Abstract
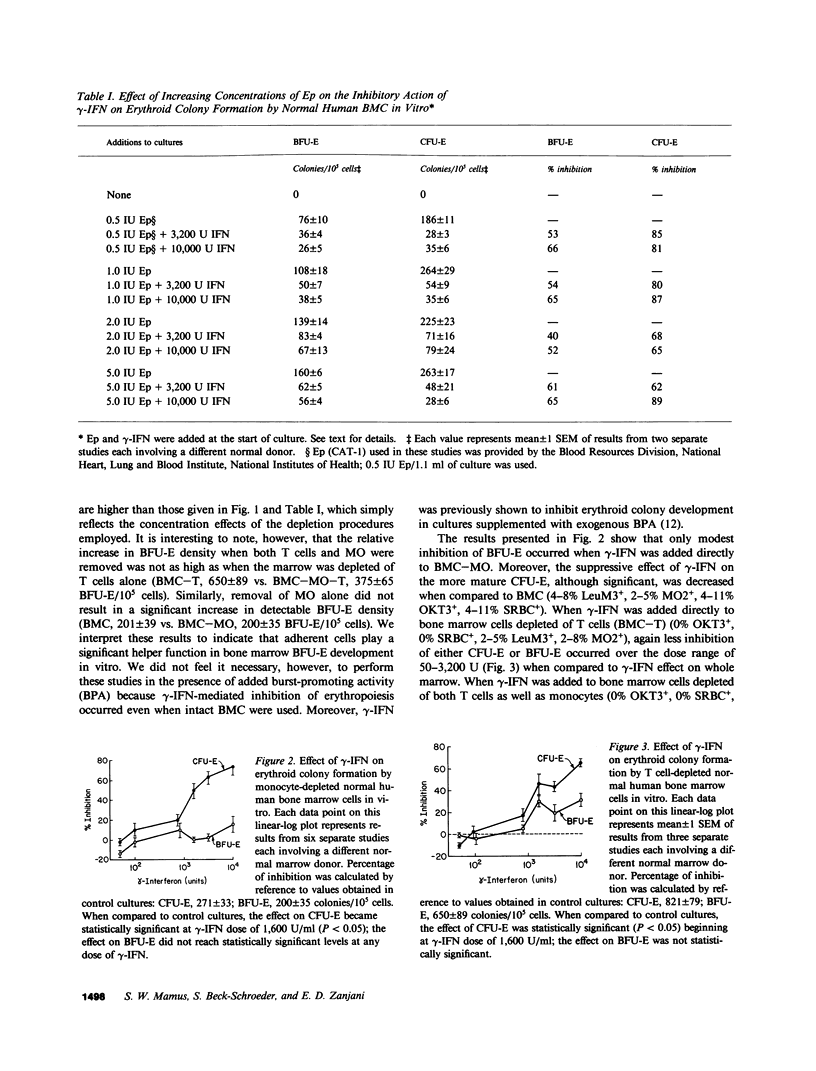
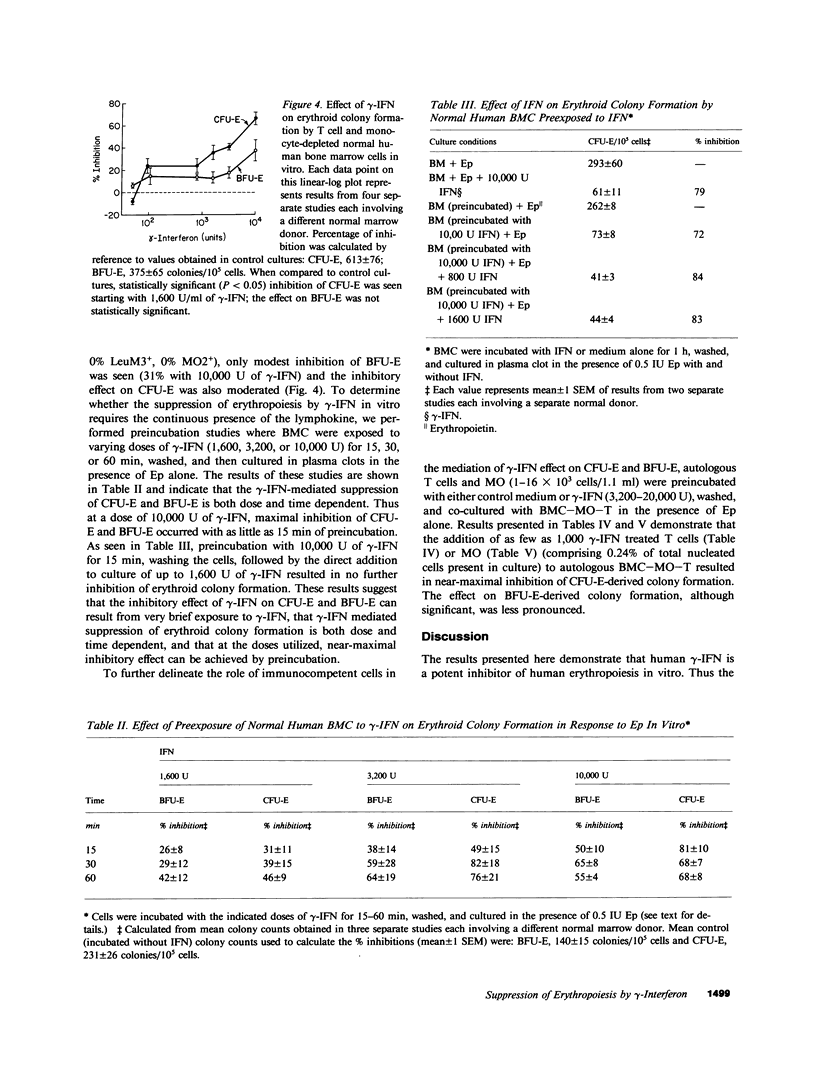
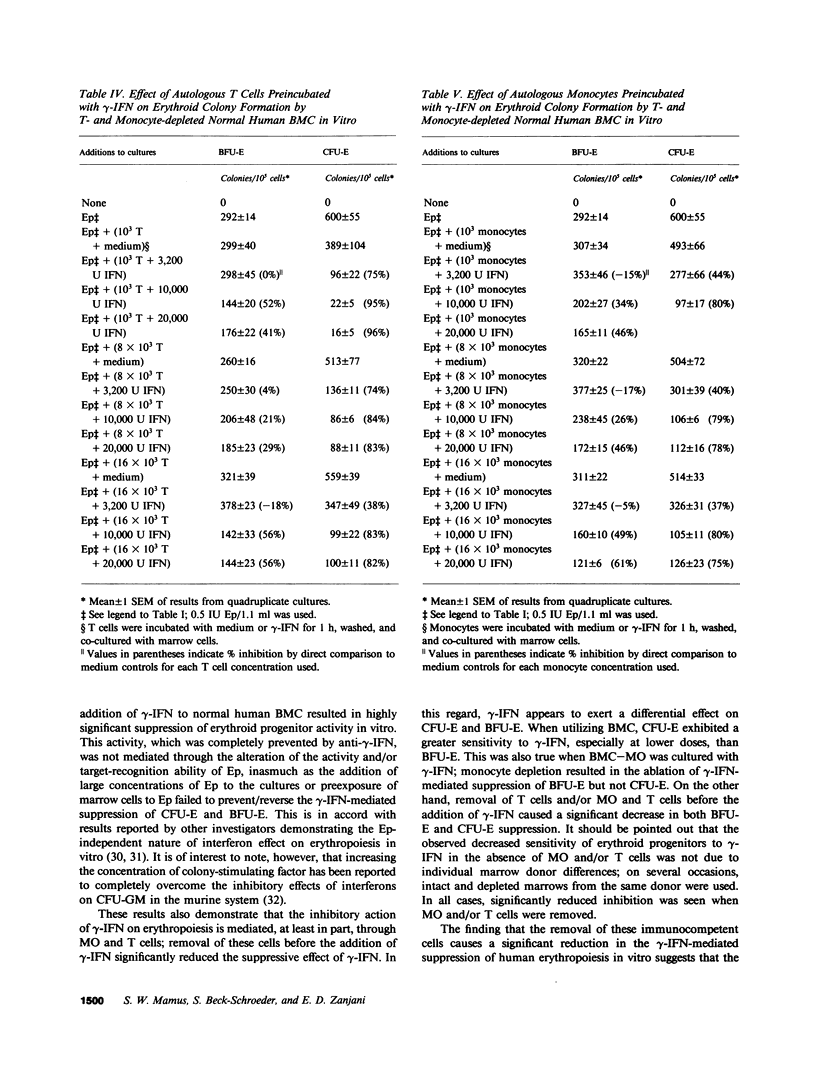
Interferons (IFN) have been shown to suppress the proliferation of human erythroid progenitors (erythroid burst-forming units [BFU-E] and colony-forming units [CFU-E]) in vitro. To examine the mechanism(s) underlying this inhibitory activity, the effect of different doses (50-10,000 U) of a highly purified preparation of recombinant DNA produced human gamma-IFN on erythroid colony formation by normal human bone marrow BFU-E and CFU-E in the presence and absence of monocytes and/or T lymphocytes was studied. The addition of gamma-IFN to whole marrow caused suppression of BFU-E (6-65%) and CFU-E (31-79%) in a dose-dependent fashion. This inhibition occurred both with the direct addition of gamma-IFN to the culture plates as well as by the preincubation of marrow cells with gamma-IFN followed by the washing of the cells; at the highest concentration of gamma-IFN (10,000 U), near-maximal inhibition of colony formation occurred with as little as 15 min of preexposure (BFU-E, 50%; CFU-E, 81%). Removal of monocytes and/or T lymphocytes before the addition of gamma-IFN significantly reduced the inhibitory effects of this lymphokine (BFU-E, -1 to 38%; CFU-E, -8 to 67%). Co-culture of purified autologous monocytes or T cells preexposed to gamma-IFN with monocyte and T cell-depleted marrow cells resulted in highly significant inhibition of erythroid colony formation even when these treated cells comprised less than 1% of the total nucleated cell populations in culture. The inhibitory action of gamma-IFN was not prevented or reversed by erythropoietin. These results demonstrate that the inhibitory effects of gamma-IFN on erythropoiesis are mediated to a significant degree through accessory cell populations, and suggest that gamma-IFN may represent a useful tool in the study of the role of immunocompetent cells in the regulation of erythropoiesis in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. D., Guyre P. M., Shen L., Glynn J. M., Maliszewski C. R., Baker P. E., Fanger M. W. Gamma interferon induces monocytoid differentiation in the HL-60 cell line. J Clin Invest. 1984 Apr;73(4):1072–1077. doi: 10.1172/JCI111292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E. Cellular interactions determine the rate and degree of interferon action. Infect Immun. 1979 Feb;23(2):496–501. doi: 10.1128/iai.23.2.496-501.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Platzer E., Feit C., Juliano L., Rubin B. Y. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983 Sep;131(3):1300–1305. [PubMed] [Google Scholar]
- Brunda M. J., Rosenbaum D. Modulation of murine natural killer cell activity in vitro and in vivo by recombinant human interferons. Cancer Res. 1984 Feb;44(2):597–601. [PubMed] [Google Scholar]
- Claeys H., Van Damme J., De Ley M., Vermylen C., Billiau A. Activation of natural cytotoxicity of human peripheral blood mononuclear cells by interferon: a kinetic study and comparison of different interferon types. Br J Haematol. 1982 Jan;50(1):85–94. doi: 10.1111/j.1365-2141.1982.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Dukes P. P., Izadi P., Ortega J. A., Shore N. A., Gomperts E. Inhibitory effects of interferon on mouse megakaryocytic progenitor cells in culture. Exp Hematol. 1980 Sep;8(8):1048–1056. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Identification of megakaryocytes, macrophages, and eosinophils in colonies of human bone marrow containing neurtophilic granulocytes and erythroblasts. Blood. 1979 May;53(5):1023–1027. [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. I., Miller W. J., Branda R. F., Zanjani E. D., Jacob H. S. Regulation of erythroid colony formation by bone marrow macrophages. Blood. 1980 Jun;55(6):1047–1050. [PubMed] [Google Scholar]
- Gresser I., Brouty-Boyé D., Thomas M. T., Macieira-Coelho A. Interferon and cell division. I. Inhibition of the multiplication of mouse leukemia L 1210 cells in vitro by interferon preparations. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1052–1058. doi: 10.1073/pnas.66.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Lutton J. D., Zalusky R., Wasserman L. R. Suppression of erythroid-colony formation by lymphocytes from patients with aplastic anemia. N Engl J Med. 1977 Jan 6;296(1):10–13. doi: 10.1056/NEJM197701062960103. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J., VALENTINE R. C. Virus interference. II. Some properties of interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):268–273. doi: 10.1098/rspb.1957.0049. [DOI] [PubMed] [Google Scholar]
- Ingimarsson S., Cantell K., Strander H. Side effects of long-term treatment with human leukocyte interferon. J Infect Dis. 1979 Oct;140(4):560–563. doi: 10.1093/infdis/140.4.560. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. M., Ernstoff M. S. Interferons in the treatment of human cancer. J Clin Oncol. 1984 Apr;2(4):336–352. doi: 10.1200/JCO.1984.2.4.336. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Fleischmann W. R., Jr, Klimpel K. D. Gamma interferon (IFN gamma) and IFN alpha/beta suppress murine myeloid colony formation (CFU-C)N: magnitude of suppression is dependent upon level of colony-stimulating factor (CSF). J Immunol. 1982 Jul;129(1):76–80. [PubMed] [Google Scholar]
- Koff W. C., Fidler I. J., Showalter S. D., Chakrabarty M. K., Hampar B., Ceccorulli L. M., Kleinerman E. S. Human monocytes activated by immunomodulators in liposomes lyse herpesvirus-infected but not normal cells. Science. 1984 Jun 1;224(4652):1007–1009. doi: 10.1126/science.6426057. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H. A., Fauser A. A. Effect of interferon on pluripotent hemopoietic progenitors (CFU-GEMM) derived from human bone marrow. Exp Hematol. 1982 Aug;10(7):587–590. [PubMed] [Google Scholar]
- Ortega J. A., Ma A., Shore N. A., Dukes P. P., Merigan T. C. Suppressive effect of interferon on erythroid cell proliferation. Exp Hematol. 1979 Mar;7(3):145–150. [PubMed] [Google Scholar]
- Preble O. T., Friedman R. M. Interferon-induced alterations in cells: relevance to viral and nonviral diseases. Lab Invest. 1983 Jul;49(1):4–18. [PubMed] [Google Scholar]
- Priestman T. J. Initial evaluation of human lymphoblastoid interferon in patients with advanced malignant disease. Lancet. 1980 Jul 19;2(8186):113–118. doi: 10.1016/s0140-6736(80)90004-5. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Fredrickson T. N., Mobraaten L. E., DeMaeyer E. The interaction of erythropoietin with fetal liver cells. II. Inhibition of the erythropoietin effect by interferon. Exp Hematol. 1977 Sep;5(5):333–340. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Van Agthoven A., Schlossman S. F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1(3):329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Verma D. S., Spitzer G., Gutterman J. U., Zander A. R., McCredie K. B., Dicke K. A. Human leukocyte interferon preparation blocks granulopoietic differentiation. Blood. 1979 Dec;54(6):1423–1427. [PubMed] [Google Scholar]
- Young N., Mortimer P. Viruses and bone marrow failure. Blood. 1984 Apr;63(4):729–737. [PubMed] [Google Scholar]
- Zanjani E. D., McGlave P. B., Davies S. F., Banisadre M., Kaplan M. E., Sarosi G. A. In vitro suppression of erythropoiesis by bone marrow adherent cells from some patients with fungal infection. Br J Haematol. 1982 Mar;50(3):479–490. doi: 10.1111/j.1365-2141.1982.tb01944.x. [DOI] [PubMed] [Google Scholar]
- Zoumbos N. C., Djeu J. Y., Young N. S. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J Immunol. 1984 Aug;133(2):769–774. [PubMed] [Google Scholar]
- Zuckerman K. S. Human erythroid burst-forming units. Growth in vitro is dependent on monocytes, but not T lymphocytes. J Clin Invest. 1981 Mar;67(3):702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Hull E., Schellekens H., Löwenberg B., de Vries M. J. Influence of interferon preparations on the proliferative capacity of human and mouse bone marrow cells in vitro. Cancer Res. 1978 Apr;38(4):911–914. [PubMed] [Google Scholar]


