Abstract
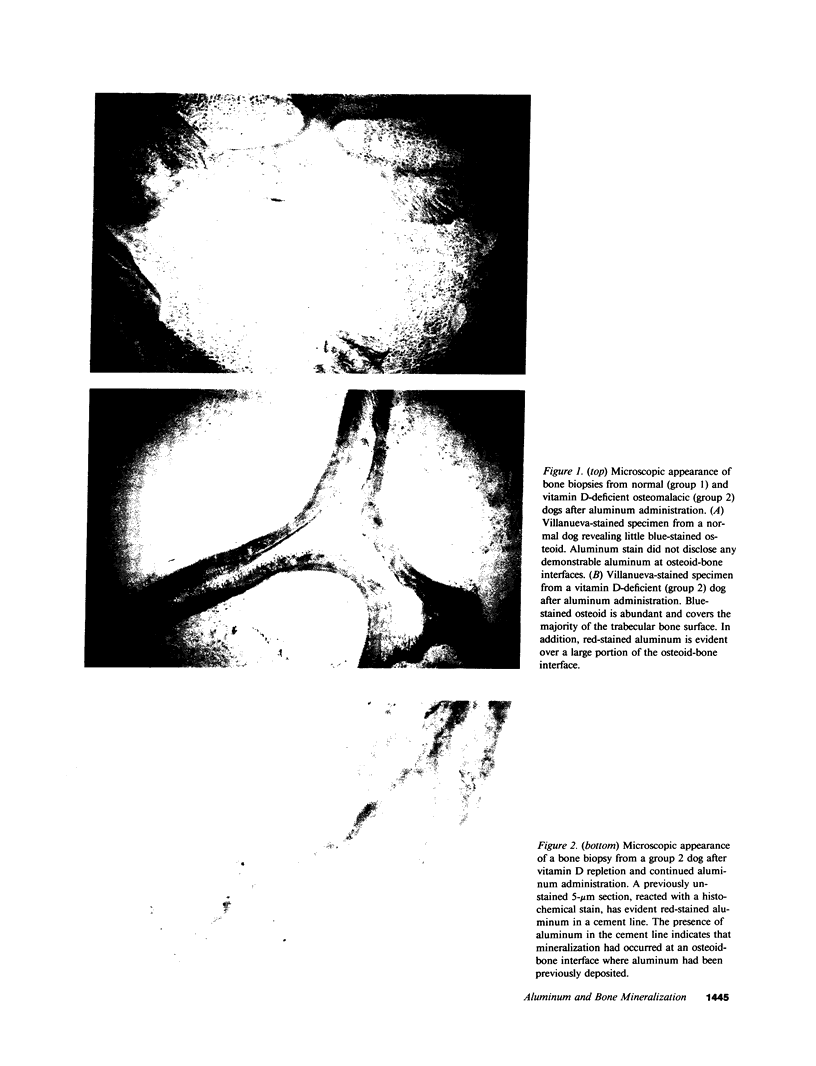
Although aluminum excess is an apparent pathogenetic factor underlying osteomalacia in dialysis-treated patients with chronic renal failure, the mechanism by which aluminum impairs bone mineralization is unclear. However, the observation that aluminum is present at osteoid-bone interfaces in bone biopsies of affected patients suggests that its presence at calcification fronts disturbs the cellular and/or physiochemical processes underlying normal mineralization. Alternatively, aluminum at osteoid-bone interfaces may reflect deposition in preexistent osteomalacic bone without direct effects on the mineralization process. We investigated whether aluminum accumulates preferentially in osteomalacic bone and, if so, whether deposition of aluminum occurs at calcification fronts and specifically inhibits mineralization. Aluminum chloride (1 mg/kg) was administered intravenously three times per week for 3 wk to five normal and five vitamin D-deficient osteomalacic dogs. Before administration of aluminum the vitamin D-deficient dogs had biochemical and bone biopsy evidence of osteomalacia. Bone aluminum content in the osteomalacic dogs (15.1 +/- 2.2 micrograms/g) and the plasma aluminum concentration (10.4 +/- 2.1 micrograms/liter) were no different than those of normal dogs (10.5 +/- 3.5 micrograms/g and 11.9 +/- 1.2 microgram/liter, respectively). After the 3 wk of aluminum administration the plasma phosphorus, parathyroid hormone, and 25-hydroxyvitamin D concentrations were unchanged in normal and vitamin D-deficient dogs. Similarly, no alteration in bone histology occurred in either group. In contrast, bone aluminum content increased to a greater extent in the vitamin D-deficient dogs (390.3 +/- 24.3 micrograms/g) than in the normal dogs (73.6 +/- 10.6 micrograms/g). Moreover, aluminum localized at the osteoid-bone interfaces of the osteomalacic bone in the vitamin D-deficient dogs, covering 42.9 +/- 9.2% of the osteoid-bone surface. Further, in spite of continued aluminum chloride administration (1 mg/kg two times per week), vitamin D repletion of the vitamin D-deficient dogs for 11 wk resulted in normalization of their biochemistries. In addition, while normal dogs maintained normal bone histology during the period of continued aluminum administration, vitamin D repletion of the vitamin D-deficient dogs induced healing of their bones. Indeed, the appearance of aluminum in the cement lines of the healed bones indicated that mineralization had occurred at sites of prior aluminum deposition. These observations illustrate that aluminum deposition in osteomalacic bone may be a secondary event that does not influence bone mineralization. Thus, although aluminum may cause osteomalacia in chronic renal failure, its presence at mineralization fronts may not be the mechanism underlying this derangement.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman F. R., Gitelman H. J. Improved electrothermal determination of aluminum in serum by atomic absorption spectroscopy. Clin Chem. 1980 Feb;26(2):258–260. [PubMed] [Google Scholar]
- Brown D. J., Dawborn J. K., Ham K. N., Xipell J. M. Treatment of dialysis osteomalacia with desferrioxamine. Lancet. 1982 Aug 14;2(8294):343–345. doi: 10.1016/s0140-6736(82)90544-x. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Alfrey A. C., Posen S., Lissner D., Hills E., Dunstan C. R., Evans R. A. Effect of aluminum on normal and uremic rats: tissue distribution, vitamin D metabolites, and quantitative bone histology. Calcif Tissue Int. 1983 May;35(3):344–351. doi: 10.1007/BF02405056. [DOI] [PubMed] [Google Scholar]
- Ellis H. A., McCarthy J. H., Herrington J. Bone aluminium in haemodialysed patients and in rats injected with aluminium chloride: relationship to impaired bone mineralisation. J Clin Pathol. 1979 Aug;32(8):832–844. doi: 10.1136/jcp.32.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld A. J., Harrelson J. M., Gutman R. A., Wells S. A., Jr, Drezner M. K. Osteomalacia after parathyroidectomy in patients with uremia. Ann Intern Med. 1982 Jan;96(1):34–39. doi: 10.7326/0003-4819-96-1-34. [DOI] [PubMed] [Google Scholar]
- Goodman W. G., Henry D. A., Horst R., Nudelman R. K., Alfrey A. C., Coburn J. W. Parenteral aluminum administration in the dog: II. Induction of osteomalacia and effect on vitamin D metabolism. Kidney Int. 1984 Feb;25(2):370–375. doi: 10.1038/ki.1984.26. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Chyu K. J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971 Dec;33(6):992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- Hodsman A. B., Sherrard D. J., Alfrey A. C., Ott S., Brickman A. S., Miller N. L., Maloney N. A., Coburn J. W. Bone aluminum and histomorphometric features of renal osteodystrophy. J Clin Endocrinol Metab. 1982 Mar;54(3):539–546. doi: 10.1210/jcem-54-3-539. [DOI] [PubMed] [Google Scholar]
- Hodsman A. B., Sherrard D. J., Wong E. G., Brickman A. S., Lee D. B., Alfrey A. C., Singer F. R., Norman A. W., Coburn J. W. Vitamin-D-resistant osteomalacia in hemodialysis patients lacking secondary hyperparathyroidism. Ann Intern Med. 1981 May;94(5):629–637. doi: 10.7326/0003-4819-94-5-629. [DOI] [PubMed] [Google Scholar]
- Lyles K. W., Harrelson J. M., Drezner M. K. The efficacy of vitamin D2 and oral phosphorus therapy in X-linked hypophosphatemic rickets and osteomalacia. J Clin Endocrinol Metab. 1982 Feb;54(2):307–315. doi: 10.1210/jcem-54-2-307. [DOI] [PubMed] [Google Scholar]
- Maloney N. A., Ott S. M., Alfrey A. C., Miller N. L., Coburn J. W., Sherrard D. J. Histological quantitation of aluminum in iliac bone from patients with renal failure. J Lab Clin Med. 1982 Feb;99(2):206–216. [PubMed] [Google Scholar]
- McClure J., Fazzalari N. L., Fassett R. G., Pugsley D. J. Bone histoquantitative findings and histochemical staining reactions for aluminium in chronic renal failure patients treated with haemodialysis fluids containing high and low concentrations of aluminium. J Clin Pathol. 1983 Nov;36(11):1281–1287. doi: 10.1136/jcp.36.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. A., Schenk R. K. Quantitative structural analysis of human cancellous bone. Acta Anat (Basel) 1970;75(1):54–66. doi: 10.1159/000143440. [DOI] [PubMed] [Google Scholar]
- Ott S. M., Maloney N. A., Coburn J. W., Alfrey A. C., Sherrard D. J. The prevalence of bone aluminum deposition in renal osteodystrophy and its relation to the response to calcitriol therapy. N Engl J Med. 1982 Sep 16;307(12):709–713. doi: 10.1056/NEJM198209163071202. [DOI] [PubMed] [Google Scholar]
- Pierides A. M., Edwards W. G., Jr, Cullum U. X., Jr, McCall J. T., Ellis H. A. Hemodialysis encephalopathy with osteomalacic fractures and muscle weakness. Kidney Int. 1980 Jul;18(1):115–124. doi: 10.1038/ki.1980.117. [DOI] [PubMed] [Google Scholar]
- Platts M. M., Goode G. C., Hislop J. S. Composition of the domestic water supply and the incidence of fractures and encephalopathy in patients on home dialysis. Br Med J. 1977 Sep 10;2(6088):657–660. doi: 10.1136/bmj.2.6088.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Felsenfeld A. J., Haygood C. C., Wilson P., Clarke C., Llach F. Animal model of aluminum-induced osteomalacia: role of chronic renal failure. Kidney Int. 1983 Feb;23(2):327–335. doi: 10.1038/ki.1983.23. [DOI] [PubMed] [Google Scholar]
- Smith P. S., McClure J. Localisation of aluminium by histochemical and electron probe x-ray microanalytical techniques in bone tissue of cases of renal osteodystrophy. J Clin Pathol. 1982 Nov;35(11):1283–1293. doi: 10.1136/jcp.35.11.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffaletti J., Blosser N., Hall T., Smith S., Tompkins D. An automated dry-slide enzymatic method evaluated for measurement of creatinine in serum. Clin Chem. 1983 Apr;29(4):684–687. [PubMed] [Google Scholar]
- Villanueva A. R. A bone stain for osteoid seams in fresh, unembedded, mineralized bone. Stain Technol. 1974 Jan;49(1):1–8. doi: 10.3109/10520297409116928. [DOI] [PubMed] [Google Scholar]
- Walker G. S., Aaron J. E., Peacock M., Robinson P. J., Davison A. M. Dialysate aluminium concentration and renal bone disease. Kidney Int. 1982 Feb;21(2):411–415. doi: 10.1038/ki.1982.37. [DOI] [PubMed] [Google Scholar]
- Ward M. K., Feest T. G., Ellis H. A., Parkinson I. S., Kerr D. N. Osteomalacic dialysis osteodystrophy: Evidence for a water-borne aetiological agent, probably aluminium. Lancet. 1978 Apr 22;1(8069):841–845. doi: 10.1016/s0140-6736(78)90191-5. [DOI] [PubMed] [Google Scholar]