in the ARIC cohort, which included middle-aged men and women without prior MI or coronary revascularization (9). In contrast to our results, the risk in ARIC was attenuated by ethnicity and was higher in African-Americans. Our study builds upon prior reports in healthy individuals by the use of the regression-derived QTcorr variable with simultaneous adjustment for age, ethnicity, sex, and heart rate, and by exclusion of participants with QRS ≥120 ms and those on QT-prolonging medications. The QT interval varies as a function of sympathetic and parasympathetic tone. Increased catecholamine levels induce QT interval prolongation in healthy individuals (12–14). Additionally, high sympathetic tone is associated with the development of atherosclerosis (12–14). Over 3 decades ago, Pauletto and colleagues proposed several mechanisms that may mediate the association of high sympathetic tone with atherosclerosis (36). First, elevated arterial pressure in the setting of high sympathetic tone may lead to arteriolar remodeling and alteration of flow with upstream impact on the large arteries. Secondly, epinephrine and norepinephrine appear to exert direct atherogenic effects, regardless of arterial pressure (37–41). Thirdly, the atherogenic effects of catecholamines may be mediated through platelet activation and subsequent platelet-derived growth factor up-regulation or platelet deposition at the arterial intima (42). Thus, prolongation of the QT interval as a surrogate of elevated sympathetic tone may explain its association with cardiovascular events. The association between QTcorr and incident HF may also be partially mediated by an association of QTcorr and left ventricular mass. However, despite the reduced sample size in the cohort of MESA participants with MRI data, the association of QTcorr with incident HF was preserved after adjustment for left ventricular mass. Nevertheless, QT prolongation may be associated with other factors, which may mediate its association with cardiovascular events. Our results did not investigate the mechanism and simply point to an association. Finally, it is important to emphasize that the association of QTcorr with incident cardiovascular events does not imply that the QTcorr should be used as an index for risk stratification in the clinical setting. Nevertheless, the C statistics for FHS risk scores modified with the QTcorr were higher than those for FHS risk scores for identification of cardiovascular events.
Study Limitations
We examined the association of only a single QT interval measurement at baseline with incident HF, CVD, and stroke events. Also, our findings represent a selected population enrolled from 6 different U.S. areas and may not be generalizable to all populations. However, the inclusion of 4 different ethnicities in the MESA cohort is a significant strength that improves the applicability of the data to various patient groups.
Conclusions
This analysis demonstrates positive associations between baseline-corrected QT intervals and risks of incident stroke, HF, and CVD events in a cohort of middle-aged participants free of CVD at baseline.
Perspectives
Competency in Medical Knowledge
Prolongation of the corrected QT interval on the ECG is associated with an elevated risk of arrhythmic and sudden death, as well as incident stroke, heart failure, and ischemic cardiovascular events in patients with and without ischemic heart disease, including otherwise healthy individuals, regardless of sex or ethnicity.
Translational Outlook
Future studies should evaluate the discriminative value of adding the QT interval to existing risk assessment tools.
Supplementary Material
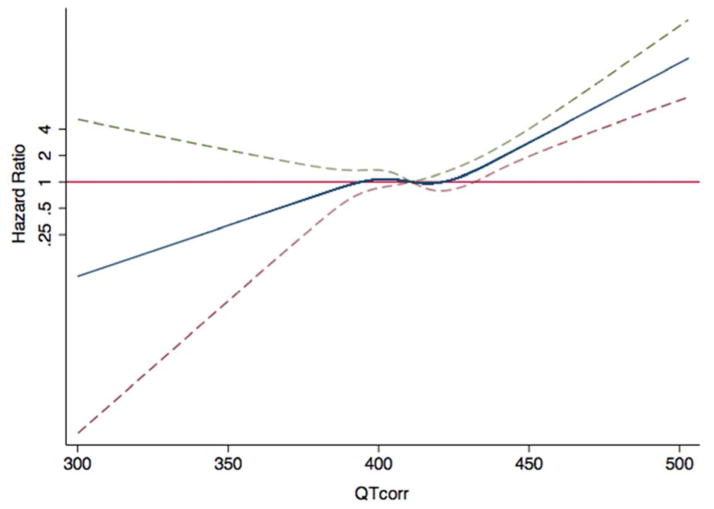
Figure 2. Hazard Ratios for CVD as a Function of the QT Interval.
The blue line indicates multivariable-adjusted hazard ratios for cardiovascular disease (CVD) endpoints as a function of QTcorr using restricted quadratic splines. The dashed lines delineate the upper and lower 95% confidence intervals. The horizontal red line indicates a hazard ratio of 1. Adjustment factors are described in Figure 1.
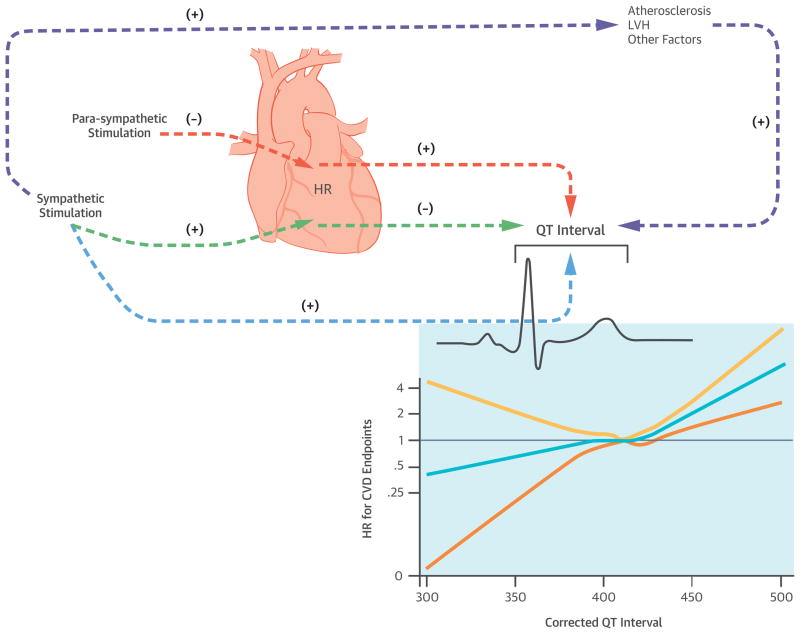
Figure 5. Central Illustration. Sympathetic and Parasympathetic Stimuli, the QT-Interval, and CVD Events.
There is a complex interaction between the central nervous system (CNS) sympathetic and parasympathetic stimuli and cardiac conduction intervals. By increasing the heart rate (HR), sympathetic stimulation (green arrows) can secondarily decrease the QT-interval. In contrast, by decreasing the heart rate, parasympathetic stimulation can increase the QT interval (orange arrows). Autonomic stimuli also exert direct effects upon the QT interval. Increased catecholamine levels typically result in QT interval prolongation in healthy individuals (light green arrow). Additionally, high sympathetic tone is associated with the development of atherosclerosis with potential secondary effects upon cardiac repolarization and the QT interval (grey arrows). Left ventricular mass is also positively associated with high sympathetic tone and the QT interval (grey arrows). In this study, we observed positive associations between baseline-corrected QT intervals and risks of incident stroke, HF, and CVD events in a cohort of middle-aged participants free of cardiovascular disease at baseline. Prolongation of the QT interval as a surrogate of elevated sympathetic tone and autonomic imbalance may explain its association with cardiovascular events. However, the QT interval may be associated with other factors, which may mediate its association with cardiovascular events. Please note that the arrows in this illustration are provided to note associations and do not imply causality.
CVD = cardiovascular disease; HR = heart rate; LV = left ventricle.
Acknowledgments
Dr. Nazarian is funded by NIH grants K23HL089333 and R01HL116280. The contents of this paper are the responsibility of the authors and do not necessarily represent the official views of the NIH. The MESA study was funded by grants N01-HC-95159 through N01-HC95169 from the National Heart, Lung and Blood Institute. The authors thank the other investigators, staff, and participants of the MESA study for their contributions.
Abbreviations
- CHD
coronary heart disease
- CVD
cardiovascular disease
- ECG
electrocardiography
- FHS
Framingham Heart Study
- HDL
high-density lipoprotein
- HF
heart failure
- LDL
low-density lipoprotein
- MI
myocardial infarction
- PVD
peripheral vascular disease
Footnotes
Disclosures: Dr. Nazarian is a scientific advisor to Biosense Webster Inc. and is the principal investigator for research funding to Johns Hopkins from Biosense Webster, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll of Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 2.Williams ES, Thomas KL, Broderick S, et al. Race and gender variation in the QT interval and its association with mortality in patients with coronary artery disease: results from the Duke Databank for Cardiovascular Disease (DDCD) Am Heart J. 2012;164:434–41. doi: 10.1016/j.ahj.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Peters RW, Byington RP, Barker A, et al. Prognostic value of prolonged ventricular repolarization following myocardial infarction: the BHAT experience. The BHAT Study Group. J Clin Epidemiol. 1990;43:167–72. doi: 10.1016/0895-4356(90)90180-w. [DOI] [PubMed] [Google Scholar]
- 4.Naas AA, Davidson NC, Thompson C, et al. QT and QTc dispersion are accurate predictors of cardiac death in newly diagnosed non-insulin dependent diabetes: cohort study. BMJ. 1998;316:745–6. doi: 10.1136/bmj.316.7133.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schillaci G, Pirro M, Ronti T, et al. Prognostic impact of prolonged ventricular repolarization in hypertension. Arch Intern Med. 2006;166:909–13. doi: 10.1001/archinte.166.8.909. [DOI] [PubMed] [Google Scholar]
- 6.Dekker JM, Schouten EG, Klootwijk P, et al. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation. 1994;90:779–85. doi: 10.1161/01.cir.90.2.779. [DOI] [PubMed] [Google Scholar]
- 7.Soliman EZ, Howard G, Cushman M, et al. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2012;59:1460–7. doi: 10.1016/j.jacc.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noseworthy PA, Peloso GM, Hwang SJ, et al. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2012;17:340–8. doi: 10.1111/j.1542-474X.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekker JM, Crow RS, Hannan PJ, et al. Heart rate-corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle-aged men and women: the ARIC study. J Am Coll Cardiol. 2004;43:565–71. doi: 10.1016/j.jacc.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Schouten EG, Dekker JM, Meppelink PJ, et al. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–23. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 11.Montanez A, Ruskin JN, Hebert PR, et al. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–8. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 12.Baumert M, Lambert GW, Dawood T, et al. QT interval variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol Heart Circ Physiol. 2008;295:H962–H968. doi: 10.1152/ajpheart.00301.2008. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman ES, Gorodeski EZ, Dettmer MM, et al. Use of autonomic maneuvers to probe phenotype/genotype discordance in congenital long QT syndrome. Am J Cardiol. 2005;96:1425–30. doi: 10.1016/j.amjcard.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 14.Vyas H, Hejlik J, Ackerman MJ. Epinephrine QT stress testing in the evaluation of congenital long-QT syndrome: diagnostic accuracy of the paradoxical QT response. Circulation. 2006;113:1385–92. doi: 10.1161/CIRCULATIONAHA.105.600445. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed September 3, 2014];MESA website. 2014 Available at: http://www.mesa-nhlbi.org.
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Rautaharju PM, Surawicz B, Gettes LS, et al. American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–50. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Post WS, Dalal D, et al. QT-interval duration and mortality rate: results from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1727–33. doi: 10.1001/archinternmed.2011.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 21.Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Wolf PA, Belanger AJ, et al. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, D’Agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 25.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. New Engl J Med. 2003;348:1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074–7. doi: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 27.Moss AJ. Prolonged QT-interval syndromes. JAMA. 1986;256:2985–7. [PubMed] [Google Scholar]
- 28.Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis. 2001;43:1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 29.de Bruyne MC, Hoes AW, Kors JA, et al. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. The Rotterdam Study. Eur Heart J. 1999;20:278–84. doi: 10.1053/euhj.1998.1276. [DOI] [PubMed] [Google Scholar]
- 30.Algra A, Tijssen JG, Roelandt JR, et al. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 31.Karjalainen J, Reunanen A, Ristola P, et al. QT interval as a cardiac risk factor in a middle aged population. Heart. 1997;77:543–8. doi: 10.1136/hrt.77.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elming H, Holm E, Jun L, et al. The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J. 1998;19:1391–400. doi: 10.1053/euhj.1998.1094. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg RJ, Bengtson J, Chen ZY, et al. Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience) Am J Cardiol. 1991;67:55–8. doi: 10.1016/0002-9149(91)90099-7. [DOI] [PubMed] [Google Scholar]
- 34.Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: the ARIC Study with 13 years of follow-up. Circulation. 2003;108:1985–9. doi: 10.1161/01.CIR.0000095027.28753.9D. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Post WS, Blasco-Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22:660–70. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauletto P, Scannapieco G, Pessina AC. Sympathetic drive and vascular damage in hypertension and atherosclerosis. Hypertension. 1991;17:III75–81. doi: 10.1161/01.hyp.17.4_suppl.iii75. [DOI] [PubMed] [Google Scholar]
- 37.Ostlund-Lindqvist AM, Lindqvist P, Brautigam J, et al. Effect of metoprolol on diet-induced atherosclerosis in rabbits. Arteriosclerosis. 1988;8:40–5. doi: 10.1161/01.atv.8.1.40. [DOI] [PubMed] [Google Scholar]
- 38.Lindqvist P, Olsson G, Nordborg C, et al. Atherosclerosis in rabbits identified as high and low responders to an atherogenic diet and the effect of treatment with a beta 1-blocker. Atherosclerosis. 1988;72:163–72. doi: 10.1016/0021-9150(88)90077-9. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan JR, Manuck SB, Clarkson TB, et al. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–5. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan JR, Manuck SB, Adams MR, et al. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–72. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- 41.Pettersson K, Bejne B, Bjork H, et al. Experimental sympathetic activation causes endothelial injury in the rabbit thoracic aorta via beta 1-adrenoceptor activation. Circ Res. 1990;67:1027–34. doi: 10.1161/01.res.67.4.1027. [DOI] [PubMed] [Google Scholar]
- 42.Armstrong ML, Peterson RE, Hoak JC, et al. Arterial platelet accumulation in experimental hypercholesterolemia. Atherosclerosis. 1980;36:89–100. doi: 10.1016/0021-9150(80)90202-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.