Abstract
Protein-carbohydrate interactions play significant role in modulating cell-cell and cell-extracellular matrix interactions, which, in turn, mediate various biological processes such as growth regulation, immune function, cancer metastasis, and apoptosis. Galectin-3, a member of the β-galactoside-binding protein family, is found multifunctional and is involved in normal growth development as well as cancer progression and metastasis, but the detailed mechanisms of its functions are not well understood. This review discusses its structure, binding properties, transcriptional regulation and roles in homotypic/heterotypic cell adhesion, angiogenesis and apoptosis.
Keywords: galectin-3, angiogenesis, apoptosis, tumorigenesis, TF-disaccharide
Introduction
Interactions between cells, between cells and the extracellular matrix (ECM) are pivotal for proper cellular function. In recent years, protein-carbohydrate interactions have been considered as very important for modulation cell-cell and cell-ECM interactions, which, in turn, mediate various biological processes such as cell activation, growth regulation, cancer metastasis, and apoptosis. Thus, the identification of carbohydrate-binding proteins (lectins) and their partners (carbohydrate ligands), and the detailed understanding of the molecular mechanisms and downstream effects of these protein-carbohydrate interactions are subjects of current intense research. Galectins (gal), a family of β-galactoside-binding proteins, are involved in growth development as well as cancer progression and metastasis1–5. However, the detailed mechanisms of these functions remain largely unknown. Of the fifteen members of the galectin family identified so far, gal1, 2, 5, 7, 10, 11, 13, 14, and 15 are examples of the “proto” type galectins (one carbohydrate-recognition domain [CRD] per subunit), while gal4, 6, 8, 9, and 12 are “tandem-repeat” type galectins, which contain two CRDs6 (Fig. 1). Gal-3 is the only representative of the “chimera” galectin type and probably the most studied member of the galectin family2, 7–10. As a multifunctional protein with increased or decreased expression in many types of human cancers, it has generated significant interest in cancer research over the past decades. Here we describe its structure and roles in various aspects of tumorigenesis.
Fig 1. Classification of galectins.
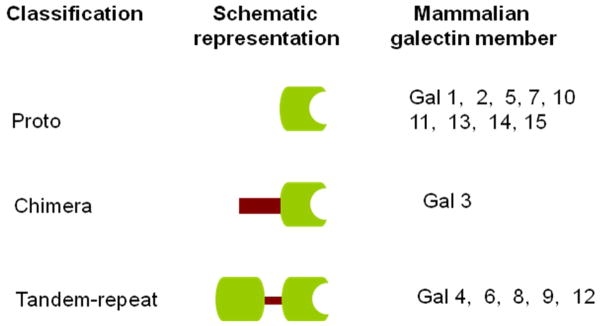
Schematic representation of proto-, chimera, and tandem-repeat type galectins. They are numbered according to the order of their discovery.
Structure of gal-3
Primary structure
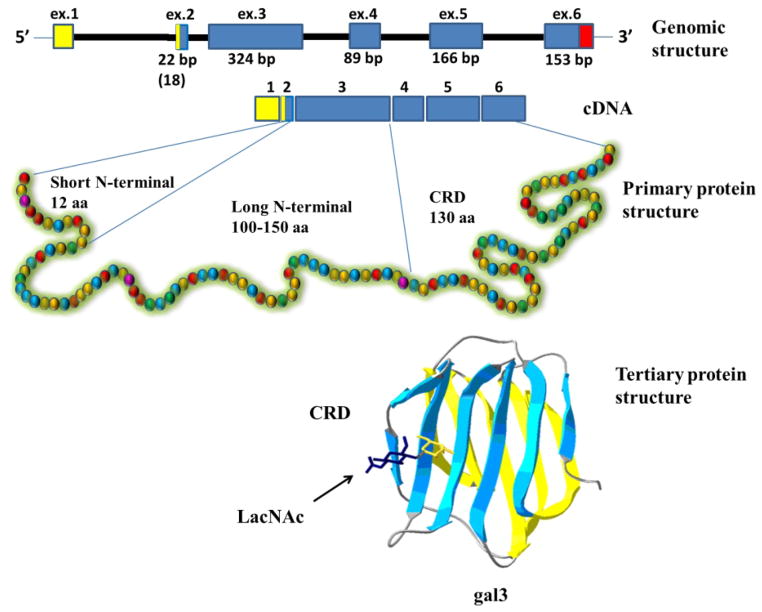
Gal-3 (previously known as Mac-2, L-29, L-31, L-34, IgE binding-protein, CBP35, and CBP30) contains three structurally distinct domains: a 12-amino acid short N-terminal domain (ND), proline and glycine rich long ND, and a C-terminal CRD11 (Fig. 2). Gal-3 is a monomer, but can form multimer at certain circumstances such as at high concentration12. The short ND is highly conserved in all mammalian gal-38 and may have at least two roles; its deletion blocks secretion of gal-39, while mutation of the conserved Ser6 affects gal-3 anti-apoptotic signaling activity13. The long ND is responsible for multimerization of gal-3 and shows positive cooperativity in carbohydrate binding12. For example, matrix metalloproteinases, MMP-2 and MMP-9 cleave gal-3 at the position Ala62Tyr63 resulting in 22 kDa fragment that fails to self associate14. The C-terminal domain of gal-3 is composed of about 130 amino acids that form a globular structure like other galectins8. It accommodates whole carbohydrate-binding site, which is responsible for lectin activity15–16. Within the CRD particularly interesting amino acid sequence is NWGR. This motif is highly conserved within the BH1 domain of the Bcl-2 family proteins, and is responsible for the anti-apoptotic activity of both Bcl-2 and gal-317. The NWGR motif is also involved in self-association of gal-3 molecules through the CRDs in the absence of saccharide ligands18.
Fig 2. Structure of galectin-3.
Schematic representation of nucleotide (genomic and cDNA) and protein (primary and tertiary) structures.
Genomic structure
The human gal-3 gene (LGALS3), located on chromosome 14, locus q21q2219, is about 17 kb long and is composed of six exons and five introns20 (see Fig. 2). Exon I contains the major part of the 5′ untranslated sequence of mRNA; while exon II encodes the remaining part of the 5′ untranslated region, the translation initiation site and codon sequence for the first six amino acids including the initial methionine. The N-terminal domain of the gal-3 is located in exon III; while the CRD is in exon V. Interestingly, the intron II of LGALS3 contains an internal promoter that drives production of alternative transcripts preferentially in peripheral blood leukocytes21, 22. These transcripts arise from an internal gene embedded within LGALS3, named galig (galectin-3 internal gene)22. Galig’s CRD is incapable of binding carbohydrates as it contains two overlapping open-reading frames within the lectin coding sequence. However, the galig protein promotes cytochrome c release upon direct interaction with the mitochondria23.
Carbohydrate-binding properties of gal-3 and its ligands
Although all galectins bind β-galactoside, their ability to discriminate among carbohydrate structures is striking. For most galectins, N-acetyllactosamine (Galβ1,4GlcNAc) is 5–10 times more active than lactose24–28 and so N-glycans are good ligands. Interestingly, the TF-disaccharide (Galβ1,3GalNAc) present in O-glycans is also a good ligand for gal-3, but not for gal125, 26. The basis for the variable binding profiles of these galectins has been explained by their 3-D structures29, 30. Although galectins lack a typical secretory signal peptide31, they are present not only in the cytosol but also in the ECM32, 33. In the extracellular space, galectins bind to β-galactoside-containing glycoproteins of ECM and cell surface. Extracellular gal-3 binds laminin34, 35, fibronectin36, CD2937, CD6638, α1β1 integrin36, and Mac-2 binding protein39. Intracellularly, gal-3 binds gemin 440, Bcl-241, nucling42, synexin43, and β-catenin44, 45 via protein-carbohydrate or protein-protein interactions.
Transcriptional regulation
Although a large body of data about gal-3 expression is available in the literature, the mechanisms of regulation of gal-3 expression are not well understood. However, the expression of gal-3 depends on cell type, external stimuli and environmental conditions and involves numerous transcription factors and signaling pathways8. Gal-3 expression may serve as differentiation marker for certain cell types. For example, the differentiation of the human monocytes or promyelocytic cell line HL-60 tomacrophage-like cells induced by phorbol ester is accompanied by increased expression of gal-346. Gal-3 expression is up-regulated in phagocytic macrophages and thus considered as a “macrophage activation marker” 47. Gal-3 expression is also elevated in microglia and macrophages activated by phagocytosis of myelin or when exposed to granulocyte-macrophage colony-stimulating factor48. In contrast, activation of human monocytes by lipopolysaccharide and interferon-γ is accompanied by decrease of gal-3 expression49. The reduced expression of gal-3 was also observed in monocytic THP-1 cells treated with non-steroidal50 or cortico-steroidal anti-inflammatory drugs51. Interestingly, gal-3 expression is absent or barely detected in the resting lymphocytes52, 53, but the activated B and T cells induce gal-3expression53. Gal-3 could be considered also as a transformation marker since the gal-3 expression is increased in fully ras-transformed fibroblasts, when cells have lost their anchorage-dependent growth54.
In the promoter region of the gal-3 gene, several regulatory elements such as five putative Sp1 binding sites (GC boxes), five cAMP-dependent response element (CRE) motifs, four AP-1- and one AP-4-like sites, two NF-κB-like sites, one sis-inducible element (SIE) and a consensus basic helixloophelix (bHLH) core sequence are found20. The presenceof multiple GC box motifs for binding ubiquitous expressed Sp1 transcription factor is a characteristic of constitutively expressed “housekeeping” genes. The activation of the Sp1 binding transcription factor is responsible for gal-3 induction by Tat protein of HIV55. The SIE that binds sis-inducible factors was suggested to be a possible candidate for the growth-induced activation of gal-3 gene expression, caused by the addition of serum. The presence of CRE and NF-κB-like site in the gal-3 promoter suggests that the activation of gal-3 expression could be regulated through the signaling pathways involving the cAMP-response element-binding protein (CREB) or the NF-κB transcription factor. The CREB/ATF and the NF-κB/Rel transcription factors pathways may be involved in the regulation of gal-3 expression by the Tax protein during HTLV-I infection of T cells56. The involvement of the NF-κB transcription factorin regulation of gal-3 expression, as well as the Jun protein, a component of AP-1 transcription factor has recently been confirmed57. The regulation of gal-3 expression through the NF-κB transcription factor was shown to be mediated by nucling, a novel apoptosis-associated protein, which interferes with NF-κB via the nuclear translocation process of NF-κB/p65, thus inhibiting galectin-3 expression on both protein and mRNA level58, 59. In skeletal tissues, the regulation of gal-3 expression is mediated by the transcription factor Runx27. Very recently, gal-3 expression is found to be regulated in pituitary and prostate tumors by methylation of CpG islands in promoter region60–63. Gal-3 was shown highly expressed in androgen independent PC-3 and DU-145 cells, but weakly expressed in androgen dependent LNCaP cells62. Treatment of LNCaP cells with azacytidine (DNA methyltransferase inhibitor) showed restored expression of gal-3 indicating that the promoter methylation is responsible for gal-3 gene silencing62. We have also demonstrated DNA methylation on the gal-3 promoter in LNCaP cells following PCR amplification of the bisulfate treated DNA and cloning and sequencing of the PCR product60.
Role of gal-3 in cell-cell and cell-ECM interactions
Gal-3 plays an important role in normal development and tumorigenesis through regulating cell proliferation, apoptosis, cell adhesion, invasion, angiogenesis and metastasis by binding to the cell surface β-galactose-containing glycoconjugates or glycolipids. Gal-3 may exert its multiple biological roles intracellularly within the nucleus or the cytoplasm, or after its secretion, at the cell surface and/or the extracellular space, mediating interactions between cells and the extracellular matrix3, 4, 6.
Gal-3 expression in normal tissues: Role of gal-3 in growth development
Gal-3 is developmentally regulated and expressed in many tissues of adults64, 65. During mouse embryogenesis, gal-3 first appears at fourth day of gestation in the trophectoderm of blastocyst, followed by its expression in the notochord cells between 8.5 and 11.5 days of gestation64. In later stages of mouse development, gal-3 is expressed in the cartilage, ribs, facial bones, suprabasal layer of epidermis, endodermallining of the bladder, larynx and oesophagus8. In adult, gal-3 is mainly expressed in the epithelial cells such as small intestine66, colon67, cornea68, 69, kidney70, lung71, thymus72, breast73, and prostate74. The expression of gal-3 is also detected in ductal cells of salivary glands75, pancreas76, kidney77, and eye78 and in intrahepaticbile ducts79. Regarding cell type, gal-3 expression is observed in fibroblasts80, chondrocytes and osteoblasts41, osteoclasts81, keratinocytes82, Schwann cells83 and gastric mucosa84, endothelial cells85, and also immune related cells such as neutrophils86, eosinophils 87, basophils and mast cells88, Langerhans cells82, 89, dendritic cells90, as well as monocytes49 and macrophages from different tissues3, 7, 91, 92.
Gal-3 promotes tumor progression and metastasis: Changes in cellular localization of gal-3
Gal-3 is expressed in many tumors and possibly plays an important role in tumor progression and metastasis7, 8, 10, 41, 73, 74, 92, 93. However, theintensity of the gal-3 expression in tumors depends on the type of tumor, its invasiveness and metastatic potential42, 43. For example, increased expression of gal-3 is observed in colon, head and neck, gastric, endometrial, thyroid, liver, bladder cancers and breast carcinomas44, 45, 73, 93–95. Gal-3 transfected human breast cancer cells BT549, which is gal-3 null, after intrasplenic injection, formed metastatic colonies in the liver, while gal-3 null BT549 cells did not96. Change in cellular localization of gal-3 is also observed during progression of various cancers. For example, down-regulation of gal-3 expression has been demonstrated in colorectal cancer, with increased cytoplasmic expression of gal-3 at more advanced stages42, 43, 97. In tongue cancer, nuclear gal-3 is decreased, but cytoplasmic gal-3 is increased during progression from normal to cancer42, 43. The decreased expression of gal-3 was also observed in prostate62, 74, 98, kidney99, and pituitary cancers61. In PCa, although gal-3 is down-regulated, its nuclear exclusion and cytoplasmic localization are correlated with disease progression62, 74, 100. Phosphorylation of gal-3 at Ser6 regulates its nuclear export101. Recent data by us and others indicated that decreased expression of gal-3 in pituitary and prostate tumors is, in part, due to its gal-3 promoter methylation60–63. Gal-3 expression in gastric, liver, lung, bladder, and head and neck cancers was significantly increased compared to the normal tissues, and correlated with the progression of clinical stages and metastases95–99.
Cytoplasmic gal-3 inhibits apoptosis
Intracellular gal-3 inhibits apoptosis by various mechanisms7. For example, gal-3 acts as a specific binding partner for activated K-Ras, which promotes strong activation of PI3K (phosphoinositide 3-kinase) 102. Gal-3 is the only member of its family that contains the NWGR anti-death domain. Bcl-2 translocation to the mitochondrial membrane blocks apoptosis and cytochrome c release103. Cytochrome c release and nitric oxide-induced apoptosis were blocked in gal-3 transfected BT549 human breast carcinoma cells104. Moreover, gal-3 binds Bcl-2 protein in vitro and inhibits mitochondrial- apoptotic response. Interestingly, synexin (annexin 7) is required for gal-3-prevention of mitochondrial damage105.
Extracellular gal-3 secreted from tumor cells induces apoptosis of cancer-infiltrating T-cells: Role of gal-3 in the immune escape mechanism during tumor progression
Recent studies revealed that gal-3 can induce apoptosis of activated T-cells37, 106, 107. Interestingly, gal-3-null T-cell lines such as Jurkat, CEM, and MOLT-4 cells were significantly more sensitive to exogenous gal-3 than gal-3-expressing lines SKW6.4 and H9. For example, gal-3 transfectedJurkat cells were found more resistant to apoptosis induced by anti-Fas antibodies or staurosporine (protein kinase inhibitor) compared to the non-transfected control cells17, 108. These differences are probably due to a balance between the anti-apoptotic activity of intracellular gal-3 and pro-apoptotic activity of extracellular gal-3. Extra-cellular gal-3 can also induce apoptosis in human T cells including human peripheral blood mononuclear cells (PBMCs) and activated mouse T-cells37. This would imply that tumor cells defend themselves against infiltrating T-cells by secreting gal-3. Two major signaling pathways, one via death receptors Fas (apo-1/CD95) and the other using TRAIL (TNF related apoptosis inducing ligand or Apo2-L), are known for extrinsic apoptotic signals109, 110.
Cell surface glycoproteins such as CD29, CD7, CD95, CD98, and T-cell receptor (TCR) have been shown to associate with gal-3, which may mediate induction of apoptosis by extracellular gal-3111–113. For example, extracellular gal-3 binds to the CD29/CD7 complex, which triggers the activation of an intracellular apoptotic signaling cascade followed by mitochondrial cytochrome c release and activation of caspase-3106.
Gal-3 mediates homotypic and heterotypic aggregation and promotes tumor cells endothelial interactions, angiogenesis, and tumor metastasis: Role of TF antigen in cancer metastasis
The formation of secondary tumors by circulating cancer cells requires embolization by aggregating with other tumor cells in micro capillaries followed by extravasation at secondary sites. In the first step of extravasation, cells bind to endothelial cells through protein-carbohydrate interactions and penetrate through the layers of endothelial cells and basement membrane. It was shown that cell surface gal-3 mediates homotypic cell adhesion by binding to soluble complementary glycoconjugates114, 115. Interactions of metastatic cancer cells with vasculatory endothelium are critical during early stages of cancer metastasis116. Gal-3 mediates homotypic and heterotypic aggregation and promotes interactions between tumor cells and endothelial cells, angiogenesis, and tumor metastasis2, 4, 7. It has been shown that gal-3 expressed in endothelium participates in docking of cancer cells including breast and prostate cancers on capillary endothelium by specifically interacting with cancer cells-associated TF-disaccharide (TFD, Galβ1,3GalNAc)117–119. The TFD, present in the core I structure of mucin-type O-linked glycan, is generally masked by sialic acid in normal cells, but is exposed or non-sialylated in malignant and premalignant epithelia118, 119. Circulating gal-3 has been shown to increase cancer cell homotypic aggregation by interaction with TFD on the cancer-associated transmembrane mucin protein MUC1120, 121. Significance of gal-3 in homotypic and heterotypic cell-cell interactions was also demonstrated by using three-dimensional co-cultures of endothelial and epithelial cells73. Gal-3 was shown to stimulate capillary tube formation of human umbilical vein endothelial cells (HUVEC) in vitro and angiogenesis in vivo, which was inhibited by specific sugars and antibodies. Overexpression of gal-3 in gal-3 non-expressing prostate cancer cell line LNCaP induced in vivo tumor growth and angiogenesis122.
Natural carbohydrate compounds to prevent gal-3-mediated tumorigenesis
There have been a few attempts to use naturally occurring substances to control and prevent cancer metastasis. Modified citrus pectin (MCP), a pH-modified soluble β-galactosyl-containing polysaccharide obtained from the peel of citrus fruits has been claimed to be an effective anti-metastatic drug for many cancers123. The MCP was shown to inhibit in vitro tumor cell adhesion to endothelium124 and homotypic aggregation as well as in vivo formation of metastatic deposits of human breast and prostate carcinoma cells in lungs and bones125.
As TFD is found exposed mostly on tumor cell surface (masked in normal cells) and also tumor-endothelial cell interactions required for metastasis are mediated by endothelium-associated gal-3 and cancer cell associated TFD, we reasoned that exogenous TFD would be more effective and specific to block gal-3-mediated tumorigenesis. We purified TFD-containing glycopeptide from cod fish and showed that TFD compound could inhibit tumor-endothelial cell interactions and angiogenesis126. Moreover, the purified TFD compound blocked gal-3 mediated T cell apoptosis126.
Concluding remarks
During cancer progression and metastasis, gal-3 renders anti-cancer activities in several ways. First, the intracellular (cytoplasmic) gal-3 is anti-apoptotic. Second, gal-3 promotes angiogenesis. Third, the extracellular gal-3 is involved in homotypic aggregation. Fourth, tumor-endothelial cell interactions required for metastasis are mediated by endothelium associated gal-3 and cancer cell-associated TFD. Fifth, tumor cell secreted gal-3 induces apoptosis of cancer-infiltrating T cells, thus, to promote immune escape and tumor progression. Thus, gal-3 plays multiple crucial roles in cancer progression and metastasis and so perturbation of gal-3 function either by blocking its expression with siRNA or by inhibiting its activity with external carbohydrates such as pectin or TFD would prevent tumorigenesis.
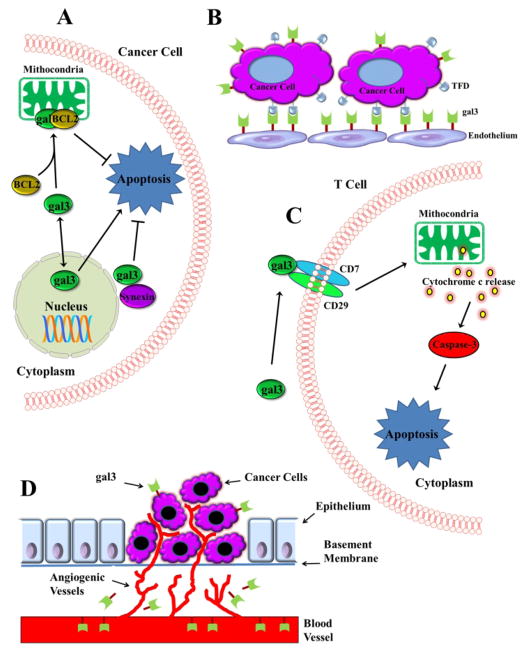
Fig 3. Function of galectin-3.
Schematic representation of (A) intracellular and (B–D) extracellular function of gal-3. A. Nuclear gal-3 is apoptotic, while cytoplasmic gal-3 is shown anti-apoptotic. B. Gal-3 promotes tumor-endothelial cell interactions. C. Gal-3 promotes apoptosis of T-cells. D. Gal-3 promotes tumor cell angiogenesis.
Acknowledgments
We apologize to the authors whose works are not cited due to the limited space. The work carried out by us was supported by the US Army Medical Research and Materiel Command grant W81XWH-07-1-0565, a start-up fund from the University of Maryland School of Medicine, and the National Institute of Health Grants RO3 CA133935-01 and R41CA141970-01A2 to H.A. E.K. is supported by a grant from “The Council of Higher Education” of Turkish Government.
References
- 1.Ahmed H, Du SJ, O’Leary N, Vasta GR. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology. 2004;14:219. doi: 10.1093/glycob/cwh032. [DOI] [PubMed] [Google Scholar]
- 2.Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605. doi: 10.1007/s10555-007-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol. 2007;66:143. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakahara S, Raz A. Biological modulation by lectins and their ligands in tumor progression and metastasis. Anticancer Agents Med Chem. 2008;8:22. doi: 10.2174/187152008783330833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64:1679. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 8.Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol Rev. 2009;230:114. doi: 10.1111/j.1600-065X.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 11.Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239. [PubMed] [Google Scholar]
- 12.Massa SM, Cooper DN, Leffler H. Barondes SH, L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- 13.Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002;277:6852. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 14.Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998;1379:97. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992;267:14167. [PubMed] [Google Scholar]
- 16.Ochieng J, Platt D, Tait L, Hogan V, Raz T, Carmi P, Raz A. Structure-function relationship of a recombinant human galactoside-binding protein. Biochemistry. 1993;32:4455. doi: 10.1021/bi00067a038. [DOI] [PubMed] [Google Scholar]
- 17.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang RY, Hill PN, Hsu DK, Liu FT. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37:4086. doi: 10.1021/bi971409c. [DOI] [PubMed] [Google Scholar]
- 19.Raimond J, Zimonjic DB, Mignon C, Mattei M, Popescu NC, Monsigny M, Legrand A. Mapping of the galectin-3 gene (LGALS3) to human chromosome 14 at region 14q2122. Mamm Genome. 1997;8:706. doi: 10.1007/s003359900548. [DOI] [PubMed] [Google Scholar]
- 20.Kadrofske MM, Openo KP, Wang JL. The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998;349:7. doi: 10.1006/abbi.1997.0447. [DOI] [PubMed] [Google Scholar]
- 21.Raimond J, Rouleux F, Monsigny M, Legrand A. The second intron of the human galectin-3 gene has a strong promoter activity down-regulated by p53. FEBS Lett. 1995;363:165. doi: 10.1016/0014-5793(95)00310-6. [DOI] [PubMed] [Google Scholar]
- 22.Guittaut M, Charpentier S, Normand T, Dubois M, Raimond J, Legrand A. Identification of an internal gene to the human Galectin-3 gene with two different overlapping reading frames that do not encode Galectin-3. J Biol Chem. 2001;276:2652. doi: 10.1074/jbc.m002523200. [DOI] [PubMed] [Google Scholar]
- 23.Duneau M, Boyer-Guittaut M, Gonzalez P, Charpentier S, Normand T, Dubois M, Raimond J, Legrand A. Galig, a novel cell death gene that encodes a mitochondrial protein promoting cytochrome c release. Exp Cell Res. 2005;302:194. doi: 10.1016/j.yexcr.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed H, Allen HJ, Sharma A, Matta KL. Human splenic galaptin: carbohydrate-binding specificity and characterization of the combining site. Biochemistry. 1990;29:5315. doi: 10.1021/bi00474a015. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed H, Vasta GR. Galectins: conservation of functionally and structurally relevant amino acid residues defines two types of carbohydrate recognition domains. Glycobiology. 1994;4:545. doi: 10.1093/glycob/4.5.545. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed H, Pohl J, Fink NE, Strobel F, Vasta GR. The primary structure and carbohydrate specificity of a β-galactosyl-binding lectin from toad (Bufo arenarum Hensel) ovary reveal closer similarities to the mammalian galectin-1 than to the galectin from the clawed frog Xenopus laevis. J Biol Chem. 1996;271:33083. doi: 10.1074/jbc.271.51.33083. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed H, Fink NE, Pohl J, Vasta GR. Galectin-1 from bovine spleen: Biochemical characterization, carbohydrate specificity and tissue-specific isoform profiles. J Biochem (Tokyo) 1996;120:1007. doi: 10.1093/oxfordjournals.jbchem.a021493. [DOI] [PubMed] [Google Scholar]
- 28.Salomonsson E, Carlsson MC, Osla V, Hendus-Altenburger R, Kahl-Knutson B, Oberg CT, Sundin A, Nilsson R, Nordberg-Karlsson E, Nilsson UJ, Karlsson A, Rini JM, Leffler H. Mutational tuning of galectin-3 specificity and biological function. J Biol Chem. 2010;285:35079. doi: 10.1074/jbc.M109.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao DI, Kapadia G, Ahmed H, Vasta GR, Herzberg O. Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside binding protein. Proc Natl Acad Sci USA. 1994;91:1428. doi: 10.1073/pnas.91.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchet MA, Ahmed H, Vasta GR, Amzel LM. A soluble β-galactosyl-binding lectin (galectin) from toad (Bufo arenarum Hensel) ovary: Crystallographic studies of two protein-sugar complexes. Proteins. 2000;40:378. doi: 10.1002/1097-0134(20000815)40:3<378::aid-prot40>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Hirabayashi J, Kasai K. The family of metazoan metal-independent β-galactoside-binding lectins:structure, function and molecular evolution. Glycobiology. 1993;3:297. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DNW, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990;110:1681. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho M, Cummings RD. Galectin-1, a β-galactoside-binding lectin in Chinese hamster ovary cells, II. Localization and biosynthesis. J Biol Chem. 1995;270:5207. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- 34.Barboni EA, Bawumia S, Hughes RC. Kinetic measurements of binding of galectin 3 to a laminin substratum. Glycoconj J. 1999;16:365. doi: 10.1023/a:1007004330048. [DOI] [PubMed] [Google Scholar]
- 35.Kariya Y, Kawamura C, Tabei T, Gu J. Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem. 2010;285:3330. doi: 10.1074/jbc.M109.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998;246:788. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 37.Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302. [PubMed] [Google Scholar]
- 38.Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592. [PubMed] [Google Scholar]
- 39.Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin. J Biol Chem. 1991;266:18731. [PubMed] [Google Scholar]
- 40.Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29:3595. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R-Y, Hsu DK, Liu F-T. Expression of galectin-3 modulates T cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Sakai T, Sano N, Fukui K. Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem J. 2004;380:31. doi: 10.1042/BJ20031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 44.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 45.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abedin MJ, Kashio Y, Seki M, Nakamura K, Hirashima M. Potential roles of galectins in myeloid differentiation into three different lineages. J Leukoc Biol. 2003;73:650. doi: 10.1189/jlb.0402163. [DOI] [PubMed] [Google Scholar]
- 47.Elliott MJ, Strasser A, Metcalf D. Selective up-regulation of macrophage function in granulocyte-macrophage colony-stimulating factor transgenic mice. J Immunol. 1991;147:2957. [PubMed] [Google Scholar]
- 48.Reichert F, Rotshenker S. Galectin-3/MAC-2 in experimental allergic encephalomyelitis. Exp Neurol. 1999;160:508. doi: 10.1006/exnr.1999.7229. [DOI] [PubMed] [Google Scholar]
- 49.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson EY., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016. [PMC free article] [PubMed] [Google Scholar]
- 50.Dabelic S, Flogel M, Dumic J. Effects of aspirin and indomethacin on galectin-3. Croat Chem Acta. 2005;178:433. [Google Scholar]
- 51.Dabelic S, Flogel M, Dumic J. Corticosteroids affect galectin-3 expression. Period Biol. 2005;107:175. [Google Scholar]
- 52.Liu FT, Albrandt K, Mendel E, Kulczycki A, Jr, Orida NK. Identification of an IgE-binding protein by molecular cloning. Proc Natl Acad Sci USA. 1985;82:4100. doi: 10.1073/pnas.82.12.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta- galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555. [PubMed] [Google Scholar]
- 54.Hebert E, Monsigny M. Galectin-3 mRNA level depends on transformation phenotype in ras-transformed NIH 3T3 cells. Biol Cell. 1994;81:73. doi: 10.1016/0248-4900(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 55.Fogel S, Guittaut M, Legrand A, Monsigny M, Hebert E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology. 1999;9:383. doi: 10.1093/glycob/9.4.383. [DOI] [PubMed] [Google Scholar]
- 56.Hsu DK, Hammes SR, Kuwabara I, Greene WC, Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. Am J Pathol. 1996;148:1661. [PMC free article] [PubMed] [Google Scholar]
- 57.Dumic J, Lauc G, Flogel M. Expression of galectin-3 in cells exposed to stress-Roles of jun and NF-kappaB. Cell Physiol Biochem. 2000;10:149. doi: 10.1159/000016345. [DOI] [PubMed] [Google Scholar]
- 58.Stock M, Schafer H, Stricker S, Gross G, Mundlos S, Otto F. Expression of galectin-3 in skeletal tissues is controlled by Runx2. J Biol Chem. 2003;278:17360. doi: 10.1074/jbc.M207631200. [DOI] [PubMed] [Google Scholar]
- 59.Costessi A, Pines A, D’Andrea P, Romanello M, Damante G, Cesaratto L, Quadrifoglio F, Moro L, Tell G. Extracellular nucleotides activate Runx2 in the osteoblast-like HOBIT cell line: a possible molecular link between mechanical stress and osteoblasts’ response. Bone. 2005;36:418. doi: 10.1016/j.bone.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed H, Banerjee PB, Vasta GR. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: Silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem Biophys Res Commun. 2007;358:241. doi: 10.1016/j.bbrc.2007.04.114. [DOI] [PubMed] [Google Scholar]
- 61.Ruebel KH, Jin L, Qian X, Scheithauer BW, Kovacs K, Nakamura N, Zhang H, Raz A, Lloyd RV. Effects of DNA methylation on galectin-3 expression in pituitary tumors. Cancer Res. 2005;65:1136. doi: 10.1158/0008-5472.CAN-04-3578. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed H, Cappello F, Rodolico V, Vasta GR. Evidence of heavy methylation in the galectin-3 promoter in early stages of prostate adenocarcinoma: Development and validation of a methylated marker for early diagnosis of prostate cancer. Trans Oncol. 2009;2:146. doi: 10.1593/tlo.09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmed H. Promoter methylation in prostate cancer and its application for the early detection of prostate cancer using serum and urine samples. Biomarkers in Cancer. 2010;2:17. doi: 10.4137/BIC.S3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colnot C, Ripoche MA, Scaerou F, Foulis D, Poirier F. Galectins in mouse embryogenesis. Biochem Soc Trans. 1996;24:141. doi: 10.1042/bst0240141. [DOI] [PubMed] [Google Scholar]
- 65.Poirier F. Roles of galectins in vivo. Biochem Soc Symp. 2002;69:95. doi: 10.1042/bss0690095. [DOI] [PubMed] [Google Scholar]
- 66.Mercer N, Guzman L, Cueto Rua E, Drut R, Ahmed H, Vasta GR, Toscano MA, Rabinovich GA, Docena GH. Duodenal intraepithelial lymphocytes of children with cow milk allergy preferentially bind the glycan-binding protein galectin-3. Int J Immunopathol Pharmacol. 2009;22:207. doi: 10.1177/039463200902200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dumont P, Berton A, Nagy N, Sandras F, Tinton S, Demetter P, Mascart F, Allaoui A, Decaestecker C, Salmon I. Expression of galectin-3 in the tumor immune response in colon cancer. Lab Invest. 2008;88:896. doi: 10.1038/labinvest.2008.54. [DOI] [PubMed] [Google Scholar]
- 68.Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 69.Kaji Y, Amano S, Usui T, Oshika T, Yamashiro K, Ishida S, Suzuki K, Tanaka S, Adamis AP, Nagai R, Horiuchi S. Expression and function of receptors for advanced glycation end products in bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 2003;44:521. doi: 10.1167/iovs.02-0268. [DOI] [PubMed] [Google Scholar]
- 70.Kang EH, Moon KC, Lee EY, Lee YJ, Lee EB, Ahn C, Song YW. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus. 2009;18:22. doi: 10.1177/0961203308094361. [DOI] [PubMed] [Google Scholar]
- 71.Won YS, Jeong ES, Park HJ, Lee CH, Nam KH, Kim HC, Park JI, Choi YK. Upregulation of galectin-3 by Corynebacterium kutscheri infection in the rat lung. Exp Anim. 2007;56:85. doi: 10.1538/expanim.56.85. [DOI] [PubMed] [Google Scholar]
- 72.Silva-Monteiro E, Reis Lorenzato L, Kenji Nihei O, Junqueira M, Rabinovich GA, Hsu DK, Liu FT, Savino W, Chammas R, Villa-Verde DM. Altered expression of galectin-3 induces cortical thymocyte depletion and premature exit of immature thymocytes during Trypanosoma cruzi infection. Am J Pathol. 2007;170:546. doi: 10.2353/ajpath.2007.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44:118. doi: 10.1002/1097-0045(20000701)44:2<118::aid-pros4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 75.Xu XC, Sola Gallego JJ, Lotan R, El-Naggar AK. Differential expression of galectin-1 and galectin-3 in benign and malignant salivary gland neoplasms. Int J Oncol. 2000;17:271. doi: 10.3892/ijo.17.2.271. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Friess H, Zhu Z, Frigeri L, Zimmermann A, Korc M, Berberat PO, Buchler MW. Galectin-1 and galectin-3 in chronic pancreatitis. Lab Invest. 2000;80:1233. doi: 10.1038/labinvest.3780131. [DOI] [PubMed] [Google Scholar]
- 77.Sasaki S, Bao Q, Hughes RC. Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol. 1999;187:481. doi: 10.1002/(SICI)1096-9896(199903)187:4<481::AID-PATH263>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 78.Fautsch MP, Silva AO, Johnson DH. Carbohydrate binding proteins galectin-1 and galectin-3 in human trabecular meshwork. Exp Eye Res. 2003;77:11. doi: 10.1016/s0014-4835(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 79.Shimonishi T, Miyazaki K, Kono N, Sabit H, Tuneyama K, Harada K, Hirabayashi J, Kasai K, Nakanuma Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol. 2001;32:302. doi: 10.1053/hupa.2001.22767. [DOI] [PubMed] [Google Scholar]
- 80.Moutsatsos IK, Wade M, Schindler M, Wang JL. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci USA. 1987;84:6452. doi: 10.1073/pnas.84.18.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niida S, Amizuka N, Hara F, Ozawa H, Kodama H. Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J Bone Miner Res. 1994;9:873. doi: 10.1002/jbmr.5650090613. [DOI] [PubMed] [Google Scholar]
- 82.Wollenberg A, de la Salle H, Hanau D, Liu FT, Bieber T. Human keratinocytes release the endogenous beta-galactoside-binding soluble lectin immunoglobulin E (IgE-binding protein) which binds to Langerhans cells where it modulates their binding capacity for IgE glycoforms. J Exp Med. 1993;178:777. doi: 10.1084/jem.178.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994;14:3231. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer. 1994;56:474. doi: 10.1002/ijc.2910560404. [DOI] [PubMed] [Google Scholar]
- 85.Lotan R, Belloni PN, Tressler RJ, Lotan D, Xu XC, Nicolson GL. Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconj J. 1994;11:462. doi: 10.1007/BF00731282. [DOI] [PubMed] [Google Scholar]
- 86.Truong MJ, Gruart V, Kusnierz JP, Papin JP, Loiseau S, Capron A, Capron M. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE dependent activation. J Exp Med. 1993;177:243. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Truong MJ, Gruart V, Liu FT, Prin L, Capron A, Capron M. IgEbinding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur J Immunol. 1993;23:3230. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- 88.Craig SS, Krishnaswamy P, Irani AM, Kepley CL, Liu FT, Schwartz LB. Immunoelectron microscopic localization of galectin-3, an IgE binding protein, in human mast cells and basophils. Anat Rec. 1995;242:211. doi: 10.1002/ar.1092420210. [DOI] [PubMed] [Google Scholar]
- 89.Smetana K, Holikova Z, Klubal R, Bovin NV, Dvorankova B, Bartunkova J, Liu FT, Gabius HJ. Coexpression of binding sites for A (B) histo-blood group trisaccharides with galectin-3 and Lag antigen in human Langerhans cells. J Leukoc Biol. 1999;66:644. doi: 10.1002/jlb.66.4.644. [DOI] [PubMed] [Google Scholar]
- 90.Dietz AB, Bulur PA, Knutson GJ, Matasic R, Vuk-Pavlovic S. Maturation of human monocyte-derived dendritic cells studied by microarray hybridization. Biochem. Biophys. Res Commun. 2000;275:731. doi: 10.1006/bbrc.2000.3372. [DOI] [PubMed] [Google Scholar]
- 91.Saada A, Reichert F, Rotshenker S. Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the upregulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J Cell Biol. 1996;133:159. doi: 10.1083/jcb.133.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 93.Song YK, Billiar TR, Lee YJ. Role of galectin-3 in breast cancer metastasis: involvement of nitric oxide. Am J Pathol. 2002;160:1069. doi: 10.1016/S0002-9440(10)64927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80:776. [PubMed] [Google Scholar]
- 95.Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519. doi: 10.1002/(sici)1097-0215(19990517)81:4<519::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 96.Miyazaki J, Hokari R, Kato S, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep. 2002;9:1307. [PubMed] [Google Scholar]
- 97.Yoshimura A, Gemma A, Hosoya Y, Komaki E, Hosomi Y, Okano T, Takenaka K, Matuda K, Seike M, Uematsu K, Hibino S, Shibuya M, Yamada T, Hirohashi S, Kudoh S. Increased expression of the LGALS3 (galectin 3) gene in human non-small-cell lung cancer. Genes Chromosomes Cancer. 2003;37:59. doi: 10.1002/gcc.10205. [DOI] [PubMed] [Google Scholar]
- 98.Merseburger AS, Kramer MW, Hennenlotter J, Simon P, Knapp J, Hartmann JT, Stenzl A, Serth J, Kuczyk MA. Involvement of decreased galectin-3 expression in the pathogenesis and progression of prostate cancer. Prostate. 2008;68:72. doi: 10.1002/pros.20688. [DOI] [PubMed] [Google Scholar]
- 99.Merseburger AS, Kramer MW, Hennenlotter J, Serth J, Kruck S, Gracia A, Stenzl A, Kuczyk MA. Loss of galectin-3 expression correlates with clear cell renal carcinoma progression and reduced survival. World J Urol. 2008;26:637. doi: 10.1007/s00345-008-0294-8. [DOI] [PubMed] [Google Scholar]
- 100.Van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89:361. doi: 10.1002/1097-0215(20000720)89:4<361::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 101.Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24:4395. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 103.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001;159:1055. doi: 10.1016/S0002-9440(10)61780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 106.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol Rev. 2009;230:114. doi: 10.1111/j.1600-065X.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 107.Li W, Jian-jun W, Xue-Feng Z, Feng Z. CD133(+) human pulmonary adenocarcinoma cells induce apoptosis of CD8(+) T cells by highly expressed galectin-3. Clin Invest Med. 2010;33:E44. doi: 10.25011/cim.v33i1.11837. [DOI] [PubMed] [Google Scholar]
- 108.Nangia-Makker P, Balan V, Raz A. Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron. 2008;1:43. doi: 10.1007/s12307-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 110.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 111.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 113.Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, Kanayama HO, Kim HR, Raz A. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64:3376. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- 114.Inohara H, Raz A. Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res. 1995;55:3267. [PubMed] [Google Scholar]
- 115.Inohara H, Akahani S, Koths K, Raz A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 1996;56:4530. [PubMed] [Google Scholar]
- 116.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 117.Yu LG. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj J. 2007;24:411. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- 118.Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 119.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851. [PubMed] [Google Scholar]
- 120.Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, Yu LG. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu LG. Circulating galectin-3 in the bloodstream: An emerging promoter of cancer metastasis. World J Gastrointest Oncol. 2010;2:177. doi: 10.4251/wjgo.v2.i4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Califice S, Castronovo V, Bracke M, van den Brule F. Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23:7527. doi: 10.1038/sj.onc.1207997. [DOI] [PubMed] [Google Scholar]
- 123.Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr Res. 2009;344:1788. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, Donat TL, Tait L, Hogan V, Raz A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus peçtin. J Natl Cancer Inst. 1995;87:348. doi: 10.1093/jnci/87.5.348. [DOI] [PubMed] [Google Scholar]
- 125.Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guha Prasun, Kaptan Engin, Bandyopadhyaya Gargi, Kalvakolanu Dhan, Vasta Gerardo R, Ahmed Hafiz. Natural Thomsen-Friedenreich disaccharide (TFD) containing compound prevents galectin-3-mediated tumor-endothelial cell adhesion, angiogenesis, and T-cell apoptosis. Submitted. [Google Scholar]