Abstract
Purpose
This Phase I dose-escalation study investigated the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), safety, pharmacokinetics (PK), pharmacodynamics (PD) and preliminary clinical activity of CH5132799.
Patients and Methods
Patients with metastatic solid tumors were eligible for the study. CH5132799 was administered orally once daily (QD) or twice daily (BID) in 28-day cycles.
Results
Thirty-eight patients with solid tumors received CH5132799 at 2-96 mg QD or 48-72 mg BID. The MTD was 48 mg on the BID schedule but was not reached on the QD schedule. DLTs were grade 3 elevated liver function tests (LFT), grade 3 fatigue, grade 3 encephalopathy, grade 3 diarrhea and grade 3 diarrhea with grade 3 stomatitis; all DLTs were reversible. Most drug-related adverse events were grade 1/2. Diarrhea (34%) and nausea (32%) were the most common events. Mean Cmax and AUC0-24 in steady state at MTD were 175 ng/ml and 1,550 ng·hr/ml respectively, consistent with efficacious exposure based on preclinical modelling. Reduction in SUVmax with [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) was observed in five of seven patients at MTD. A patient with PIK3CA-mutated clear cell carcinoma of the ovary achieved a partial response by GCIG CA125 criteria and further, a heavily pre-treated patient with triple negative breast cancer had marked improvement in her cutaneous skin lesions lasting 6 cycles.
Conclusion
CH5132799 is well tolerated at the MTD dose 48 mg BID. At this dose the drug had a favorable PK and PD profile and preliminary evidence of clinical activity.
Keywords: Phase I, Class I PI3K inhibitor, CH5132799, dose-escalation, advanced/metastatic solid tumors
Introduction
The intracellular phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway regulates cellular functions such as cell survival, proliferation, growth, apoptosis, protein synthesis and glucose metabolism (1-6). Of the three classes of PI3K (I to III), class IA is implicated most in cancer (5). Class IA PI3K heterodimers comprise a p85-regulatory and a p110-catalytic subunit with several isoforms (5). Mutation or amplification of PIK3CA, the gene encoding the catalytic subunit of PI3K (p110α), promotes oncogenic activation of the PI3K pathway and occurs frequently in human cancers such as ovarian cancer and breast cancer (1, 6-12). Phosphatase and tensin homolog (PTEN), a key negative regulator of AKT, can be inactivated via mutations, down-regulation, or loss of protein expression, and is associated with tumorigenesis in prostate, gastric and other cancers (1, 13-19). Moreover, it is likely that PI3K pathway activation is associated with resistance to both chemotherapy (20-22) and targeted agents (23-26). Selective inhibition of the PI3K/AKT/mTOR pathway in cancer represents a promising therapeutic approach and has been the focus of significant research efforts including the clinical development of novel agents targeting this pathway (1, 19, 27-37).
CH5132799 (Chugai Pharmaceutical Co Ltd, Tokyo, Japan) is an oral PI3K inhibitor with specific and potent activity against class I PI3Ks, especially demonstrated in wild-type and mutant PI3Kα isoforms, and PI3Kγ at nanomolar concentrations(38). CH5132799 has no inhibitory activity against class III PI3K, or mammalian target of rapamycin (mTOR) (38). In vitro experiments demonstrated a strong antiproliferative effect of CH5132799 on human cancer cell lines with alterations in the PI3K pathway (38, 39). In vivo, CH5132799 demonstrated significant antitumor activity in human tumor xenograft models with PIK3CA mutations, with good correlation between CH5132799 exposure and inhibition of PI3K signalling (39).
The primary objective of this first-in-human, Phase I, dose escalation study was to determine the MTD of CH5132799 using a continuous oral schedule in patients with advanced solid tumors. Secondary objectives included the characterisation of CH5132799 PK, and the PD profile of PI3K inhibition in tumor and in surrogate tissues such as peripheral blood samples, and by [18F] fluorodeoxyglucose positron emission tomography (FDG-PET).
Patients and Methods
Study population
Patients had been diagnosed with advanced solid tumors that were not amenable or were refractory to standard therapy. Patients were aged ≥18 years with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 and adequate bone marrow, renal, hepatic and cardiac function were enrolled (see supplementary data for detailed inclusion and exclusion criteria) and a life expectancy of ≥12 weeks.
This study was approved by an independent ethics committee (The Royal Marsden Research Ethics Committee, London, United Kingdom) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP). Written informed consent was obtained from all patients before carrying out any study-related procedures.
Study design and CH5132799 dose escalation
This open-label dose-escalation study was conducted at four centers. Prior to the first treatment cycle, CH5132799 was administered as a single oral dose followed by a 5–7 day washout (run-in period). A classical “3+3” design was used for dose-escalation with QD to the early patient cohorts, and then BID to others, continuously in 4-week cycles. Dose escalation was determined by the nature and grade/severity of toxicities.
The primary objective was to determine the MTD of CH5132799 based on DLTs observed during the run-in period and first 4-week cycle. The MTD was defined as the highest dose level at which no more than one of six patients experienced a DLT. A starting dose of 2 mg was chosen based on the highest non-severe toxic dose in a non-rodent species and the severely toxic dose (10% lethal dose) in rat, which means 7.8-fold and 30-fold safety margins were applied to the two metabolically/kinetically relevant animal species, respectively (40).
Assessments
Medical history and demographic data were collected at baseline. Physical examination, monitoring of vital signs and other safety assessments were performed throughout the study. All toxicities were documented using Common Terminology Criteria for Adverse Events (CTCAE) V4.03 (41). DLTs were defined as grade ≥3 non-hematologic toxicity despite adequate treatment, grade 4 neutropenia lasting ≥7 days, febrile neutropenia, grade 4 thrombocytopenia lasting ≥7 days or requiring a platelet transfusion.
Tumor response was assessed according to Response Evaluation Criteria In Solid Tumors (RECIST; version 1.1) with imaging at baseline and every two cycles (42).
Pharmacokinetics and Pharmacodynamics
Plasma PK samples were collected on Cycle 0, Day 1, followed by Cycle 1, Days 1, 8, 15 and 22. Plasma concentrations of CH5132799 were measured by a validated LC/MS/MS assay method (Chugai Pharmaceutical Co. Ltd., Kamakura, Japan, data on file) and PK parameters calculated by non-compartmental analysis with first-order oral absorption (WinNonlin Ver 5.3 and Phoenix WinNonlin Ver.6.1 (Pharsight Corporation, NC, USA). The CH5132799-related inhibition of AKT phosphorylation (pAKT) was studied in platelet-rich plasma (PRP). Blood for PRP samples was collected at 0 (pre-dose), 1, 2, 6, 24, 48 and 72 hrs post-dose on Day 1 of the run-in period (Cycle 0, Day 1) and at 0 (pre-dose) on Cycle 1, Day 15. Blood samples were collected into BD Vacutainer sodium citrate coagulation tubes and centrifuged at 200 × g at 4°C for 15 minutes. The isolated PRP layer was incubated with PhosSTOP (Roche) to stabilize the phosphorylation signals and then lysed using Cell Lysis Buffer (Cell Signaling Technology) containing phenylmethane sulfonyl fluoride (Sigma-Aldrich) and then snap-frozen on dry ice. PD analysis with PRP was performed by The Institute of Cancer Research. All PRP samples were analysed for the levels of phosphorylated and total forms of PD biomarker AKT by a Meso Scale Discovery® electrochemiluminescent assay (ECLA). The assay was modified into a GCP-compliant quantitative assay for AKT by inclusion of a standard curve of recombinant active AKT protein on every plate. The quality, accuracy and precision of the assays were monitored using quality control (QC) samples created by spiking three known quantities of recombinant AKT into 10% human plasma.
Biopsies were performed at screening and at Cycle 1, Day 15. Flash frozen tumor biopsies were homogenized using a micro tissue grinder in Cell Lysis Buffer (w/v) containing PhosSTOP. The tumor lysates were centrifuged to remove debris and the protein concentration measured using the BCA assay (Pierce). Tumor biopsy samples were analysed for the levels of phosphorylated and total forms of PD biomarker AKT by a quantitative ICR modified GCP compliant Meso Scale Discovery® electrochemiluminescent assay (ECLA). [18F] Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging was performed within 28 days prior to the first dosing of CH5132799, Cycle 1, Day 8, and Cycle 3, Day 1. Lesions with the highest degree of FDG uptake were selected for quantitative analysis and a circular/spherical region of interest drawn. A SUVmax was measured for each selected lesion and the delta change in SUVmax was calculated. Tumor biopsies were taken after FDG-PET scanning to avoid interference of biopsy on FDG uptake.
Detection of mutations
PIK3CA/KRAS/BRAF mutations were studied with the OncoCarta panel v1.0 and detected by mass ARRAY System.
Statistical analyses
Descriptive statistics were used for the analysis of PK, PD, safety and tumor response data.
Results
Thirty-eight patients received at least one dose of CH5132799 (Table 1), of whom 23 received CH5132799 QD (2-96 mg/day). The decision to dose BID was made as there had been no DLTs and no significant increase in drug concentrations at doses above 56 mg QD along with PD measures i.e. recovery of p-AKT after 12 hours. Subsequently 15 further patients then received CH5132799 BID (96–144 mg/day). Overall, patients received a median of 2 cycles (range 0–6), with a median duration of 52 days of treatment (range 1–164 days). Archival tumor samples were available in 30 patients. PIK3CA mutations were found in 10% (3/30) of samples studied (Table 1).
Table 1. Patient demographics and clinical characteristics.
| Characteristic | Total patients (N=38) |
|---|---|
| Sex | |
| Male | 10 (26%) |
| Female | 28 (74%) |
| Age, years | |
| Median | 58.5 |
| Range | 41-76 |
| Race | |
| Asian | 3 (8%) |
| Black | 3 (8%) |
| White | 32 (84%) |
| Baseline ECOG performance status | |
| 0 | 12 (32%) |
| 1 | 25 (66%) |
| 2 | 1 (3%) |
| Prior anti-cancer therapies, median (range) | 3 (1–8) |
| Primary tumor site and mutational status | |
| Breast | 10 (26%) |
| No mutation | 8 |
| Unknown mutational status | 2 |
| Ovarian | 6 (16%) |
| KRAS G12D | 1 |
| PIK3CA H1047R | 1 |
| No mutation | 3 |
| Unknown mutational status | 1 |
| Oesophageal and oesophageal-gastric adenocarcinoma | 5 (13%) |
| KRAS G12D | 1 |
| EGFR S768I | 1 |
| No mutation | 3 |
| Duodenal and large intestine | 4 (11%) |
| KRAS G12A | 1 |
| KRAS G12D and PIK3CA E545K | 1 |
| No mutation | 2 |
| Gastric* | 2 (5%) |
| Lung* | 2 (5%) |
| Endometrial* | 1 (3%) |
| Vagina* | 1 (3%) |
| Myonetrium* | 1 (3%) |
| Vulva* | 1 (3%) |
| Bladder* | 1 (3%) |
| Pancreas* | 1 (3%) |
| Prostate* | 1 (3%) |
| Unknown primary origin | 1 (3%) |
| PIK3CA E545K | 1 |
Dose-limiting toxicities
DLTs were observed in five of 38 evaluable patients who received CH5132799 BID and completed the first cycle (Table 2A). Of seven patients at 48 mg BID, one patient with hepatocellular carcinoma experienced grade 3 elevated transaminases. Although the patient had liver metastases with elevated LFTs (grade 2) at baseline, a causal link to CH5132799 could not be excluded because clear evidence of disease progression in liver metastasis was not observed. At the next dose level, two of three patients receiving 72 mg BID had DLTs. One had grade 3 fatigue that was resolved following cessation of CH5132799 and she restarted CH5132799 with reduced dose when fatigue had been resolved and she did not experience the event later on, whilst the other developed grade 3 posterior reversible encephalopathy syndrome (PRES) that was presented with seizures. The diagnosis was made on characteristic MRI findings and the symptoms resolved on cessation of CH5132799 as well as instigation of supportive care without restarting CH5132799. Two of five patients receiving the intermediate dose of 56 mg BID had grade 3 diarrhea, the other grade 3 diarrhea with grade 3 stomatitis, which resolved following cessation of CH5132799 and instigation of supportive treatment, in both cases study treatment was then restarted at a reduced dose without further DLTs. Therefore, the MTD and recommended phase 2 dose (RP2D) of CH5132799 administered orally was established at 48 mg BID.
Table 2 A. Dose limiting toxicities seen on the study.
| Cohor | Dose | N | DLT 1 |
|---|---|---|---|
| 7b | 48 mg BID | 7 | grade 3 elevated LFT at Cycle 1 Day 8 |
| 8 | 72 mg BID | 3 | grade 3 cerebral encephalopathy at Cycle 1, Day 14 grade 3 Fatigue at Cycle 1 Day8 |
| 9 | 56 mg BID | 5 | grade 3 Diarrhea grade 3 Diarrhea and stomatitis (<75% of the total scheduled dose) |
Safety
The most common AEs were: diarrhea, nausea, stomatitis, fatigue, and rash which occurred in 68%, 32%, 32%, 29% and 24% of patients, respectively (Table 2B). Diarrhea was predominantly grade 1 or 2, with no grade 4 diarrhea reported. Four patients experienced grade 3 diarrhea (96 mg QD, n=2; 56 mg BID, n=2). One patient experienced grade 3 nausea (96 mg QD). No grade ≥3 gastrointestinal AEs occurred in the cohort who received CH5132799 at the R2PD (48 mg BID). Hyperglycemia was predicted due to the mechanism of action of CH5132799. Five patients experienced Grade ≥3 hyperglycemia in the BID dosing schedule suggesting a dose-dependent effect (48 mg BID, n=1; 56 mg BID, n=2; 72 mg BID; n=2 including one Grade 4). No episodes of diabetic ketoacidosis occurred and hyperglycemia was controlled with oral metformin as required. No drug-related deaths were reported.
Table 2 B. Treatment-related toxicities occurring in ≥ 10% patients by CH5132799 dose level.
| Adverse Event | QD | BID | Total (n=38) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mg (n=3) | 4 mg (n=3) | 8 mg (n=3) | 16 mg (n=3) | 32 mg (n=4) | 56 mg (n=3) | 96 mg (n=4) | 48 mg (n=7) | 56 mg (n=5) | 72 mg (n=3) | ||||||||||||
| Grade | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | |
| Diarrhea | 2 | 3 | 2 | 4 | 2 | 2 | 2 | 13 (34%) | |||||||||||||
| Nausea | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 1 | 12 (32%) | |||||||||||
| Stomatitis | 1 | 2 | 3 | 4 | 1 | 1 | 11 (29%) | ||||||||||||||
| Fatigue | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 11 (29%) | |||||||||||
| Rash | 1 | 1 | 1 | 2 | 1 | 3 | 9 (24%) | ||||||||||||||
| Decreased appetite | 1 | 2 | 1 | 2 | 2 | 8 (21%) | |||||||||||||||
| Vomiting | 2 | 3 | 1 | 6 (16%) | |||||||||||||||||
| Dry skin | 1 | 1 | 2 | 2 | 6 (16%) | ||||||||||||||||
| Hyperglycemia | 1 | 2 | 1 | 5 (13%) | |||||||||||||||||
| Anemia | 1 | 1 | 1 | 1 | 1 | 5 (13%) | |||||||||||||||
| Abdominal pain | 1 | 1 | 1 | 1 | 4 (11%) | ||||||||||||||||
Pharmacokinetics
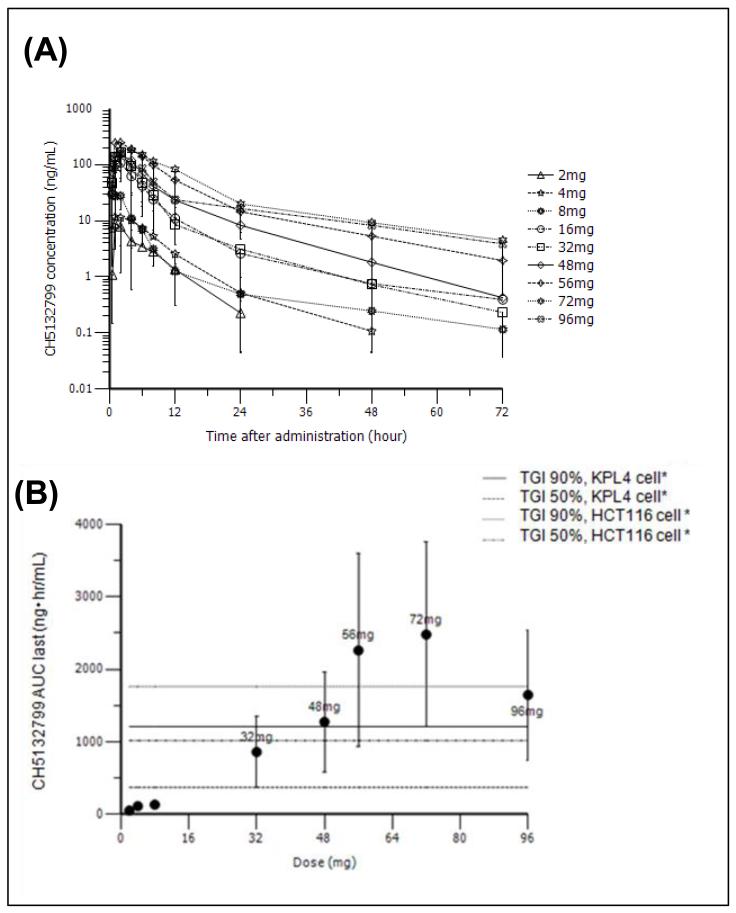
Following oral administration of a single dose of CH5132799, plasma concentrations peaked with a Tmax at 2.60 hours and t1/2 of 10.2 hours at the MTD (Figure 1A). Dose proportionality of AUC was shown in the dose range of 2 to 56 mg (Figure 2B). Steady state was reached by Cycle 1, Day 8 at the latest with little subsequent accumulation; there was no further increase in AUC at higher doses. At the MTD of 48 mg BID, mean Cmax and AUClast were 172 ng/mL and 1,270 ng·hr/mL after a single dose, respectively. Mean Cmax and AUC0-24h were 175 ng/mL and 1,550ng·hr/mL at steady state, respectively (Table 3).
Figure 1. Pharmacokinetic parameters of CH5132799.
A) Plasma concentration of CH5132799 following a single administration across the dose range of 2 mg – 96 mg. B) The correlation of the AUC (area under the curve) of CH5132799 and dose. The horizontal lines indicate effective AUC calculated to cause TGI (tumor grown inhibition) of 50% and 90% of the KPL4 and HCT116 xenograft models.
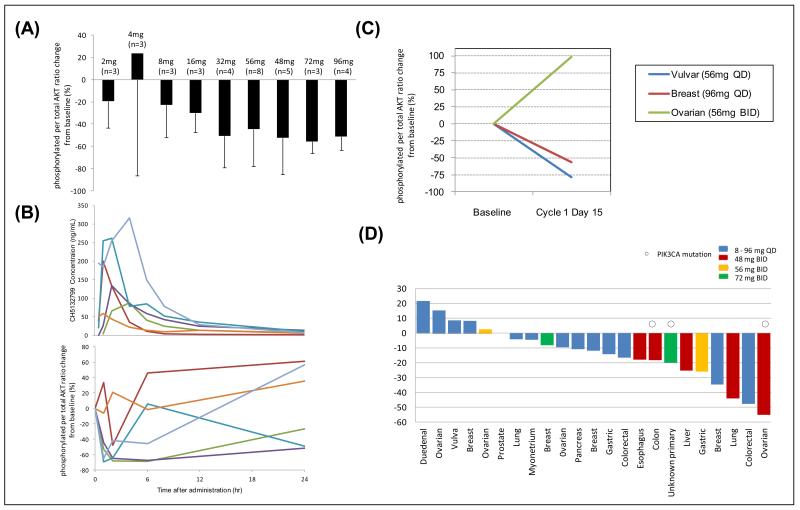
Figure 2. Pharmacodynamic parameters of CH5132799.
A) Mean percentage change of phosphorylated AKT normalized to total AKT compared to pre-treatment control values, 2 hrs following a single dose of CH5132799. B) The temporal course of plasma concentration of CH5132799 and the percentage change of p-AKT normalized to total AKT compared to pre-treatment control values following a single dose of the recommended phase II dose of CH5132799 (48 mg). C) Percentage change of p-AKT normalized to total AKT in tumor biopsies when compared to pre-treatment samples. D) SUVmax changes on FDG-PET scans in patients who had evaluable pre- and post-treatment FDG-PET scans.
Table 3. Summary of pharmacokinetics of CH5132799 in patients following oral administration.
| Regimen | Single dose |
Repeat dose |
***Accumulation ratio | |||
|---|---|---|---|---|---|---|
| *AUClast (ng·hr/mL) | Cmax (ng/mL) | t1/2 (hr) | **AUC0-24h (ng·hr/mL) | Cmax (ng/mL) | ||
| 2mg (QD) | 53.0(3) | 10.0(3) | 7.52(3) | 48.8(3) | 9.92(3) | 0.912(3) |
| 4 mg (QD) | 112(3) | 17.2(3) | 8.30(3) | 167(3) | 22.5(3) | 1.54(3) |
| 8 mg (QD) | 133(3) | 41.1(3) | 23.7(3) | 159(2) | 46.3(2) | 1.22(2) |
| 16 mg (QD) | 883(1) | 113(1) | 13.7(1) | 621(2) | 100(3) | 0.972(1) |
| 32 mg (QD) | 859(4) | 165(4) | 12.2(4) | 1740(2) | 345(3) | 1.57(2) |
| 56 mg (QD) | 969(3) | 163(3) | 14.5(3) | 748(3) | 184(3) | 1.07(3) |
| 96 mg (QD) | 1650(4) | 206(4) | 23.2(3) | 691(3) | 103(3) | 0.640(3) |
| 48 mg (BID) | 1270(4) | 172(5) | 10.2(4) | 1550(5)** | 175(5) | 1.10(2) |
| 56 mg (BID) | 3030(5) | 428(5) | 16.3(5) | 5940(2)** | 331(3) | 1.72(2) |
| 72 mg (BID) | 2480(3) | 265(3) | 18.3(3) | 5580(1)** | 631(1) | 2.25(1) |
Footnote to Table 2: Mean value and number (noted in parenthesis) are represented for each pharmacokinetic parameter.
AUClast in single dose represented AUC0-72h.
AUC0-24h in BID regimen of repeat dose were calculated based on the data from 0 to 12 hours (AUC0-12h).
Accumulation ratio represents AUC0-24h/0-12h in repeat dose / AUC0-24h/0-12h in single dose. AUC: area under the plasma concentration time curve, Cmax: maximum concentration measured, t1/2: elimination half-life
Pharmacodynamics
Significant inhibition of PI3K signalling was observed in PRP, with reduction of AKT phosphorylation at doses of 32 mg and above (Figure 2A). The temporal relationship between reduction in phosphorylation of AKT and dosing administration with CH5132799 suggested target engagement for less than 24 hours (Figure 2B). This observation contributed to the decision to increase dosing frequency to BID.
Pre- and post-treatment tumor biopsies were optional and the minimum number to be conducted during the study was not based on statistical assumptions. Tumor biopsy samples were collected before and after CH5132799 from three patients (one patient each on 56 mg QD, 96 mg QD and 56 mg BID). There was a more than 50% reduction in normalized p-AKT levels in two of the three patients (Figure 2C).
A decrease in FDG avidity between baseline and Cycle 1, Day 8 was observed in 74% of patients (17/23) who underwent serial PET imaging (Figure 2D). It was interesting that five of seven patients at the RP2D who had pre-and post- (Cycle1, Day 8) treatment PET scans showed a reduction in maximum standardised uptake value (SUVmax); two had a reduction in SUVmax of 55% and 44%.
There was no correlation between the PD changes in the limited number of biopsies, PET responses or PRP inhibition.
Efficacy
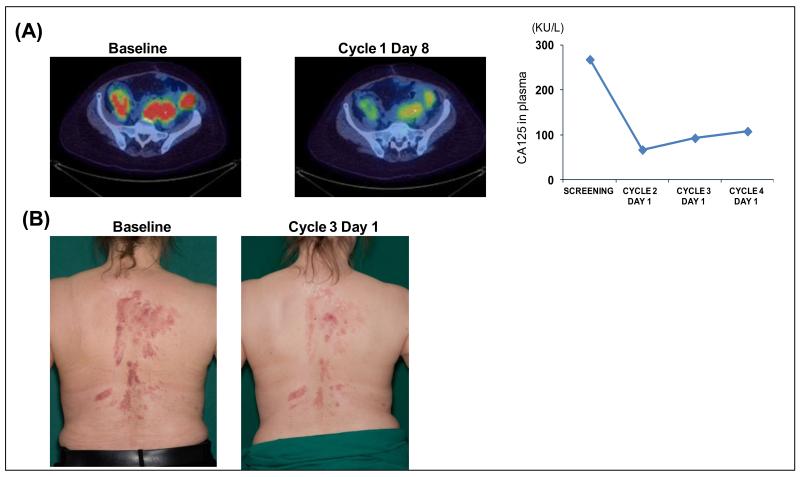
There were no RECIST partial or complete responses but one patient with PIK3CA H1047R-mutated clear cell ovarian cancer had a GCIG CA125 response having been treated at 48 mg BID and remained on study for 6 cycles (Figure 3A). A second patient with heavily pre-treated triple negative breast cancer bearing no PIK3CA or AKT mutations who was treated at the 72 mg BID dose level had marked symptomatic and objective improvement in her cutaneous skin lesions and remained on treatment for 6 cycles (Figure 3B). Disease stabilisation was seen in eight patients up to week 16 (approximately 4 cycles), including two patients with PIK3CA mutation.
Figure 3. Clinical efficacy of CH5132799.
A) FDG-PET-CT showing reduction in uptake of tracer in pelvic masses and a reduction of CA125 following treatment with CH5132799. The patient had a clear cell ovarian cancer which harbored a PIK3CA mutation. B) Representative skin lesions, before and after treatment with CH5132799, of a patient with triple-negative breast cancer.
Discussion
We report the first-in-human study of an oral PI3K class I inhibitor CH5132799. No DLTs occurred at the QD schedule, but the BID schedule was selected based on the duration of target inhibition observed in platelet-rich plasma and the lack of dose dependent incremental elevation in concentrations of drug above 56 mg QD. The BID schedule of CH5132799 was well tolerated and showed evidence of clinical and PD activity with a RP2D of 48 mg BID.
The most frequently observed toxicities were gastrointestinal, including diarrhea, nausea and stomatitis. These have been described previously in studies of PI3K, AKT and m-TOR inhibitors (28, 30-38). Reversible grade 3 LFT elevation was observed in one of seven patients treated at the MTD of 48 mg BID. This patient with hepatocellular carcinoma had liver metastases and had previously undergone hepatic resection, therefore the event was possibly caused by disease progression and/or overload of a liver with insufficient metabolic capability. No other cases of drug-related LFT abnormalities were noted although they have been described in trials of other PI3K inhibitors (30-32, 34, 43). Of note, we observed one case of PRES at a dose above the MTD. This has not been reported previously in the trials of other drugs in this class, nevertheless, it raised the possibility that CH5132799 crosses the blood-brain barrier, which may be of future relevance in the treatment of patients with intracerebral malignancies. Mood alterations have been described as adverse events in with other PI3 kinase inhibitors (30, 43), however was not seen in this trial. Although skin-related toxicities have been frequently observed in the trials of other PI3K inhibitors (28-30, 32, 33, 35-37, 43-45), CH5132799 was associated with only mild to moderate skin toxicities at a lower frequency. On-target toxicity such as hyperglycemia was observed with CH5132799 as with other PI3K, AKT and m-TOR inhibitors (28-30, 35-37, 44, 45) although did not result in ketoacidosis was well-controlled by metformin. The favorable toxicity profile of CH5132799 may make it suitable to combine with other targeted agents such as epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) or MAPK/ERK kinase (MEK) inhibitors.
The PK data show it was possible to attain CH5132799 concentrations in patients that had been efficacious in xenograft models(39). The AUC increased proportionally with dose from 2-56 mg. The PD data indicated target modulation using phosphorylation of AKT at Sr473 as a proof of mechanism biomarker. Levels of p-AKT were reduced in PRP consistently at dose levels 8 – 96 mg. The maximal inhibition approximately 50% compared to baseline and this was achieved at dose levels of 32 mg. p-AKT levels in PRP frequently recovered by 24 hrs following treatment. The duration of PD biomarker changes in normal tissue and the fact that there were no significant further reduction in p-AKT levels after the 32 mg cohort, along with the fact that the AUC of CH5132799 did not rise significantly above 56 mg, led us to explore a twice-weekly schedule, despite not reaching an conventional MTD at 96 mg on the once-daily dosing cohorts. At the recommended phase II dose of 48 mg BID levels of p-AKT were reduced in PRP and in a limited number of post-treatment tumor biopsies. There was a reduction in levels of p-AKT (Ser473) in 2/3 tumor at the higher dose levels but there was considerable variation in reduction between patients (Figure 2c). The degree of p-AKT reduction in the tumor from patients were less than what was seen in xenograft models (39), however this could reflect different platforms to assess AKT phosphorylation and other factors such as intra-tumoral heterogeneity.
Further evidence of tumor target modulation was observed with FDG-PET scans with all patients at recommended dose. All five patients scanned at the RP2D having a reduction in SUVmax and two of them achieved a PET response by PERCIST criteria (46). Although the number of patients is too small to draw definitive conclusions, these PD data are highly encouraging. There was no correlation between the PD changes in the limited number of biopsies and PET responses or PRP inhibition. The FDG PET was done pre-treatment and on day 8 of treatment while tumor biopsies were performed pre-treatment and day 15 of treatment while the detailed p-AKT studies in PRP were done on day 1 of treatment. The small number of biopsies and the disparities in the days when tests were conducted could have led to the lack of correlation between p-AKT levels in tumor, PRP and changes in SUVmax on PET.
The eight study participants (21%) showing a best response of stable disease by RECIST included two participants who with clear clinical responses. In reports of other PI3K, AKT and mTOR inhibitors as single agent in patients with solid tumors, disease stabilization was observed, but only a few cases of radiological response have been reported (28-32, 34-36, 44). Therefore, there was clinical evidence as well as PD activity for CH5132799. These results indicated that CH5132799 was comparable to other PI3K, AKT and m-TOR inhibitors in terms of single agent activity.
Three patients were found to have PIK3CA mutations. Although there were no objective radiologic responses, it was interesting that one of these patients (with clear cell ovarian cancer) had a GCIG-defined partial response in her CA125 tumor marker. Another patient with triple negative breast cancer had a visible response in cutaneous metastases. This patient with breast cancer did not have any PIK3CA mutation, although triple negative breast cancer was known frequently to diseases associated with other perturbations in PI3K pathway (47). Pre-clinical models suggest that PIK3CA mutations predict for sensitivity to PI3K inhibitors as either single agents (48, 49) or in combination (50). This was also seen with preclinical studies of CH5132799(39). However, the clinical correlation of between PIK3CA mutations and PI3K inhibitors to this has not been fully realised (51) in the clinic and it is possible that other activating mutations (52) or intra-tumoral heterogeneity (53) may play a role.
We conclude that CH5132799 at RP2D of 48 mg BID is well tolerated and attains drug concentrations which show evidence of achieving target engagement in both normal tissues and tumor. CH5132799 has clinical as well as PD activity which was one with evidence of proof-of-mechanism from a clinical response in one patient with PIK3CA mutant ovarian cancer patient. The favorable toxicity profile and activity observed with single-agent CH5132799 suggests it is suitable for combination with other targeted therapies, and for exploration in subsets of patients with cancers harboring pre-defined mutations.
Supplementary Material
Translational Relevance.
CH5132799 is an oral pan-PI3 kinase inhibitor designed to target both alpha and beta isoforms of PI3K, which are frequently deregulated in cancer due to activating mutations in the oncogene PIK3CA or loss of function of the tumor suppressor gene, PTEN. The dosing schedule was to be determined by toxicity, PK and PD parameters and started with a QD schedule followed if necessary by a BID schedule. The trial started with CH5132799 administered orally once a day (QD) but a formal MTD was not reached at 96 mg however as there were no significant increments in AUC above a dose of 56 mg QD and p-AKT showed recovery at 24 hrs. Therefore, the dosing schedule was modified to include twice a day dosing (BID). A dose of 48 mg BID was declared the recommended phase II dose, at which dose a patient with PIK3CA mutant clear cell ovarian cancer responded.
Acknowledgements
The authors thank all the patients who took part in this study, together with their families and carers.
Research support: This study was funded by Chugai Pharmaceutical Co. Ltd. Infrastructure support was provided by a Cancer Research UK Joint Phase I Clinical Core Grant [grant number C51/A6883] to The Institute of Cancer Research and The Royal Marsden. The recruiting sites acknowledge infrastructural funding from the Experimental Cancer Medicine Centres and NIHR Biomedical Research Centre initiatives.
Footnotes
Authors’ conflicts of interest: S Blagden, D Josephs, C Stavraka, A Zivi, DJ Pinato, A Anthoney, C Twelves and J Spicer have declared no conflict of interest. K Noguchi, R Shiokawa and M Inatani are employees of Chugai Pharmaceutical Co Ltd, Japan; J Prince and K Jones are employees of Chugai Pharma Europe Ltd, London, UK; A Omlin, S Decordova, K Swales, R Riisnaes, L Pope and U Banerji are employees of The Institute of Cancer Research, which has received research funding from Chugai Pharmaceutical Co Ltd, Japan.
Previous presentation of data: This work has been presented in part at the 2012 ASCO Annual Meeting, Chicago (Omlin A et al. J Clin Oncol 30, 2012 [suppl; abstract 3022]) and the 2013 Targeted Anticancer Therapies meeting, Paris (Suder A et al. Ann Oncol 24,i33-i35, 2013 [suppl 1; abstract P06.08]).
References
- 1.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–75. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 4.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 6.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 8.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 9.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 10.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875–8. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 11.Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–41. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci U S A. 2008;105:2652–7. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 14.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 16.Salvesen HB, MacDonald N, Ryan A, Jacobs IJ, Lynch ED, Akslen LA, et al. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int J Cancer. 2001;91:22–6. doi: 10.1002/1097-0215(20010101)91:1<22::aid-ijc1002>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 19.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 20.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 21.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–92. [PubMed] [Google Scholar]
- 22.Carden CP, Stewart A, Thavasu P, Kipps E, Pope L, Crespo M, et al. The association of PI3 kinase signaling and chemoresistance in advanced ovarian cancer. Mol Cancer Ther. 2012;11:1609–17. doi: 10.1158/1535-7163.MCT-11-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–8. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–7. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 27.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 28.Baird RD, Kristeleit RS, Sarker D, Olmos D, Sandhu SK, Yan Y, et al. A phase I study evaluating the pharmacokinetics (PK) and pharmacodynamics (PD) of the oral pan-phosphoinositide-3 kinase (PI3K) inhibitor GDC-0941. J Clin Oncol. 2010;28(15 suppl):2613. [Google Scholar]
- 29.Wagner AJ, Von Hoff DH, LoRusso PM, Tibes R, Mazina KE, Ware JA, et al. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol. 2009;27(15 suppl):3501. [Google Scholar]
- 30.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 31.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O’Bryant CL, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–82. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 32.Brana I, LoRusso P, Baselga J, Heath EI, Patnaik A, Gendreau S, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(15 suppl):3030. [Google Scholar]
- 33.Edelman G, Bedell C, Shapiro G, Pandya SS, Kwak EL, Scheffold C, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(15 suppl):3004. [Google Scholar]
- 34.Furman RR, Byrd JC, Flinn IW, Coutre SE, Benson DM, Brown JR, et al. Interim results from a phase I study of CAL-101, a selective oral inhibitor of phosphatidylinositol 3-kinase p110d isoform, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2010;29(15 suppl):3032. [Google Scholar]
- 35.Moreno Garcia V, Baird RD, Shah KJ, Basu B, Tunariu N, Blanco M, et al. A phase I study evaluating GDC-0941, an oral phosphoinositide-3 kinase (PI3K) inhibitor, in patients with advanced solid tumors or multiple myeloma. J Clin Oncol. 2011;29(15 suppl):3021. [Google Scholar]
- 36.Wagner AJ, Bendell JC, Dolly S, Morgan JA, Ware JA, Fredrickson J, et al. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. J Clin Oncol. 2011;29(15 suppl):3020. [Google Scholar]
- 37.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 38.Ohwada J, Ebiike H, Kawada H, Tsukazaki M, Nakamura M, Miyazaki T, et al. Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg Med Chem Lett. 2011;21:1767–72. doi: 10.1016/j.bmcl.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka H, Yoshida M, Tanimura H, Fujii T, Sakata K, Tachibana Y, et al. The selective class I PI3K inhibitor CH5132799 targets human cancers harboring oncogenic PIK3CA mutations. Clin Cancer Res. 2011;17:3272–81. doi: 10.1158/1078-0432.CCR-10-2882. [DOI] [PubMed] [Google Scholar]
- 40.Chugai data on file: 2011.
- 41.National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 4.03. 2010 Jun 14; Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 42.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Rodon J, Bendell J, Abdul RA, Homji N, Trandafir L, Quadt C, et al. P3-16-01: Safety Profile and Clinical Activity of Single-Agent BKM120, a Pan-Class I PI3K Inhibitor, for the Treatment of Patients with Metastatic Breast Carcinoma. Cancer Res. 2011;(24 suppl) Abstract nr.P3-16-01. [Google Scholar]
- 44.Hudis C, Swanton C, Janjigian YY, Lee R, Sutherland S, Lehman R, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. doi: 10.1186/bcr3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J, Infante JR, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(15 suppl):3005. [Google Scholar]
- 46.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon V, Banerji S. Molecular pathways: PI3K pathway targets in triple-negative breast cancers. Clin Cancer Res. 2013;19:3738–44. doi: 10.1158/1078-0432.CCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–83. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 49.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther. 2014;13:1117–29. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 50.Spoerke JM, O’Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18:6771–83. doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- 51.Janku F, Hong David S, Fu S, Piha-Paul Sarina A, Naing A, Falchook Gerald S, et al. Assessing PIK3CA and PTEN in Early-Phase Trials with PI3K/AKT/mTOR Inhibitors. Cell Reports. 6:377–87. doi: 10.1016/j.celrep.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganesan P, Janku F, Naing A, Hong DS, Tsimberidou AM, Falchook GS, et al. Target-based therapeutic matching in early-phase clinical trials in patients with advanced colorectal cancer and PIK3CA mutations. Mol Cancer Ther. 2013;12:2857–63. doi: 10.1158/1535-7163.MCT-13-0319-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.