Summary
While the prime function of classical MHC I molecules is to present peptide antigens to pathogen-specific cytotoxic T cells, non-classical MHC-I antigens perform a diverse array of functions in both innate and adaptive immunity. In this review we summarize recent evidence that non -classical MHC molecules are not only recognized by pathogen-specific T cells but that they also serve as immunoregulatory molecules by stimulating a number of distinct non-conventional T cell subsets.
Introduction
The highly polymorphic “classical” or “class Ia” MHC molecules serve an integral role in adaptive immunity by presenting peptides to cytotoxic T cells. However, there are also a number of “non-classical” or “class Ib” MHC molecules that, while structurally similar to class Ia molecules, frequently have quite distinct functions. Class Ib molecules are typically far less polymorphic than their classical counterparts, can have a more limited pattern of expression and in some cases bind non-peptide ligands (for a review see [1]). MHC class Ib molecules have a diverse range of functions, including in the presentation of lipid antigens (CD1d) for recognition by natural killer T (NKT) cells, as ligands (MR1) for mucosal-associated invariant T (MAIT) cells and serving as dual ligands for both NK cell and αβ T cell receptors (HLA-E and Qa-1b). A rapidly expanding body of literature highlights the diverse roles played by class Ib molecules in pathogen recognition, virus-induced autoimmune disease, tumor immunosurveillance and regulation of autoimmunity. Here, we summarize recent key work in these areas.
MHC Class Ib as Innate Pathogen Recognition Molecules
Specific MHC class Ib molecules serve as ligands for T cells expressing “semi-invariant” T cell receptors (TCR). These cells operate early in the course of an immune response and may modulate the subsequent differentiation of the adaptive response [1,2]. From this perspective, these MHC Ib-restricted T cells constitute components of the cellular innate immune response and their TCRs may arguably be regarded as pattern recognition receptors.
The non-MHC encoded CD1d molecule can present lipid antigens, such the marine sponge derived alpha-galactosylceramide (α–GalCer), to NKT cells. These cells express a semi-invariant TCR consisting of a fixed TCRα-chain (Vα14 in mice, Vα24 in humans) that can pair with a limited number of TCRβ-chains (Vβ11 in humans, Vβ8.2, Vβ7 or Vβ2 in mice). Interestingly, while the CDR3α of the NKT cell TCR is completely fixed, the CDR3β regions are highly diverse. Recent evidence suggests that variability in the TCRβ chain of NKT cells may play a role in the specificity for distinct lipid ligands [3*,4]. For example, while the CDR2β region is critical for recognition of α–GalCer, both the CDR1β and CDR3β also contribute to recognition of isoglobotriaosylceramide (iGb3). Therefore, it appears that although the NKT cell TCR may have a common mode of docking on CD1d, specific beta chain residues may impart distinct fine specificities for different glycolipids.
Recognition of CD1d by NKT cells can result in the production of both Th1 and Th2 cytokines, as well as various chemokines [5]. These factors can then orchestrate the activation of other cells of the immune system, including dendritic cells (DCs), natural killer (NK) cells, and lymphocytes. While many studies and even clinical trials have utilized α-GalCer to efficiently activate NKT cells, the relative importance of more physiological ligands remains a subject of intense interest. In this connection, pathogen-derived glycolipids such as α-galacturonosylceramide (α–GalACer) from Sphingomonas and α-galactosyldiacylglycerol (α–GalDAG) from Borrelia burgdorferi have been shown to bind to CD1d and activate NKT cells [6]. In addition, TLR ligation during bacterial infections can drive the synthesis of endogenous glycosphingolipids (GSLs), such as iGb3 that can also be presented to NKT cells by CD1d [6-8]. Moreover, the repertoire of CD1d-bound ligands may be further modulated during infection as TLR signaling through the MyD88 adaptor molecule blocks lysosomal α-galactosidase A–mediated degradation of iGb3 [9*]. Taken together, these data are consistent with a model in which stimulation of innate pattern recognition receptors by bacterial products leads to upregulation of iGb3, elevated CD1d ligand density, and activation of NKT cells.
The ability of NKT cells to produce both Th1 and Th2 cytokines makes these cells attractive targets for manipulating adaptive immune responses. Several studies have shown that NKT cells vary in the profile of cytokines produced in response to alterations in the structure of α-GalCer [10-15]. Im et al. [16*] recently reported that derivatives of α-GalCer that drive Th2 responses can directly bind CD1d on the cell surface. In contrast, α-GalCer and derivatives that produce mixed Th1/Th2 responses require lipid transfer factors to load them onto CD1d in an endosome. As a result, these CD1d:glycolipid complexes take longer to appear on the cell surface. Thus the endosomal loading process itself may force association of CD1d:glycolipid complexes with molecules that direct them to lipid rafts within the immunological synapse, whereas glycolipids that directly bind cell surface CD1d are excluded from lipid rafts. This difference in cell surface localization of CD1d may be responsible for the distinct cytokine profiles elicited by different glycolipid ligands.
Another specialized T cell population with a semi-invariant TCR utilizes a fixed TCRα (Vα19-Jα33 in mice and Vα7.2-Jα33 in humans) that pairs with a limited repertoire of TCRβ chains [17]. These cells, simultaneously identified in mice and humans [18], are found in the circulation and intestinal lamina propria [19], and therefore have been termed „mucosa-associated invariant T cells’ or MAIT cells. MAIT cells are largely CD4−CD8− or CD8αα+ are selected in the thymus and seed the intestinal mucosa [20], where they expand upon encounter with B cells expressing the MHC class Ib molecule MR1. Gut bacterial flora is an additional essential determinant for the expansion of MAIT cells in intestinal mucosal tissues, as MAIT cells are absent in germ free mice [19]. MR1 is highly conserved genetically and functionally in mammals, with ~90% identity in the predicted amino acid sequences of mouse and human α1-α2 domains and evidence for cross-species TCR-MR1 interaction [21**,22]. Earlier work suggested that the MR1-associated ligand may be an α-mannosyl ceramide [23]. Based on this, Shimamura tested a series of α-mannosyl ceramide derivatives and found that certain sphingosine glycolipids such as (α-Man)2-PI and α-Man-α-GlcNH2-PI preferentially stimulated murine MAIT cells [24]. Interestingly, depending on the type of lipid moiety used, MAIT cells, like NKT cells, variably produced IFN-γ and IL-4, raising the possibility that specific glycolipids could be used to direct MAIT cells in order to skew CD4 T cell responses.
Recent investigations into the intracellular pathways for MR1 antigen uptake and processing suggest that MAIT cells contribute to host anti-bacterial immunity. Antigen presentation by mouse MR1 is independent of TAP and the MHC class I peptide-loading complex, but facilitated by the MHC class II chaperone HLA-DM, and dependent on the MHC class II chaperone Ii. Confocal and cryoimmunoelectron microscopy studies have shown MR1 to be present in late endosomes, raising the possibility that antigens including endocytosed microbial products may be acquired from this compartment [25]. Circulating MAIT cells in healthy individuals recognize antigens derived from Mycobacterium tuberculosis (Mtb) in an MR1-dependent manner, providing evidence that microbial antigens are presented by MR1 [26]. Moreover, Le Bourhis et al. demonstrated that mouse MAIT cells recognize cells infected by unrelated strains of bacteria and yeast in an MR1-dependent manner and that these T cells mediate protection from certain bacterial infections in vivo [27**]. Taken together, current evidence implicates MAIT cells as important participants in host microbial immunity of the gut and respiratory mucosa.
Adaptive Immune Recognition of MHC Class Ib Molecules
Although a number of MHC class Ib molecules are ligands for receptors expressed by innate immunocytes, recent literature points toward an important role for these molecules in adaptive immune responses to pathogens. Class Ib-restricted αβ TCR-expressing CD8 T cells have been identified as participants in the overall adaptive T cell response in a number of mouse disease models [28-30], and have been shown to be protective in vivo in the case of Qa-1b- and H2-M3-restricted responses to Listeria monocytogenes [31-33], an H2-M3-restricted response to Mtb [34], and a class Ia-independent CD8 T cell response to a mouse γ-herpesvirus [35]. Swanson et al. recently defined a protective antiviral CD8 T cell response directed toward an oligopeptide derived from the mouse polyomavirus (MPyV) presented by Q9, a murine Qa-2 family member [36,37**]. As Q9 is nonpolymorphic, we have been able to generate Q9-restricted MPyV-specific CD8 T cell responses across MHC class Ia haplotype barriers (ARH and AEL, unpublished data). The finding that class Ib-restricted CD8 T cells mediate protection against a mouse viral infection will hopefully spur efforts to identify additional microbe-specific class Ib-restricted T cell responses in humans that might be effective across a range of different HLA haplotypes.
The existence of viral immunoevasins that impair expression of class Ib molecules also implies a role for these MHC molecules in host defense. Renukaradhya et al. provided evidence that the matrix protein of vesicular stomatitis virus (VSV) downregulates mouse CD1d expression in a p38 MAPK-dependent manner, implying that VSV has devised tactics to offset immunity conferred by NKT cells [38]. Similarly vaccinia virus inhibits CD1d expression [39], indicating that this molecule, and possibly its human homolog, are recognized by immune effector cells involved in surveillance of this viral infection. While the function of human class Ib molecule HLA-G is unclear, Park et al. recently showed that the US10 protein of human cytomegalovirus (HCMV) downregulates its expression [40], suggesting that HLA-G may have a role in anti-HCMV immunity. Taken together, these studies argue that elucidation of class Ib-specific responses to viral infections could provide new insights for viral vaccine development.
Qa-1b/HLA-E: double duty MHC Class Ib molecules
As ligands for both inhibitory and activating receptors, Qa-1b and its human ortholog HLA-E have diverse immunological roles (Figure 1). The peptide-binding groove of Qa-1b is predominantly occupied by the highly conserved Qa-1b determinant modifier (Qdm) 9mer peptide that is derived from the signal sequence of class Ia molecules [41,42]. The Qdm:Qa-1b complex is the ligand for CD94-NKG2A [43] an inhibitory signaling receptor on NK cells and CD8 T cells, as well as activating receptors such as CD94-NKG2C [44]. Similarly, the peptide-binding groove of its human ortholog, HLA-E, is also largely occupied by conserved peptides derived from other HLA-class I molecules [45] and recognition by CD94-NKG2 receptors is acutely dependent on the sequence of such peptides [46]. CD94-NKG2A appears to operate as a sensor for the expression of MHC class Ia molecules and the functional integrity of the antigen processing machinery [47,48] both of which can be impaired in neoplastic cells or targeted by immunoevasins expressed by a variety of pathogens.
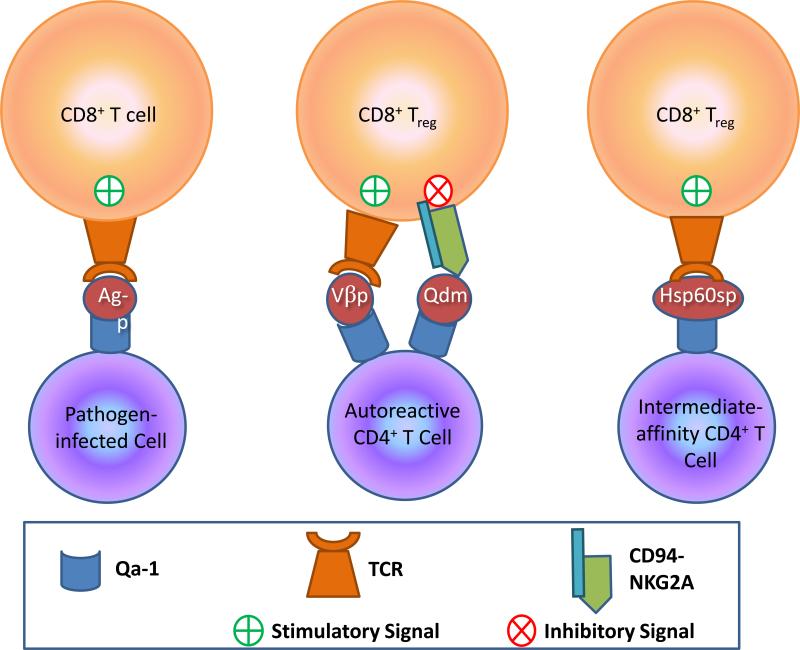
Figure 1. Different peptide:Qa-1b ligands trigger varied responses by Qa-1b-specific CD8 T cells.
Left, CD8 T cells expressing TCRs specific for pathogen-derived peptide bound to Qa-1b are stimulated to employ anti-pathogen effector activities. Middle, Dynamic interplay between TCR activation and CD94-NKG2A inhibition on a CD8 Treg determines the fate of the autoreactive CD4 T cell. Here, TCRs of CD8 Treg cells recognize a peptide from the TCR-Vβ of an autoreactive CD4 T cell presented by Qa-1b. Qa-1b also presents Qdm peptide that engages inhibitory CD94-NKG2A receptors. Right, TCR mediates CD8 Treg recognition of a peptide derived from the signal sequence of Hsp60 presented by Qa-1b. The CD8 Treg is signaled to control an intermediate-affinity, potentially self-reactive CD4 T cell.
While the function of the activating isoforms of the CD94-NKG2 family are only beginning to be understood, it is believed they may have a role in the control of pathogen infection, particularly HCMV. For example, positive serology for HCMV is strongly associated with high proportions of CD94-NKG2C expressing NK cells [49]. Similarly, co-culture of NK cells with HCMV-infected fibroblasts results in the expansion of CD94-NKG2C positive NK cells [50].
Antiviral CD8 effector T cells can also express CD94-NKG2A, which can act to balance anti-viral immunity with virus-associated immunopathology. Inhibition of virus-specific cytotoxic effector activities is critical in situations of widespread viral antigen expression by parenchymal cells as well as expression by valuable nonrenewable somatic cells. In the MPyV infection model, high load infection is associated with protracted expression of CD94-NKG2A by antiviral CD8 T cells, which serves to dampen antigen-specific cytotoxicity. For MPyV, the unwitting consequence of this host defense against excessive cell death is a high set point of persistent viral load and the elevated risk for polyomavirus-induced tumors [51]. Similarly, Zhou et al. [52] showed that Qa-1b:CD94-NKG2A engagement negatively regulates TNF-α production by influenza virus-specific CD8 effector T cells that, in turn, limits pulmonary immunopathology. Alternatively, herpes simplex virus-specific CD8 T cells in proximity to latently infected trigeminal ganglion cells express CD94-NKG2A, which engages Qa-1b expressed by neurons and thereby protects infected neurons from CD8 T cell-mediated cytotoxicity [53]. From these models of CD94-NKG2A-mediated inhibition of effector CD8 T cells, it is also apparent that this receptor regulates discrete effector activities and may do so in a manner that controls virus replication without destruction of virus-infected host cells [54]. Because IFN-γ upregulates Qa-1b:Qdm expression [55], it is also possible that IFN-γ produced by activated NK cells and T cells serves to modulate the strength of CD94-NKG2A signaling and the range of effector activities that are inhibited.
In a recent important study, Oliveira et al. showed that Qa-1b also binds a diverse array of peptides derived from improperly processed housekeeping proteins that are generated by impairments in antigen processing [56**]. These peptides replace Qdm and are immunogenic for a novel population of Qa-1b-restricted CD8 T cells that mediate cytotoxicity against target cells expressing these antigens. Importantly, these Qa-1b-restricted T cells are readily detected in the immune response to tumors deficient in antigen processing. This study raises the exciting possibility that humans may possess a similar population of HLA-E-restricted CD8 T cells in their T cell repertoire that could be harnessed for tumor immunotherapy.
A sizeable body of literature shows that Qa-1b and HLA-E operate in host microbial adaptive immune responses. In humans, HLA-E-restricted CD8 T cell responses to Mycobacterium tuberculosis (Mtb), Salmonella typhi and HCMV have been observed [57-59]. Interestingly, presentation of Mtb-derived antigens by HLA-E but not class Ia molecules was shown to be largely resistant to cycloheximide treatment of APC, suggesting that HLA-E can capture peptide antigens from distinct intracellular compartments, possibly utilizing recycled HLA-E [60**].
The UL40 protein of HCMV encodes a sequence that mimics the leader sequence of most HLA-C alleles. Consequently UL40 may facilitate the interaction between HLA-E and CD94-NKG2A following HCMV-infection. However in individuals in whom the UL40-derived peptide differs from self-encoded HLA-C sequences as a result of HLA polymorphism, HCMV infection results in a robust UL40-specific, HLA-E restricted T cell response. These responses do not appear to be subdominant to class Ia-restricted HCMV responses and in some individuals comprise in excess of 20% of the CD8 T cell population [61,62]. Such populations in the setting of HLA-C-mismatched transplantation, could negatively impact graft survival as the UL40 sequence is identical to that found in most HLA-C alleles. To this end, we have recently identified expansions of HLA-E restricted T cells in lung transplant recipients at various stages post-transplant (LCS and AGB, unpublished data).
Qa-1b also serves as a restriction element for a population of CD8 T cells that control autoreactive CD4 T cells. Expression of Qa-1b is transiently upregulated on activated CD4 T cells, which can be loaded with peptides derived from TCR Vβ chains and recognized by these CD8 regulatory T cells (Treg). Because these CD8 Treg may also express CD94-NKG2A, there is an opportunity for dynamic interplay between activating and inhibitory receptors depending on the relative occupancy of Qdm or Vβ peptides by Qa-1b (Figure 1). This dynamic regulation was investigated in an elegant paper by Lu et al. [63*] utilizing the murine experimental autoimmune encephalomyelitis (EAE) model for multiple sclerosis. By using two different mutations in Qa-1b, D227K, which disrupts binding of Qa-1b to CD8 (mitigating TCR engagement), and R72A, which disrupts Qa-1b binding to NKG2A, this group was able to distinguish the impact of these two interactions on CD8 Treg control of autoreactive CD4 T cells. Transgenic mice expressing the D227K mutation exhibited increased susceptibility to EAE, while those transgenic for the R72A mutation were EAE-resistant [64]. Kumar and colleagues [65] recently described a population of CD8αα+ TCRα+ Treg cells which recognize Qa-1b-associated TCR peptides presented by dendritic cells that have ingested apoptotic CD4 T cells. This cross-presentation was driven by inflammatory stimuli. These CD8 Treg were shown to suppress EAE and apparently do so by killing myelin basic protein-reactive CD4 T cells. This study raises the possibility that “tolerogenic” DCs may be harnessed to ameliorate autoimmune diseases by eliciting such Qa-1b/HLA-E-restricted CD8 Treg effectors.
The identification of a non-Qdm peptide that enables CD8 Treg cells to recognize autoreactive T cells suggests that Qa-1b/HLA-E-restricted responses may be put to therapeutic use to control autoimmunity or enhance vaccine efficiency. In a series of papers exploring this possibility [66-68], the Chess and Jiang group determined that intermediate affinity T cells that expand following exposure to nominal antigens preferentially express Qa-1b bearing the signal peptide from heat shock protein 60 (Hsp60sp), the ligand for a novel population of CD8 T cells with immunoregulatory capability. Qa-1b:Hsp60sp-specific CD8 T cells appear to constrain the expansion of intermediate affinity T cells, which include those that cross-react with self antigens, while sparing high affinity T cells specific for cognate antigen. Importantly, Hsp60sp peptide vaccination induced these Qa-1b-restricted CD8 T cells that then mediated cross protection in two autoimmune disease models [69*]. Thus, Qa-1b:Hsp60sp is a common structure expressed by activated T cells having TCRs of intermediate affinity for self, which allows these potentially autoreactive cells to be recognized by a generic population of Qa-1b-restricted regulatory CD8 T cells (Figure 1). While there is evidence that HLA-E can bind an Hsp60-derived peptide [70], whether or not this is expressed by T cells bearing TCRs with intermediate affinity for self antigens and is similarly recognized by such class Ib-restricted CD8 Treg remains to be determined.
Conclusions
In summary, it is increasingly evident that MHC class Ib molecules can act as restricting elements for effector T cells which can limit the spread of pathogens. Consequently, given the limited polymorphism in these genes, particularly in humans, pathogen-derived peptides that bind these molecules may represent attractive vaccine candidates. However, molecules such as CD1d, MR1, HLA-E and Qa-1b also appear to have distinct roles in immunity through the stimulation of specialized T cell populations that can both orchestrate the quality of the immune response and regulate auto-reactive T cell responses. Further insights into the antigens presented by these molecules, the mechanisms by which they acquire such antigens and the way in which they are recognized by T cells may provide novel approaches to improving pathogen-specific responses and ameliorating autoimmune disease.
Acknowledgments
This work was supported by NIH grants R01 CA139220 and R01 CA71971 (AEL), and by the National Health and Medical Research Council (NHMRC). LCS is supported by a NHMRC Career Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 2.Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8:563–568. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- 3*.Florence WC, Xia C, Gordy LE, Chen W, Zhang Y, Scott-Browne J, Kinjo Y, Yu KO, Keshipeddy S, Pellicci DG, et al. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. EMBO J. 2009;28:3579–3590. doi: 10.1038/emboj.2009.286. [This paper provides evidence that the beta chain of NKT TCR is important in the recognition of different lipid ligands.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallevaey T, Scott-Browne JP, Matsuda JL, Young MH, Pellicci DG, Patel O, Thakur M, Kjer-Nielsen L, Richardson SK, Cerundolo V, et al. T cell receptor CDR2 beta and CDR3 beta loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31:60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middendorp S, Nieuwenhuis EE. NKT cells in mucosal immunity. Mucosal Immunol. 2009;2:393–402. doi: 10.1038/mi.2009.99. [DOI] [PubMed] [Google Scholar]
- 6.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 7.De Libero G, Moran AP, Gober HJ, Rossy E, Shamshiev A, Chelnokova O, Mazorra Z, Vendetti S, Sacchi A, Prendergast MM, et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 2005;22:763–772. doi: 10.1016/j.immuni.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9*.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [This paper furthers our understanding of the processing and loading of glycolipid antigens on CD1d for presentation to iNKT cells by showing that TLR signaling regulates the production of endogenous CD1d-binding glycolipids.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 11.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, 3rd, Teyton L, Bendelac A, Savage PB. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 13.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, Ho DD, Tsuji M. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci U S A. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [This paper links the structure of a CD1d-binding glycolipid to CD1d localization in cell membrane lipid rafts, which in turn influences the cytokine profile of responding NKT cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfrey DI, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Rossjohn J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin Immunol. 2010;22:61–67. doi: 10.1016/j.smim.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 20.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [This study demonstrates the evolutionary conservation of MR1 presentation to MAIT cells. Cross reactivity of human and mouse MAIT cells was observed on bovine, rat, human and mouse MR1 proteins. In addition, it was shown that acid eluates from MR1 enhance MAIT activation across different species.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998;161:4066–4077. [PubMed] [Google Scholar]
- 23.Okamoto N, Kanie O, Huang YY, Fujii R, Watanabe H, Shimamura M. Synthetic alpha-mannosyl ceramide as a potent stimulant for an NKT cell repertoire bearing the invariant Valpha19-Jalpha26 TCR alpha chain. Chem Biol. 2005;12:677–683. doi: 10.1016/j.chembiol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Shimamura M. Non-reducing end alpha-mannosylated glycolipids as potent activators for invariant Valpha19 TCR-bearing natural killer T cells. Carbohydr Res. 2008;343:2010–2017. doi: 10.1016/j.carres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Gilfillan S, Kim S, Thompson B, Wang X, Sant AJ, Fremont DH, Lantz O, Hansen TH. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J Exp Med. 2008;205:1201–1211. doi: 10.1084/jem.20072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [This paper shows that mucosal-associated invariant T (MAIT) cells contribute to host defense by targeting highly conserved microbial antigens presented by MR1.] [DOI] [PubMed] [Google Scholar]
- 28.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 29.Milligan GN, Flaherty L, Braciale VL, Braciale TJ. Nonconventional (TL-encoded) major histocompatibility complex molecules present processed viral antigen to cytotoxic T lymphocytes. J Exp Med. 1991;174:133–138. doi: 10.1084/jem.174.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerksiek KM, Busch DH, Pilip IM, Allen SE, Pamer EG. H2-M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J Exp Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Orazio SE, Halme DG, Ploegh HL, Starnbach MN. Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection. J Immunol. 2003;171:291–298. doi: 10.4049/jimmunol.171.1.291. [DOI] [PubMed] [Google Scholar]
- 32.Seaman MS, Wang CR, Forman J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J Immunol. 2000;165:5192–5201. doi: 10.4049/jimmunol.165.9.5192. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doi T, Yamada H, Yajima T, Wajjwalku W, Hara T, Yoshikai Y. H2-M3-restricted CD8+ T cells induced by peptide-pulsed dendritic cells confer protection against Mycobacterium tuberculosis. J Immunol. 2007;178:3806–3813. doi: 10.4049/jimmunol.178.6.3806. [DOI] [PubMed] [Google Scholar]
- 35.Braaten DC, McClellan JS, Messaoudi I, Tibbetts SA, McClellan KB, Nikolich-Zugich J, Virgin HW. Effective control of chronic gamma-herpesvirus infection by unconventional MHC Class Ia-independent CD8 T cells. PLoS Pathog. 2006;2:e37. doi: 10.1371/journal.ppat.0020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson PA, II, Hofstetter AR, Wilson JJ, Lukacher AE. Cutting edge: shift in antigen dependence by an antiviral MHC class Ib-restricted CD8 T cell response during persistent viral infection. J Immunol. 2009;182:5198–5202. doi: 10.4049/jimmunol.0900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Swanson PA, II, Pack CD, Hadley A, Wang CR, Stroynowski I, Jensen PE, Lukacher AE. An MHC class Ib-restricted CD8 T cell response confers antiviral immunity. J Exp Med. 2008;205:1647–1657. doi: 10.1084/jem.20080570. [The authors demonstrate that infection by mouse polyomavirus elicits CD8 T cells that recognize a viral peptide presented by the mouse Class Ib molecule Qa-2, and that these T cells control infection in vivo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renukaradhya GJ, Khan MA, Shaji D, Brutkiewicz RR. Vesicular stomatitis virus matrix protein impairs CD1d-mediated antigen presentation through activation of the p38 MAPK pathway. J Virol. 2008;82:12535–12542. doi: 10.1128/JVI.00881-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renukaradhya GJ, Webb TJ, Khan MA, Lin YL, Du W, Gervay-Hague J, Brutkiewicz RR. Virus-induced inhibition of CD1d1-mediated antigen presentation: reciprocal regulation by p38 and ERK. J Immunol. 2005;175:4301–4308. doi: 10.4049/jimmunol.175.7.4301. [DOI] [PubMed] [Google Scholar]
- 40.Park B, Spooner E, Houser BL, Strominger JL, Ploegh HL. The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation. J Exp Med. 2010;207:2033–2041. doi: 10.1084/jem.20091793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeCloux A, Woods AS, Cotter RJ, Soloski MJ, Forman J. Dominance of a single peptide bound to the class I(B) molecule, Qa-1b. J Immunol. 1997;158:2183–2191. [PubMed] [Google Scholar]
- 42.Cotterill LA, Stauss HJ, Millrain MM, Pappin DJ, Rahman D, Canas B, Chandler P, Stackpoole A, Simpson E, Robinson PJ, et al. Qa-1 interaction and T cell recognition of the Qa-1 determinant modifier peptide. Eur J Immunol. 1997;27:2123–2132. doi: 10.1002/eji.1830270902. [DOI] [PubMed] [Google Scholar]
- 43.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b). J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vance RE, Jamieson AM, Raulet DH. Recognition of the class Ib molecule Qa-1(b) by putative activating receptors CD94/NKG2C and CD94/NKG2E on mouse natural killer cells. J Exp Med. 1999;190:1801–1812. doi: 10.1084/jem.190.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrie EJ, Clements CS, Lin J, Sullivan LC, Johnson D, Huyton T, Heroux A, Hoare HL, Beddoe T, Reid HH, et al. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J Exp Med. 2008;205:725–735. doi: 10.1084/jem.20072525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoare HL, Sullivan LC, Clements CS, Ely LK, Beddoe T, Henderson KN, Lin J, Reid HH, Brooks AG, Rossjohn J. Subtle changes in peptide conformation profoundly affect recognition of the non-classical MHC class I molecule HLA-E by the CD94-NKG2 natural killer cell receptors. J Mol Biol. 2008;377:1297–1303. doi: 10.1016/j.jmb.2008.01.098. [DOI] [PubMed] [Google Scholar]
- 47.Bland FA, Lemberg MK, McMichael AJ, Martoglio B, Braud VM. Requirement of the proteasome for the trimming of signal peptide-derived epitopes presented by the nonclassical major histocompatibility complex class I molecule HLA-E. J Biol Chem. 2003;278:33747–33752. doi: 10.1074/jbc.M305593200. [DOI] [PubMed] [Google Scholar]
- 48.Braud VM, Allan DS, Wilson D, McMichael AJ. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr Biol. 1998;8:1–10. doi: 10.1016/s0960-9822(98)70014-4. [DOI] [PubMed] [Google Scholar]
- 49.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 50.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 51.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Matsuoka M, Cantor H, Homer R, Enelow RI. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 53.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 54.Divito S, Cherpes TL, Hendricks RL. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36:119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 55.Ota T, Takeda K, Akiba H, Hayakawa Y, Ogasawara K, Ikarashi Y, Miyake S, Wakasugi H, Yamamura T, Kronenberg M, et al. IFN-gamma-mediated negative feedback regulation of NKT-cell function by CD94/NKG2. Blood. 2005;106:184–192. doi: 10.1182/blood-2004-11-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Oliveira CC, van Veelen PA, Querido B, de Ru A, Sluijter M, Laban S, Drijfhout JW, van der Burg SH, Offringa R, van Hall T. The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigen-processing defects. J Exp Med. 2010;207:207–221. doi: 10.1084/jem.20091429. [This paper shows that Qa-1b presents a surprisingly diverse repertoire of peptides in cells deficient in the transporter associated with antigen processing (TAP) that are specifically recognized by Qa-1b-restricted CD8 T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196:1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pietra G, Romagnani C, Mazzarino P, Falco M, Millo E, Moretta A, Moretta L, Mingari MC. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 2003;100:10896–10901. doi: 10.1073/pnas.1834449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 60**.Grotzke JE, Harriff MJ, Siler AC, Nolt D, Delepine J, Lewinsohn DA, Lewinsohn DM. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [This paper examines the processing and presentation of Mycobacterium antigens by MHC class Ia and class Ib (HLA-E). Interestingly, it appears that the presentation of these antigens by class Ia molecules require newly synthesized HLA whereas HLA-E does not. This implies that presentation by HLA-E could utilize recycled HLA-E molecules.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoare HL, Sullivan LC, Pietra G, Clements CS, Lee EJ, Ely LK, Beddoe T, Falco M, Kjer-Nielsen L, Reid HH, et al. Structural basis for a major histocompatibility complex class Ib-restricted T cell response. Nat Immunol. 2006;7:256–264. doi: 10.1038/ni1312. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan LC, Clements CS, Rossjohn J, Brooks AG. The major histocompatibility complex class Ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens. 2008;72:415–424. doi: 10.1111/j.1399-0039.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 63*.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [Using two Qa-1 mutant knock-in mice, the authors separated the disparate effects of Qa-1 engaging CD94-NKG2A versus TCRs, and thereby show that the ratio of inhibitory and activating receptors expressed by CD8 T cells governs the cell's overall response to Qa-1-expressing cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leavenworth JW, Schellack C, Kim HJ, Lu L, Spee P, Cantor H. Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti-NKG2A F(ab′)2. Proc Natl Acad Sci U S A. 2010;107:2562–2567. doi: 10.1073/pnas.0914732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith TR, Tang X, Maricic I, Garcia Z, Fanchiang S, Kumar V. Dendritic cells use endocytic pathway for cross-priming class Ib MHC-restricted CD8alphaalpha+TCRalphabeta+ T cells with regulatory properties. J Immunol. 2009;182:6959–6968. doi: 10.4049/jimmunol.0900316. [DOI] [PubMed] [Google Scholar]
- 66.Chen W, Zhang L, Liang B, Saenger Y, Li J, Chess L, Jiang H. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci U S A. 2007;104:20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang H, Kashleva H, Xu LX, Forman J, Flaherty L, Pernis B, Braunstein NS, Chess L. T cell vaccination induces T cell receptor Vbeta-specific Qa-1-restricted regulatory CD8(+) T cells. Proc Natl Acad Sci U S A. 1998;95:4533–4537. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang H, Wu Y, Liang B, Zheng Z, Tang G, Kanellopoulos J, Soloski M, Winchester R, Goldstein I, Chess L. An affinity/avidity model of peripheral T cell regulation. J Clin Invest. 2005;115:302–312. doi: 10.1172/JCI23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Wu Y, Zheng Z, Jiang Y, Chess L, Jiang H. The specificity of T cell regulation that enables self-nonself discrimination in the periphery. Proc Natl Acad Sci U S A. 2009;106:534–539. doi: 10.1073/pnas.0811843106. [Previously (ref. 66), these authors showed that intermediate affinity, but not high affinity, CD4 T cells express Qa-1 loaded with a peptide from heat shock protein 60 (Hsp-60sp). In this paper, Qa-1-restricted CD8 T cells are shown to cross-protect in two mouse autoimmune disease models.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403–1414. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]