Abstract
Background
Parathyroid carcinoma is a rare cancer. Unlike other more common malignancies, the significance of lymph node (LN) status remains controversial. The purpose of this study was to determine the relative importance of LN metastases in disease-specific survival (DSS).
Methods
A retrospective review of the Surveillance, Epidemiology, and End Result (SEER) database was performed on parathyroid carcinoma cases diagnosed between 1988 and 2010.
Results
405 parathyroid carcinoma patients were identified. Among 114 patients with LNs examined at surgery, only 12 (10.5%) had positive LNs. Sensitivity analysis found that a tumor size threshold of 3 cm best divided the cohort by DSS. Only tumors ≥3 cm and distant metastasis but not LN metastases were independent prognostic factors on multivariate analysis. When examining factors associated with LN status, only tumors ≥3 cm predicted LN metastasis. LN metastases were 7.5 times more likely in patients with tumors ≥3 cm than those with tumors <3 cm.
Conclusions
Tumors ≥3 cm were associated with LN metastases in parathyroid carcinoma but positive LN status was not associated with DSS. Tumor size can potentially risk stratify patients by their risk of LN metastases.
Introduction
Parathyroid cancer is a rare disease, whose major morbidity and mortality are attributed to metabolic complications from hypercalcemia, including bone disease, nephrolithiasis, pancreatitis and peptic ulcer disease, accounting for 0.005% of all malignancies1 and 0.74% to 4.7% of hyperparathyroidism.2–4 Unlike parathyroid adenoma, where the female to male ratio is approximately 4:1, parathyroid carcinoma affects both genders equally.
Several studies in the past used population data to analyze the prognostic factors of parathyroid carcinoma. The earliest United States population based study was performed by Hundahl et al using the National Cancer Data Base (NCDB) with 286 patients diagnosed with parathyroid cancer between 1985 and 1996.1 The survey reported relative 5-year overall survival of 85.5% and 10-year survival of 49.1%. A second study by Lee et al., using the SEER database, with 224 patients diagnosed between 1988 and 2003 reported 5-year cancer-related survival of 91% and 10-year cancer-related survival of 87.6%.5 There was a 60% increase in incidence between the periods of 1988–1991 and 2000–2003 but an improvement in survival was observed between the two population studies.1, 5 Potential explanations for the increase in parathyroid carcinoma incidence include increased screening, changes in diagnostic techniques, an increase in referral for surgery due to availability of minimally invasive procedures, and possibly a true increase in the incidence.6 While younger age, female gender, absence of distant metastasis at diagnosis and recent year of diagnosis, were associated with improved survival, tumor size and LN status did not influence DSS.1, 5 The incidence of regional LN involvement at initial diagnosis varied widely, ranging between 6.5% and 32.1%.7
The therapy that offers the best outcome remains surgical resection. Current standard of treatment dictates parathyroidectomy and en bloc resection with surrounding tissues, including the ipsilateral thyroid lobe, isthmus, and central neck lymph node compartment.3, 8–11 However, even with surgical resection, recurrence rate has been reported to be between 42–72%,8, 9, 12–14 frequently requiring one or more re-operations. In addition, central neck dissection carries added risks such as injury to the recurrent laryngeal nerve, affecting voice and swallow function, bleeding, and inadvertent damage or removal of the other normal parathyroid glands.15, 16
The purpose of this study was to determine how metastatic lymph nodes impact DSS in parathyroid carcinoma. Because of the rare nature of parathyroid carcinoma, a population based database allowed us to have a large enough sample size to answer the question of whether the regional LN status necessarily affected DSS.
Material and Methods
We used data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry between 1988 and 2010 because tumor size and lymph node status was reported beginning from 1988. Patients were first identified using primary site code of C750 (parathyroid) in combination with the International Classification of Disease for Oncology, 3rd Edition (ICD-O-3),17 in combination with histology codes 800 (neoplasm), 801 (carcinoma, not otherwise specified), 802 (carcinoma, undifferentiated, not otherwise specified) and 814 (adenocarcinoma, not otherwise specified). We included all patients ≥18 years old with active follow up and excluded patients without histology confirmation or autopsy only cases. In addition, we obtained patient demographic information, tumor characteristics, treatment options, and survival information. We divided the patients into two age groups: <45 years old and ≥45 years old. Diagnostic years were grouped into four periods: 1988–1993, 1994–1999, 2000–2005, and 2006–2010. The last cut-off point for follow-up was December 31, 2009.
Statistics analysis was carried out using Stata (Stata 12 for Windows; StataCorp LP., College Station, TX). To evaluate potential factors affecting survival time, taking survival time and censoring into account, Cox proportional hazards regression was used to report hazard ratio (HR) with 95% confidence intervals. Logistic regression was used to evaluate potential factors that predicted positive lymph node status. Factors with p<0.2 in univariate analysis were included in multivariate analysis. Sensitivity analysis was performed to obtain the smallest tumor size threshold that differentiated survival. Unless otherwise stated, all tests were 2 sided with p<0.05 defined as statistically significant.
The study was approved by the Institutional Review Board of University of Wisconsin-Madison.
Results
Patient characteristics
405 patients with parathyroid carcinoma meeting our inclusion criteria were identified in the SEER registries between 1988 and 2010 with median follow-up of 68 months (interquartile range 29–106 months). There was a slight male dominance with 52.3% of the patients being male and 47.7% of the patients being female (Table 1). 75.8% of the patients were White, followed by Black (15.8%), Asian (7.4%) and others (0.9%). The median patient age was 56 years (interquartile range 46–66 years). 47.2% of the cases were diagnosed in the third diagnostic period (2000–2005), following by the 27.2% of the cases in the last diagnostic period (2006–2010). 329 patients underwent parathyroidectomy, 42 patients had en bloc resection, and 7 patients had debulking procedures.
Table 1.
* Patient and Tumor Characteristics (n = 405)
| Characteristics | Number (%) |
|---|---|
| Age, year | 56 (46–66) |
| Age ≥ 45 | 319 (78.8) |
| Female | 193 (47.7) |
| Race | |
| White | 307 (75.8) |
| Black | 64 (15.8) |
| Asian | 30 (7.4) |
| Others | 4 (0.9) |
| Histology | |
| Neoplasm | 10 (2.5) |
| Carcinoma, NOS | 385 (95.1) |
| Adenocarcinoma, NOS | 10 (2.5) |
| Grade | |
| I | 39 (9.6) |
| II | 10 (2.5) |
| III | 3 (0.7) |
| IV | 0 (0) |
| † Unknown | 353 (87.2) |
| LN examined | |
| Yes | 114 (28.1) |
| No | 274 (67.7) |
| Unknown | 17 (4.2) |
| Tumor size, cm | 2.8 (1.9–3.8) |
| Local invasion | 60 (14.8) |
| Metastatic LN | 12 (3.0) |
| Distant metastasis | 7 (1.7) |
| Surgery | |
| None | 12 (3.0) |
| Parathyroidectomy | 329 (81.2) |
| En bloc resection | 42 (10.4) |
| ‡ Others | 8 (1.9) |
| Unknown | 14 (3.5) |
Continuous variables are represented as the median followed by interquartile range in the parenthesis. LN, lymph node; NOS, not otherwise specified.
Tumor grade was reported in only 52 cases.
Others include debulking surgery and local destruction of tumors.
Tumor characteristics
Histology was recorded as neoplasm in 10 patients (2.5%), carcinoma, not otherwise specified in 385 patients (95.1%), and adenocarcinoma, not otherwise specified in 10 patients (2.5%). All tumors in the study were malignant. Tumor grades were recorded in 52 patients, including grade I in 39 (9.6%), grade II in 10 (2.5%), and grade III in 3 (0.7%). Median tumor size removed was 2.8 cm (interquartile range 1.9–3.8 cm). Regional lymph nodes were examined in 114 patients, of whom, 12 patients (10.5%) were found to have positive lymph node. The parathyroid carcinoma was confined to the gland in 318 patients (78.5%), locally invasive in 60 patients (14.8%), and metastatic in 7 patients (1.7%).
Prognostic factors
The DSS rates were 94.1% at 5 years and 89.9% at 10 years. However, the DSS remained stable between 10 and 20 years. The overall survival rates were 82.5% at 5 years and 65.4% at 10 years. Sensitivity analysis revealed that 3 cm tumor size cut off was the lowest threshold that best divided he cohort by DSS (HR 3.62; p=0.03) (Figure 1). On multivariate Cox proportional hazards analysis, controlling for gender, age and diagnostic year, tumor size ≥3 cm (HR 5.35; p=0.01), and distant metastasis (HR 45.10; p<0.01) remained significant prognostic factors of DSS (Table 2). Importantly, metastatic lymph nodes did not independently predict worse DSS (HR 3.72; p=0.19).
Figure 1.
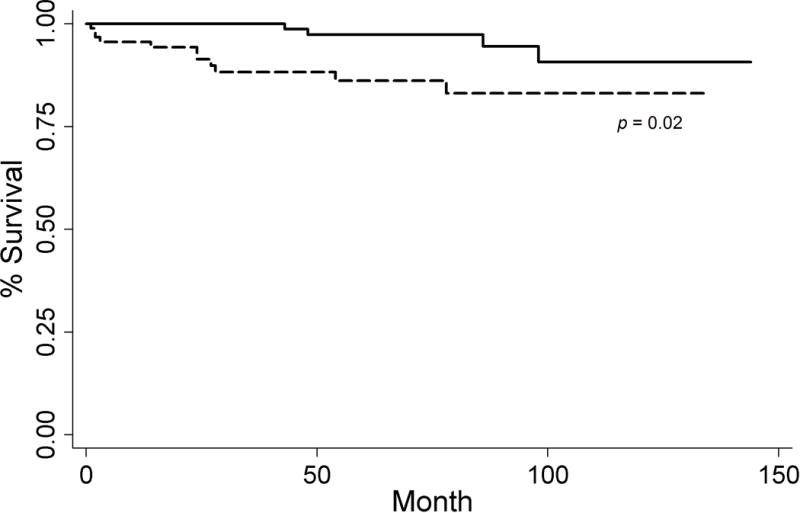
Kaplan-Meier curve with 3 cm threshold
Table 2.
* Multivaraite Cox proportional hazards model on disease-specific survival
| Prognostic Factors | Multivariate
|
||
|---|---|---|---|
| HR | 95% CI | p | |
| Female | 0.64 | 0.27–2.46 | 0.71 |
| Age ≥45 | 1.84 | 0.38–8.83 | 0.45 |
| Tumor ≥3 cm | 5.35 | 1.47–19.5 | 0.01 |
| Positive LN | 3.72 | 0.52–26.4 | 0.19 |
| Metastasis | 45.10 | 2.70–77.6 | <0.01 |
Positive LN represents regional lymph node metastasis and metastasis represents distal metastasis. LN, lymph node; HR, hazards ratio; CI, confidence interval
Lymph node status
Lymph node metastasis were 7.5 times more likely in patients with tumors ≥3 cm than those with tumors <3 cm (21% vs. 2.8%; p=0.02) (Figure 2). On multivariate logistic regression, tumors ≥3 cm (HR 27.78; p=0.02) was an independently predictive factor of positive lymph nodes after controlling for gender, age, and diagnostic year (Table 3). When we compared patients with LN data to those without LN data, there were no differences in distributions of gender, age ≥45 years old, tumors ≥3 cm, diagnostic period, local invasion, and distant metastasis (Table 4). The predominant race in both groups was White with 94 patients (82%) with lymph node examination and 200 (73%) without lymph node examination (p=0.048). Regarding surgical options, debulking were 4 times and en bloc resection was 3 times more frequently performed in patients with lymph node examined (p<0.01). Radiation therapy was performed in 17 patients (15%) with lymph node examination and 20 patients (7%) without lymph node examination.
Figure 2.
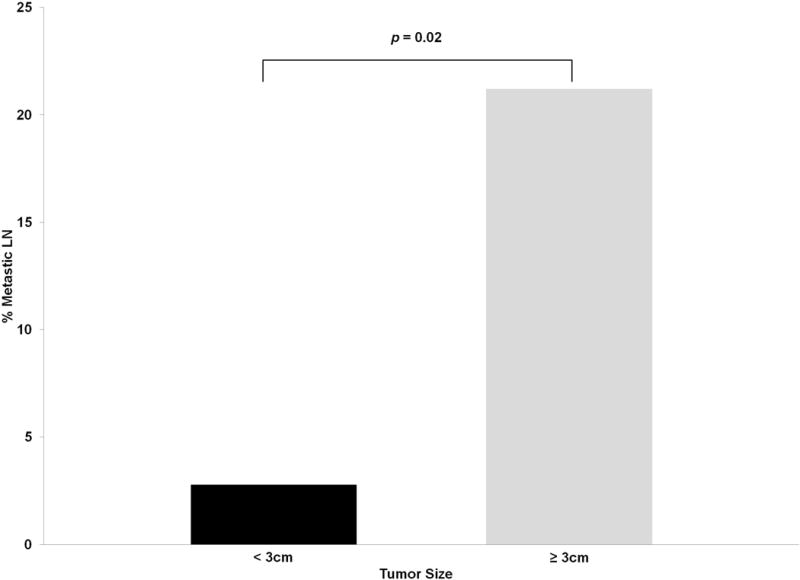
Percentage metastatic LN with 3 cm threshold
Table 3.
† Multivariate logistic regression for metastatic lymph nodes
| Predictors | Multivariate
|
||
|---|---|---|---|
| OR | 95% CI | p | |
| Female | 0.31 | 0.05–2.04 | 0.22 |
| Age ≥45 | 2.88 | 0.37–22.27 | 0.31 |
| Dx Periods | 0.21 | 0.06–0.65 | <0.01 |
| Tumor ≥3 cm | 19.48 | 1.48–256.62 | 0.02 |
Dx Periods, diagnostic periods; OR, odds ratio; CI, confidence interval
Table 4.
‡ Comparison between patients with and without regional lymph node examination
| Factors | LN Examined
|
§p | |
|---|---|---|---|
| No (%) | Yes (%) | ||
| Female | 132 (48%) | 52 (46%) | 0.65 |
| Age ≥45 | 221 (81%) | 84 (74%) | 0.13 |
| White | 200 (73%) | 94 (82%) | <0.05 |
| Dx Periods | |||
| 1988–1993 | 26 (9%) | 8 (7%) | |
| 1994–1999 | 41 (15%) | 20 (18%) | 0.77 |
| 2000–2005 | 124 (45%) | 49 (43%) | |
| 2006–2010 | 83 (30%) | 37 (32%) | |
| Tumor ≥3 cm | 75 (45%) | 34 (49%) | 0.58 |
| Local Invasion | 36 (14%) | 21 (19%) | 0.23 |
| Metastasis | 4 (2%) | 3 (3%) | 0.45 |
| Radiation | 20 (7%) | 17 (15%) | 0.02 |
| Death | 73 (27%) | 25 (22%) | 0.33 |
Results are expressed as raw number and with percentage in parenthesis. Dx Periods, diagnostic periods; LN, lymph node.
X2 test
Discussion
For patients with parathyroid carcinoma, surgery provides the best survival benefit,1, 3, 8–10, 15, 18, 19 but there is no consensus on surgical approach due to the low incidence of the disease. While the current parathyroid carcinoma standard surgical treatment involves en bloc removal of the entire parathyroid tumor along with the ipsilateral thyroid lobe, isthmus, and the ipsilateral central lymph node compartment,11 our results suggest that regional lymph node status does not impact DSS except for tumors ≥3 cm.
In our study, 81.2% of patients underwent parathyroidecomy and 10.4% of patients underwent en bloc resections with DSS of 90% at 10 years. As many studies demonstrated the advantage of en bloc resection on survival and recurrence,5, 8–10, 14 our finding could be interpreted as that simple parathyroidecomy was adequate for survival. However, since the surgical treatment coding in the SEER was not specific enough to delineate the exact extend of resection, we do not feel that this interpretation should be definitive. “En bloc resection” is not specifically stated in the SEER coding scheme except under radical surgery in the 3rd edition manual. Therefore, in our study, we only categorized surgical surgery as en bloc resection with the understanding that the frequency of en bloc resection in the database was likely an underestimation.
Regional LN metastases were not common, and mainly involved the central compartment of the neck.7 Schulte et al. found lymph node metastasis in 1 out of 11 patients (9.1%) and advocated for en bloc resection with clearance of the central compartment for suspicious parathyroid lesions.7 Others have reported metastatic lymph nodes associated with parathyroid carcinoma between 3% and 32%.1, 20 Of the patients who had surgeries in our analysis, only 42 patients (11.1%) received the appropriate en bloc resection, indicating the difficulties to recognize the disease pre-operatively or intra-operatively. Therefore, whenever possible, it is very critical that the physician has a high index of suspicion prior to resection to ensure the most optimal therapy.
One possible confounding factor is the association between tumor size and metastatic LN, such that patients with larger tumors are more likely to have their lymph nodes examined more frequently. Our result showed that although there was slightly higher but not statistically significant proportion of larger tumors in patients with LN examined. Therefore, we do not feel that larger tumors increase the propensity of LN examination. In addition, there were no other significant differences in patient or disease features when comparing those with and without LNs examined. However, we did find that patients with LN examined were significantly more likely to receive debulking surgery, en bloc resection, or radiation therapy. The most likely explanation is that for patients who underwent more aggressive and complete resection, regional LN are more likely to be included in the resected specimen. Similarly, we speculate that patients with a larger tumor would be more likely to go on to receive additional therapies such as radiation.
Metastatic LNs were associated with earlier diagnostic period and tumors ≥3cm. It appeared that more recent year of diagnosis was protective of metastatic LNs, most likely due to earlier detection of parathyroid carcinoma in the later years. A decrease in the rate of clinically palpable cervical mass from 29% to 15%over 35 years in patients with parathyroid carcinoma was reported in two different studies, indicating a trend toward earlier detection in the modern era21, 22 The introduction of calcium screening and parathyroid hormone (PTH) assay allowed increased detection of smaller tumors with less advanced stage.18
Unlike other more common malignancies, tumor size and LN metastases were not found to be prognostic factors in the past.1, 5, 8 Because of the lack of prognostic values, the National Comprehensive Cancer Network (NCCN) and the American Joint Committee on Cancer (AJCC) have not established a staging system for parathyroid carcinoma. However, Shaha from Memorial Sloan-Kettering, in response to the study conducted by Hundahl et al., using the NCDB,1 proposed a parathyroid carcinoma staging system, which used tumor size, local tumor extension, regional LN metastases, and distant metastases as prognostic factors.23 They chose 3 cm as the cut-off between T1 and T2 because Hundahl et al. reported a mean tumor size of 3.3 cm.1 Our analysis confirmed that 3 cm was an appropriate cut-off for differentiating prognosis.
In a meta-analysis, Talat and Schulte proposed two staging system – Schulte a and Schulte b.14 Specifically, the Schulte b system simply divided the patients into low risk and high risk groups, according to vascular invasion, LN, and distant metastasis status. The authors reported that high risk patients had 3.5 times higher risk for cancer recurrence and 4.9 times higher risk of overall death with univariate analysis. Our univariate analysis (data not shown) demonstrated that patients with regional LN metastases had 5 times higher risk of disease-specific death, although the lymph node status became non-significant on multivariate analysis. Furthermore, regional LN metastases had no significant predictive value on overall mortality in our study, which was in contrast to what Talat and Schulte found.
Several studies indicate that although the incidence of parathyroid carcinoma continued to increase,5, 6 long-term survival remained favorable, with 5-year survival ranged from 85% to 91% and the 10-year survival ranged from 49.1% to 87.6%.1, 5, 12, 21 Our study showed DSS rates of 94.1% at 5 years and 90% at 10 years. As we previous stated, the NCDB study reported 5-year and 10-year overall relative survival of 85.5% and 49.1%. However, the authors did not report disease-specific survival. The other large study by Tatal and Schulte reported that 96% of low risk patients and 72% of high risk patients survived at 5 years. However, our survival results were similar to those of the study conducted by Lee et al, likely because the same database was utilized. Due to this long survival period, recurrence instead of death became the main concern after the initial surgical treatment with reported rate between 30.5% and 100%.4, 9, 10, 24, 25 In this regard, one explanation of the higher survival rate in the studies using the SEER database could be the incomplete capture of death due to complications of parathyroid carcinoma, specifically, the sequalae of hypercalcemia, including renal failure, coma, or cardiac arrest secondary to arrhythmia from shortened QT interval and flattened T wave. Death due to complications of parathyroid carcinoma may be coded as death due to other cause (i.e, arrhythmia, renal failure) instead of death due to cancer. The overall survival (82.5% at 5 years and 65.4% at 10 years) may serve as a better estimation of disease specific survival since SEER could potentially overestimate the survival rates for this particular cancer.
As with any population based database study, our study has its limitations. First there are several patient, disease, or tumor variables that were not in the database, including PTH, serum calcium level, detailed surgical record, disease recurrence, or specific follow-up information. Recurrence, as mentioned above, is an important outcome measurement for parathyroid carcinoma, as the consequences of hypercalcemia are the main cause of death, but we were unable to evaluate this point due to the lack of disease recurrence record in the SEER database. Secondly, there was also limited information on follow-up except for the length of follow-up and the type of follow-up (autopsy/death certificate only or active). Third, the codes used in SEER were not very specific regarding type of surgery or degrees of local invasion, making misclassification or miscoding a potential limitation. It was difficult to discern the specific extent of surrounding tissue or organ removal with the coding schemes. In addition, due to the retrospective nature of the study, SEER does not contain data on tumor characteristics and treatments that many experts would consider important, specifically, completeness of resection (R0 vs. R1) or fracture of the tumor. Finally, missing data points decreases the number of patients available with data on all variables. Nevertheless, given the rare nature of parathyroid carcinoma, SEER provided large enough data to have the statistical power and objectivity not available from previous single institution studies.
We were able to demonstrate that there was an association between tumor size and DSS as well as regional LN metastases. Given the limitations of SEER, we cannot make specific treatment recommendations regarding lymph node dissection in parathyroid carcinoma. However, the results reported here can serve as a starting point toward identifying which patients would benefit from LN dissection and can direct future studies on the optimal therapeutic approach to the lymph nodes.
Acknowledgments
This work was supported by the NIH National Center for Advancing Translational Sciences (NCATS) grant UL1TR000427.
Footnotes
Disclosure statement: No conflict of interest or financial interests
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985–1995: a National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–44. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Ruda JM, Hollenbeak CS, Stack BC., Jr A systematic review of the diagnosis and treatment of primary hyperparathyroidism. Otolaryngol Head Neck Surg. 2005;132:359–72. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Hong SW, Jeong JJ, Nam KH, Chung WY, Chang HS, et al. Parathyroid carcinoma: a 16-year experience in a single institution. Endocr J. 2010;57:493–7. doi: 10.1507/endocrj.k09e-365. [DOI] [PubMed] [Google Scholar]
- 4.Iacobone M, Lumachi F, Favia G. Up-to-date on parathyroid carcinoma: analysis of an experience of 19 cases. J Surg Oncol. 2004;88:223–8. doi: 10.1002/jso.20152. [DOI] [PubMed] [Google Scholar]
- 5.Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–41. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]
- 6.Brown S, O’Neill C, Suliburk J, Sidhu S, Sywak M, Gill A, et al. Parathyroid carcinoma: increasing incidence and changing presentation. ANZ J Surg. 2011;81:528–32. doi: 10.1111/j.1445-2197.2010.05594.x. [DOI] [PubMed] [Google Scholar]
- 7.Schulte KM, Talat N, Miell J, Moniz C, Sinha P, Diaz-Cano S. Lymph node involvement and surgical approach in parathyroid cancer. World J Surg. 2010;34:2611–20. doi: 10.1007/s00268-010-0722-y. [DOI] [PubMed] [Google Scholar]
- 8.Sandelin K, Auer G, Bondeson L, Grimelius L, Farnebo LO. Prognostic factors in parathyroid cancer: a review of 95 cases. World J Surg. 1992;16:724–31. doi: 10.1007/BF02067369. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman SM, Rigual NR, Hicks WL, Jr, Popat SR, Lore JM, Jr, Douglas WG, et al. Parathyroid carcinoma: a multicenter review of clinicopathologic features and treatment outcomes. Ear Nose Throat J. 2004;83:491–4. [PubMed] [Google Scholar]
- 10.Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13:11–23. doi: 10.1007/s11864-011-0171-3. [DOI] [PubMed] [Google Scholar]
- 11.Mitmaker D, Shen W. Chapter II.B.4: Parathyroid Carcinoma. In: Sippel R, Chen H, editors. The Handbook of Endocrine Surgery. Hackensack, NJ: World Scientific; 2011. pp. 211–21. [Google Scholar]
- 12.Munson ND, Foote RL, Northcutt RC, Tiegs RD, Fitzpatrick LA, Grant CS, et al. Parathyroid carcinoma: is there a role for adjuvant radiation therapy? Cancer. 2003;98:2378–84. doi: 10.1002/cncr.11819. [DOI] [PubMed] [Google Scholar]
- 13.Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. [PubMed] [Google Scholar]
- 14.Talat N, Schulte KM. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol. 2010;17:2156–74. doi: 10.1245/s10434-010-1003-6. [DOI] [PubMed] [Google Scholar]
- 15.Harari A, Waring A, Fernandez-Ranvier G, Hwang J, Suh I, Mitmaker E, et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab. 2011;96:3679–86. doi: 10.1210/jc.2011-1571. [DOI] [PubMed] [Google Scholar]
- 16.Rao SR, Shaha AR, Singh B, Rinaldo A, Ferlito A. Management of cancer of the parathyroid. Acta Otolaryngol. 2002;122:448–52. doi: 10.1080/00016480260000184. [DOI] [PubMed] [Google Scholar]
- 17.Percy C, Fritz A, J A, Shanmugarathan S, Sobin L, P DM, et al. International Classification of Diseases for Oncology (ICD-O) 3. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 18.Owen RP, Silver CE, Pellitteri PK, Shaha AR, Devaney KO, Werner JA, et al. Parathyroid carcinoma: a review. Head Neck. 2011;33:429–36. doi: 10.1002/hed.21376. [DOI] [PubMed] [Google Scholar]
- 19.Adler JT, Sippel RS, Chen H. New trends in parathyroid surgery. Curr Probl Surg. 2010;47:958–1017. doi: 10.1067/j.cpsurg.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Holmes EC, Morton DL, Ketcham AS. Parathyroid carcinoma: a collective review. Ann Surg. 1969;169:631–40. doi: 10.1097/00000658-196904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26:716–26. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 22.Anderson BJ, Samaan NA, Vassilopoulou-Sellin R, Ordonez NG, Hickey RC. Parathyroid carcinoma: features and difficulties in diagnosis and management. Surgery. 1983;94:906–15. [PubMed] [Google Scholar]
- 23.Shaha AR, Shah JP. Parathyroid carcinoma: a diagnostic and therapeutic challenge. Cancer. United states. 1999:378–80. [PubMed] [Google Scholar]
- 24.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–5. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Placzkowski K, Christian R, Chen H. Radioguided parathyroidectomy for recurrent parathyroid cancer. Clin Nucl Med. 2007;32:358–60. doi: 10.1097/01.rlu.0000259623.79805.2d. [DOI] [PubMed] [Google Scholar]


